Acute Toxicity of an Emerging Insecticide Pymetrozine to Procambarus clarkii Associated with Rice-Crayfish Culture (RCIS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Test Organisms and Chemical
2.3. Test Conditions
2.4. Acute Toxicity Tests
2.5. Histopathology Test
2.6. Statistical Analysis
3. Results
3.1. Mortality, LC50 Values and MAC
3.2. Behavioral Responses
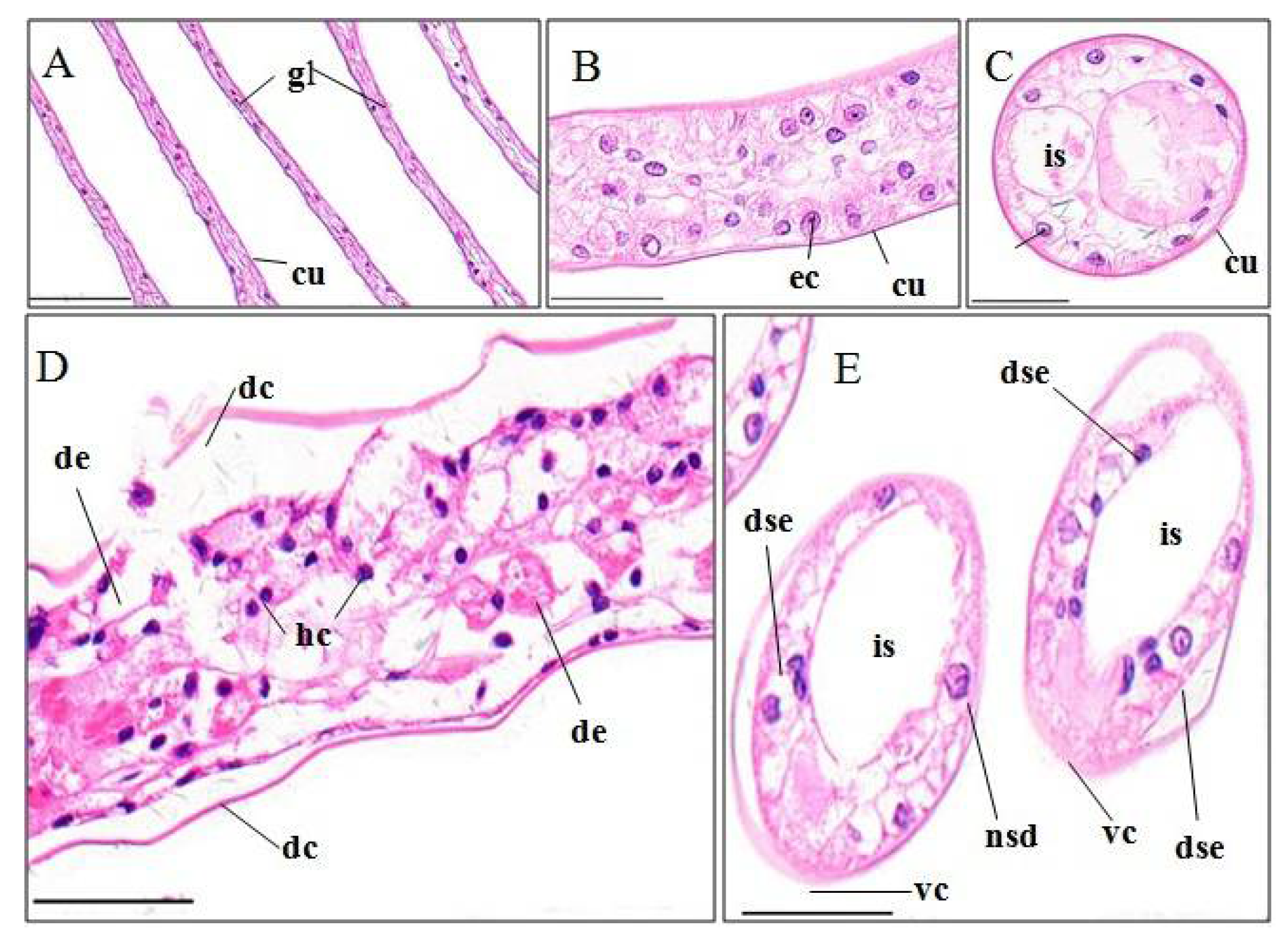
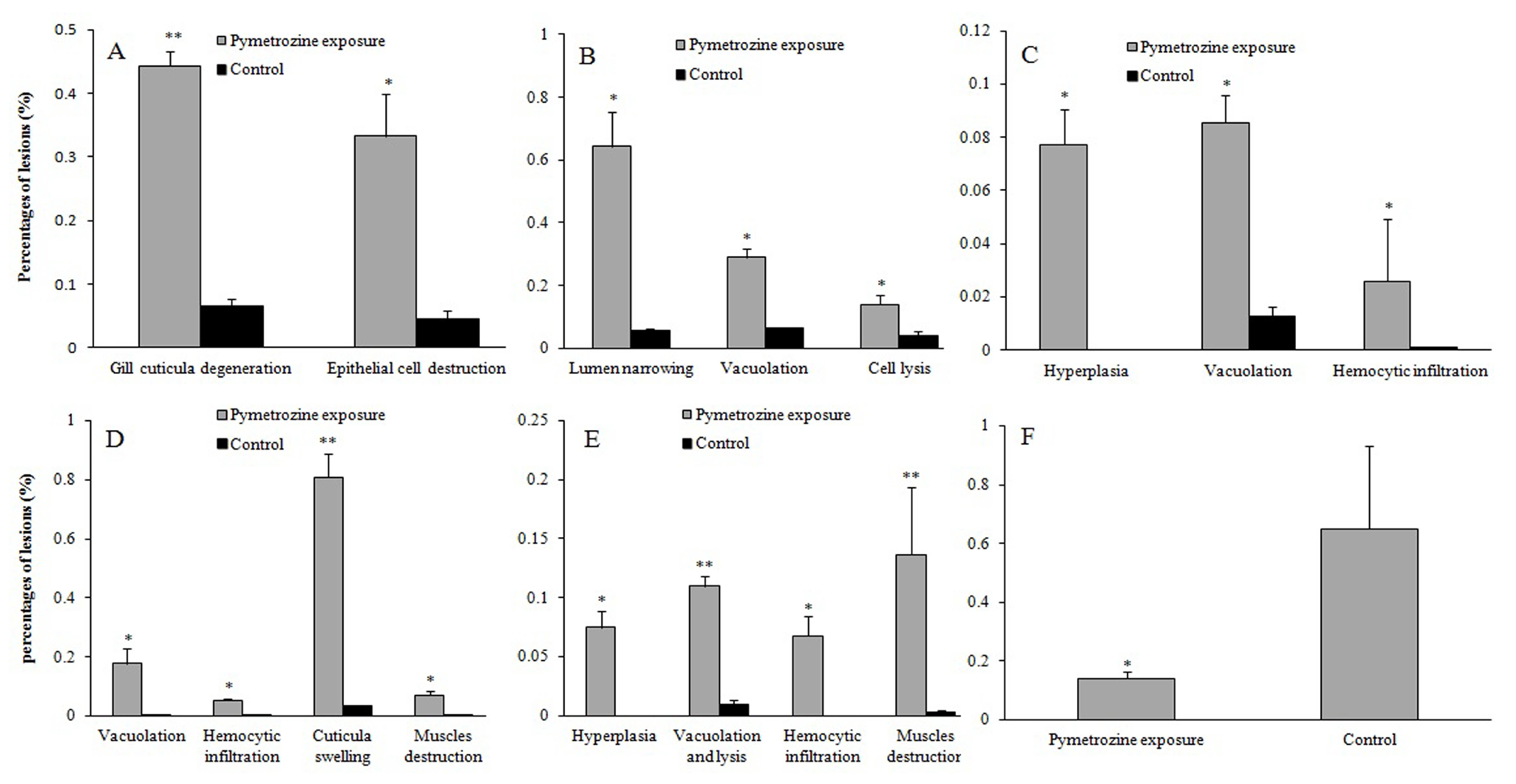
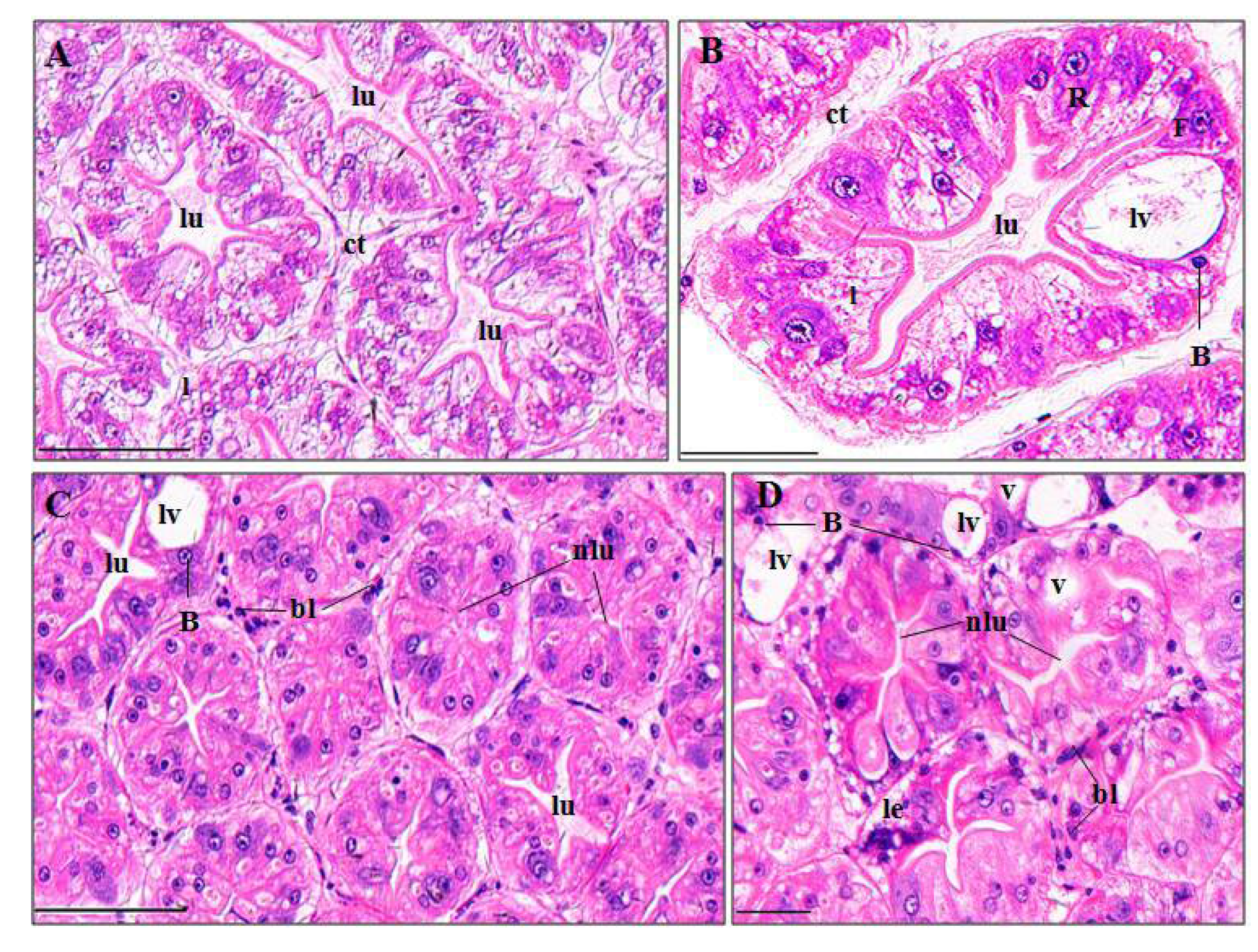
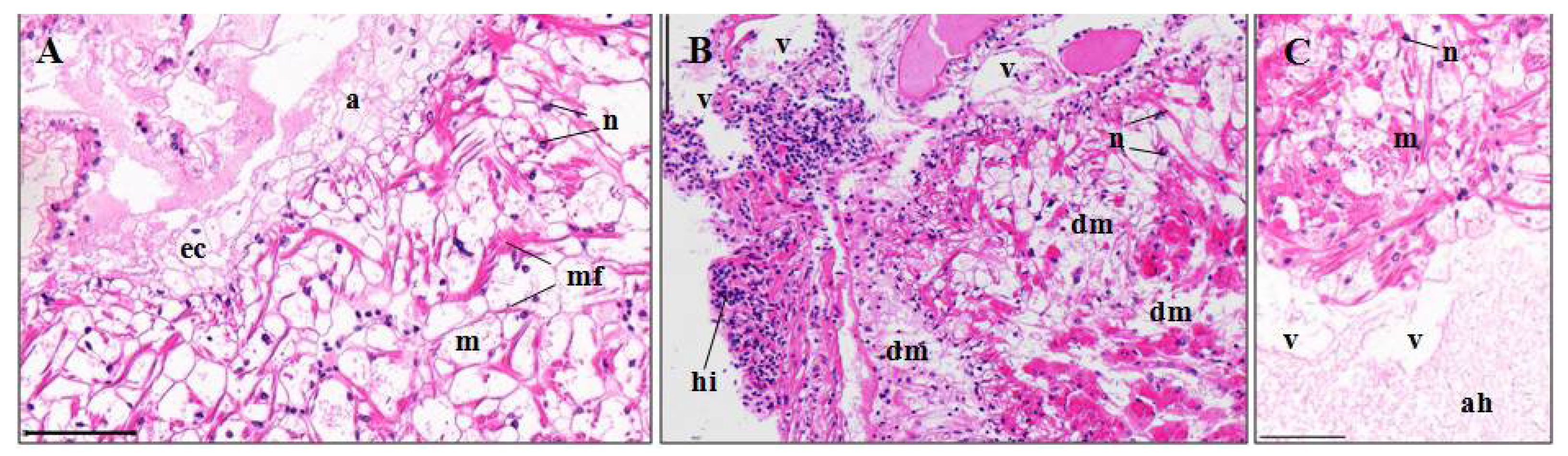
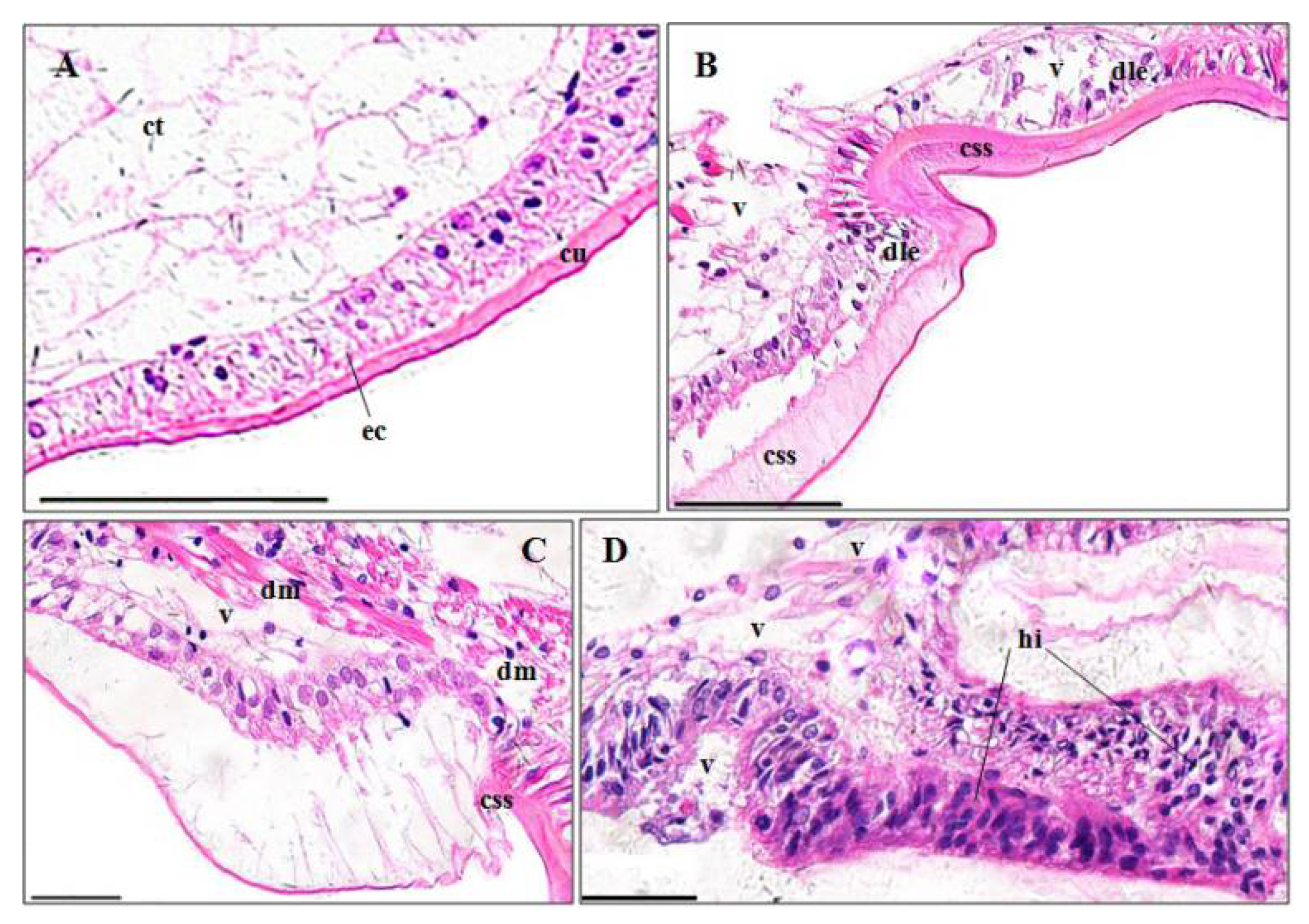
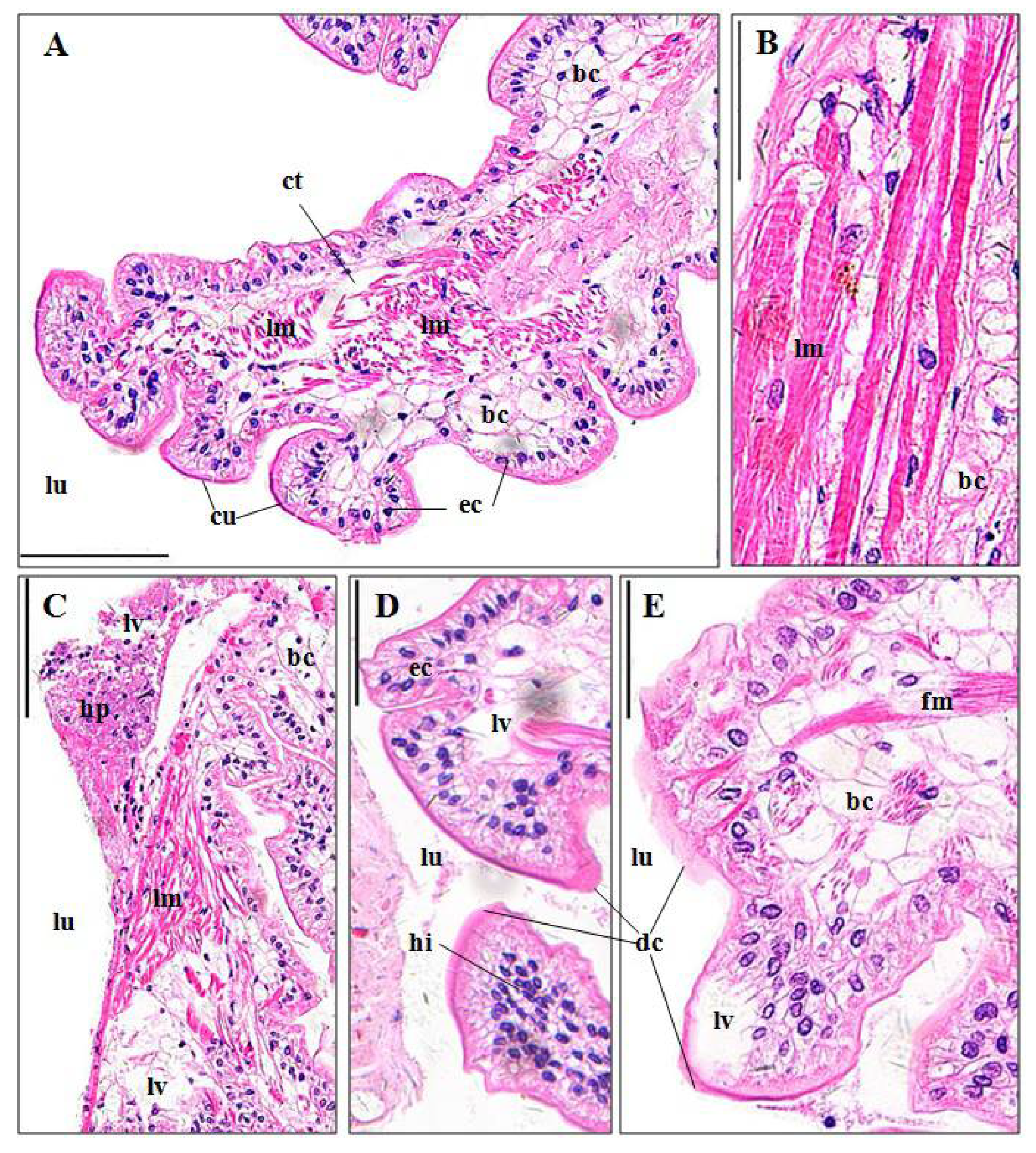
3.3. Histopathological Effects of Pymetrozine
3.3.1. Gills
3.3.2. Perigastric Organs
3.3.3. Hearts
3.3.4. Stomachs
3.3.5. Midguts
3.3.6. Abdominal Muscles
4. Discussion
4.1. Lethal Effects of Pymetrozine
4.2. Behavioral Effects of Pymetrozine
4.3. Histopathological Effects of Pymetrozine
4.3.1. Gill
4.3.2. Perigastric Organ
4.3.3. Heart
4.3.4. Stomach
4.3.5. Midgut
4.3.6. Abdominal Muscle
4.4. Pymetrozine Safety Evaluation for RCIS
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fornstrom, C.B.; Landrum, P.F.; Weisskopf, C.P.; La Point, T.W. Effects of terbufos on juvenile red swamp crayfish (Procambarus clarkii): Differential routes of exposure. Environ. Toxicol. Chem. 1997, 16, 2514–2520. [Google Scholar] [CrossRef]
- Morolli, C.; Quaglio, F.; Rocca, G.D.; Malvisi, J.; Salvo, A.D. Evaluation of the toxicity of synthetic pyrethroids to red swamp crayfish (Procambarus clarkii, Girard 1852) and Common carp (Cyprinus carpio, L. 1758). Bull. Fr. Pêche Piscic. 2006, 380–381, 1381–1394. [Google Scholar] [CrossRef]
- Desouky, M.M.A.; Abdel-Gawad, H.; Hegazi, B. Distribution, fate and histopathological effects of ethion insecticide on selected organs of the crayfish, Procambarus clarkii. Food Chem. Toxicol. 2013, 52, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, C.; Andreu-Moliner, E.S.; Fernández-Casalderrey, A.; Ferrando, M.D. Acute toxicity and oxygen consumption in the gills of Procambarus clarkii in relation to chlorpyrifos exposure. Bull. Environ. Contam. Toxicol. 1992, 49, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Stara, A.; Zuskova, E.; Kouba, A.; Velisek, J. Effects of terbuthylazine-desethyl, a terbuthylazine degradation product, on red swamp crayfish (Procambarus clarkii). Sci. Total Environ. 2016, 566–567, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Xu, E.G.; Ren, Y.; Jin, S.Y.; Zhang, T.L.; Liu, J.S.; Li, Z.J. Mixture toxicity of bensulfuron-methyl and acetochlor to red swamp crayfish (Procambarus clarkii): Behavioral, morphological and histological effects. Int. J. Environ. Res. Public Health 2017, 14, 1466. [Google Scholar] [CrossRef] [PubMed]
- FAO. Cultured Aquatic Species Information Programme. Procambarus clarkii (Girard, 1852); FAO: Rome, Italy, 2016; Available online: http://www.fao.org/fishery/culturedspecies/Procambarus_clarkii/en (accessed on 27 April 2018).
- Guo, Y.F.; Zhu, W.Z.; Ma, D.W.; Tang, J.Q. China Crayfish Industry Development Report. Fish. Adv. Mag. 2017, 7, 85–91. (In Chinese) [Google Scholar]
- Cheah, M.L.; Avault, J.W., Jr.; Graves, J.B. Acute toxicity of selected rice pesticides to crayfish Procambarus clarkii. Prog. Fish Cult. 1980, 42, 169–172. [Google Scholar] [CrossRef]
- Barbee, G.C.; McClain, W.R.; Lanka, S.K.; Stout, M.J. Acute toxicity of chlorantraniliprole to non-target crayfish (Procambarus clarkii) associated with rice-crayfish cropping systems. Pest Manag. Sci. 2010, 66, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Sommer, T.R. Laboratory and field studies on the toxic effects of thiobencarb (Bolero) to the crawfish Procambarus clarkii. J. World Aquac. Soc. 2010, 14, 434–440. [Google Scholar] [CrossRef]
- Biever, R.C.; Hoberg, J.R.; Jacobson, B.; Dionne, E.; Sulaiman, M.; Mccahon, P. Icon® rice seed treatment toxicity to crayfish (Procambarus clarkii) in experimental rice paddies. Environ. Toxicol. Chem. 2003, 22, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, T.; Huangfu, W.G.; Wu, Y.L. Residues and dynamics of pymetrozine in rice field ecosystem. Chemosphere 2011, 82, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Preetha, G.; Stanley, J.; Suresh, S.; Kuttalam, S.; Samiyappan, R. Toxicity of selected insecticides to Trichogramma chilonis: Assessing their safety in the rice ecosystem. Phytoparasitica 2009, 37, 209–215. [Google Scholar] [CrossRef]
- Ma, J.H.; Jiang, Y.P.; Wang, W.M.; Zhu, H.L.; Qiao, D.F. Primary study of acute toxicity of pymetrozine to Penaeus vannamei. Shanghai Agric. Sci. Technol. 2012, 3, 66–68. (In Chinese) [Google Scholar]
- Barbee, G.C.; Stout, M.J. Comparative acute toxicity of neonicotinoid and pyrethroid insecticides to non-target crayfish (Procambarus clarkii) associated with rice-crayfish crop rotations. Pest Manag. Sci. 2009, 65, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Zhang, L.; Xu, P.; Li, J.Z.; Wang, H.L. Dissipation and residue of pymetrozine in rice field ecosystem. Environ. Monit. Assess. 2015, 187, 78. [Google Scholar] [CrossRef] [PubMed]
- Sechser, B. Pymetrozine (CGA 215944): Suitability for Integrated Pest Management (IPM); No. 22; Ciba Insect Control Newsletter: Vienna, Austria, 1993; p. 16. [Google Scholar]
- US EPA. United States Environmental Protection Agency. 2000. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-101103_01-Aug-00.pdf (accessed on 27 April 2018).
- Hu, S.Q.; Yin, D.Q.; Chen, L.Y. Safety Evaluation of Four New Pesticides in Aquatic Ecosystem. Rural Eco-Environ. 2002, 18, 23–26. (In Chinese) [Google Scholar]
- Chen, C.N.; Huang, Y.Y.; Chen, X.J.; Liu, Y.; Du, J. Acute toxicity of three pesticides to juvenile allogynogenetic crucian carp. Southwest China. J. Agric. Sci. 2014, 27, 1309–1313. (In Chinese) [Google Scholar]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. Histopathology of lambda-cyhalothrin on tissues (gill, kidney, liver and intestine) of Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2007, 24, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.F.; Xue, H.; Wang, X.F.; Tang, J.Q. Acute toxicity of chlorpyrifos (CPF) to crayfish (Procambarus clarkii) and the histopathological observation. J. Ecol. Rural Environ. 2012, 28, 462–467. (In Chinese) [Google Scholar]
- US Environmental Protection Agency (US EPA). Hazard Evaluation Division, Standard Evaluation Procedure: Acute Toxicity for Freshwater Fish; (PB86-129277) US EPA: Washington, DC, USA, 1985. [Google Scholar]
- Medina, P.; Morales, J.J.; Budia, F.; Adan, A.; Estal, P.D.; Viñuela, E. Compatibility of endoparasitoid Hyposoter didymator (Hymenoptera: Ichneumonidae) protected stages with five selected insecticides. J. Econ. Entomol. 2007, 100, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Hebel, D.K.; Jones, M.B.; Depledge, M.H. Responses of crustaceans to contaminant exposure: A holistic approach. Estuar. Coast. Shelf Sci. 1997, 44, 177–184. [Google Scholar] [CrossRef]
- Benli, A.Ç.K.; Sarıkaya, R.; Sepici-Dincel, A.; Selvi, M.; Şahin, D.; Erkoç, F. Investigation of acute toxicity of (2,4-dichlorophenoxy)acetic acid (2,4-D) herbicide on crayfish (Astacus leptodactylus, Esch. 1823). Pestic. Biochem. Phys. 2007, 88, 296–299. [Google Scholar] [CrossRef]
- Benli, A.C. The influence of etofenprox on narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823): Acute toxicity and sublethal effects on histology, hemolymph parameters, and total hemocyte counts. Environ. Toxicol. 2015, 30, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.S.; Naqvi, S.M.; Naqvi, N.Z. Paraquat toxicity to louisiana crayfish (Procambarus clarkii). Bull. Environ. Contam. Toxicol. 1980, 25, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Browne, A.M.; Moore, P.A. The effects of sublethal levels of 2,4-dichlorophenoxyacetic acid herbicide (2,4-D) on feeding behaviors of the crayfish O. rusticus. Arch. Environ. Contam. Toxicol. 2014, 67, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.R.; Doughtie, D.G. Histopathological changes in grass shrimp exposed to chromium, pentachlorophenol and dithiocarbamates. Mar. Environ. Res. 1984, 14, 371–395. [Google Scholar] [CrossRef]
- Chang, C.C.; Lee, P.P.; Hsu, J.P.; Yeh, S.P.; Cheng, W. Survival, and biochemical, physiological, and histopathological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to short-term trichlorfon exposure. Aquaculture 2006, 253, 653–666. [Google Scholar] [CrossRef]
- Lignot, J.H.; Trilles, J.P.; Charmantier, G. Effect of an organophosphorus insecticide, fenitrothion, on survival and osmoregulation of various developmental stages of the shrimp Penaeus japonicus (Crustacea: Decapoda). Mar. Biol. 1997, 128, 307–316. [Google Scholar] [CrossRef]
- Vogt, G.; Quinitio, E.T. Accumulation and excretion of metal granules in the prawn, Penaeus monodon, exposed to water-borne copper, lead, iron and calcium. Aquat. Toxicol. 1994, 28, 223–241. [Google Scholar] [CrossRef]
- Bhavan, P.S.; Geraldine, P. Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposed to endosulfan. Aquat. Toxicol. 2000, 50, 331–339. [Google Scholar] [CrossRef]
- Wei, K.Q.; Yang, J.X. Histological alterations and immune response in the crayfish Procambarus clarkii given rVP28-incorporated diets. Fish Shellfish Immun. 2011, 31, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.J.; Du, Y.B.; Lam, P.K.S.; Wu, R.S.S.; Zhou, B.S. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol. Appl. Pharm. 2008, 230, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Suvarchala, G.; Philip, G.H. Toxicity of 3,5,6-trichloro-2-pyridinol tested at multiple stages of zebrafish (Danio rerio) development. Environ. Sci. Pollut. Res. 2016, 23, 15515–15523. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.G.; Mager, E.M.; Grosell, M.; Pasparakis, C.; Schlenker, L.S.; Stieglitz, J.D.; Benetti, D.; Hazard, E.S.; Courtney, S.M.; Diamante, G.; et al. Time- and oil-dependent transcriptomic and physiological responses to Deepwater Horizon oil in mahi-mahi (Coryphaena hippurus) embryos and larvae. Environ. Sci. Technol. 2016, 50, 7842–7851. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jia, Y.L.; Su, G.Y.; Sun, Y.K.; Letcher, R.J.; Giesy, J.P.; Yu, H.X.; Han, Z.H.; Liu, C.S. Parental transfer of tris(1,3-dichloro-2-propyl) phosphate and transgenerational inhibition of growth of zebrafish exposed to environmentally relevant concentrations. Environ. Pollut. 2017, 220, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y. Toxic Effects of Triazophos on Penaeus monodon and Two Bivalves. Master’s Thesis, Shanghai Ocean University, Shanghai, China, June 2013. (In Chinese). [Google Scholar]
- Kaushik, N.; Kumar, S. Midgut pathology of aldrin, monocrotophos, and carbaryl in the freshwater crab, Paratelphusa masoniana (Henderson). Bull. Environ. Contam. Toxicol. 1998, 60, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, P.; Fox, P.J.; Douthwaite, R.J.; Wood, A.B. Accumulation of endosulfan residues in fish and their predators after aerial spraying for the control of tsetse fly in Botswana. Pest Manag. Sci. 1982, 13, 39–48. [Google Scholar] [CrossRef]
- Ballesteros, M.L.; Gonzalez, M.; Wunderlin, D.A.; Bistoni, M.A.; Miglioranza, K.S.B. Uptake, tissue distribution and metabolism of the insecticide endosulfan in Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Environ. Pollut. 2011, 159, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Sierra, A.; Alonso, Á.; Camargo, J.A. Fluoride bioaccumulation and toxic effects on the survival and behavior of the endangered white-clawed crayfish Austropotamobius pallipes (Lereboullet). Arch. Environ. Contam. Toxicol. 2013, 65, 244–250. [Google Scholar] [CrossRef] [PubMed]


| Organism | LC50/EC50 (mg/L) | US EPA Toxicity Category | Reference |
|---|---|---|---|
| Oncorhynchus mykiss | >128 (96 h LC50) | Practically non | [17] |
| Lepomis macrochirus | >134 (96 h LC50) | Practically non | [17] |
| Cyprinodon variegatus | >117 (96 h LC50) | Practically non | [17] |
| Brachydanio rerio | 119.84 (96 h LC50) | Practically non | [18] |
| Carassais auratus gibebio | 387 (96 h LC50) | Practically non | [19] |
| Lemna gibba | >109 (EC50) | Slightly | [17] |
| Daphnia magna | 87 (48 h EC50) | Slightly | [17] |
| Kirchneria subcapitata | 17 (EC50) | Slightly | [17] |
| Hyposoter didymator | – | Harmless | [20] |
| Trichogramma chilonis | 0.96 (48 h LC50) | Slightly–moderately | [21] |
| Time (h) | Regression Equation | R2 | LC50 (mg/L) | 95% Confidence Limits (mg/L) |
|---|---|---|---|---|
| 24 | P = -0.050 + 3.472C | 0.927 | 1.034 | 0.875–1.392 |
| 48 | P = 0.316 + 2.254C | 0.904 | 0.724 | 0.588–0.942 |
| 72 | P = 0.650 + 2.515C | 0.942 | 0.551 | 0.452–0.671 |
| 96 | P = 0.880 + 2.753C | 0.973 | 0.479 | 0.393–0.571 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xu, E.G.; Li, W.; Jin, S.; Yuan, T.; Liu, J.; Li, Z.; Zhang, T. Acute Toxicity of an Emerging Insecticide Pymetrozine to Procambarus clarkii Associated with Rice-Crayfish Culture (RCIS). Int. J. Environ. Res. Public Health 2018, 15, 984. https://doi.org/10.3390/ijerph15050984
Yu J, Xu EG, Li W, Jin S, Yuan T, Liu J, Li Z, Zhang T. Acute Toxicity of an Emerging Insecticide Pymetrozine to Procambarus clarkii Associated with Rice-Crayfish Culture (RCIS). International Journal of Environmental Research and Public Health. 2018; 15(5):984. https://doi.org/10.3390/ijerph15050984
Chicago/Turabian StyleYu, Jixin, Elvis Genbo Xu, Wei Li, Shiyu Jin, Ting Yuan, Jiashou Liu, Zhongjie Li, and Tanglin Zhang. 2018. "Acute Toxicity of an Emerging Insecticide Pymetrozine to Procambarus clarkii Associated with Rice-Crayfish Culture (RCIS)" International Journal of Environmental Research and Public Health 15, no. 5: 984. https://doi.org/10.3390/ijerph15050984
APA StyleYu, J., Xu, E. G., Li, W., Jin, S., Yuan, T., Liu, J., Li, Z., & Zhang, T. (2018). Acute Toxicity of an Emerging Insecticide Pymetrozine to Procambarus clarkii Associated with Rice-Crayfish Culture (RCIS). International Journal of Environmental Research and Public Health, 15(5), 984. https://doi.org/10.3390/ijerph15050984





