Adsorption Performance Analysis of Alternative Reactive Media for Remediation of Aquifers Affected by Heavy Metal Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Materials Tested
2.1.1. Cabuya Fibers
- Water absorption: 60.0% as average value [33];
2.1.2. Ecuador Limestone
2.1.3. Natural Zeolite
2.2. Synthetic Materials Tested
2.2.1. Synthetic Zeolite
2.2.2. Zero Valent Iron (ZVI)
2.3. Heavy Metals Investigated
2.4. Laboratory Investigations
2.5. Characterization of the Investigated Materials
2.5.1. Cabuya Fibers
2.5.2. Ecuador Limestone
2.5.3. Natural Zeolite
2.5.4. Synthetic Zeolite
2.5.5. ZVI
3. Results and Discussion
3.1. Adsorption Trends Analysis
3.1.1. Copper
3.1.2. Zinc
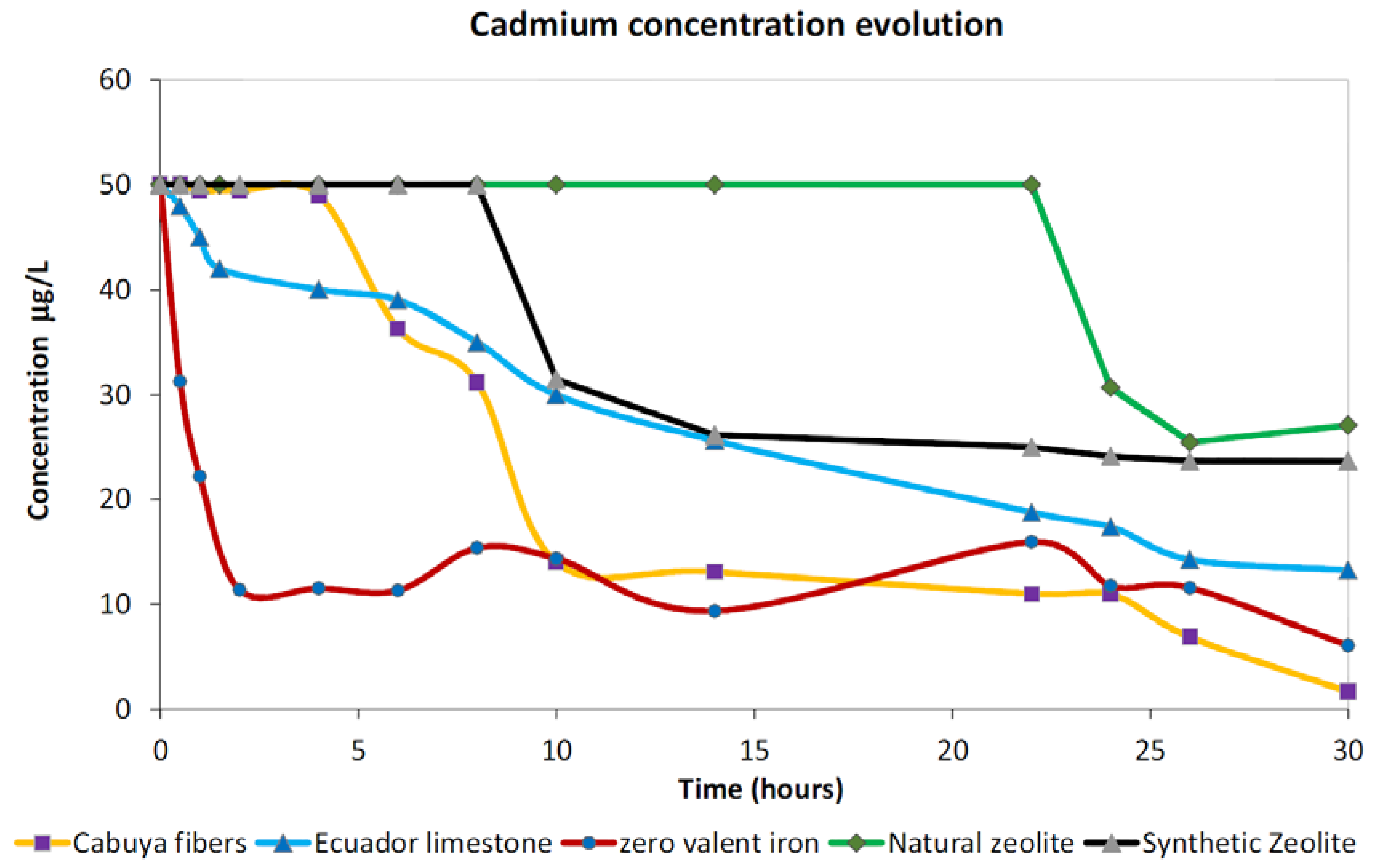
3.1.3. Cadmium
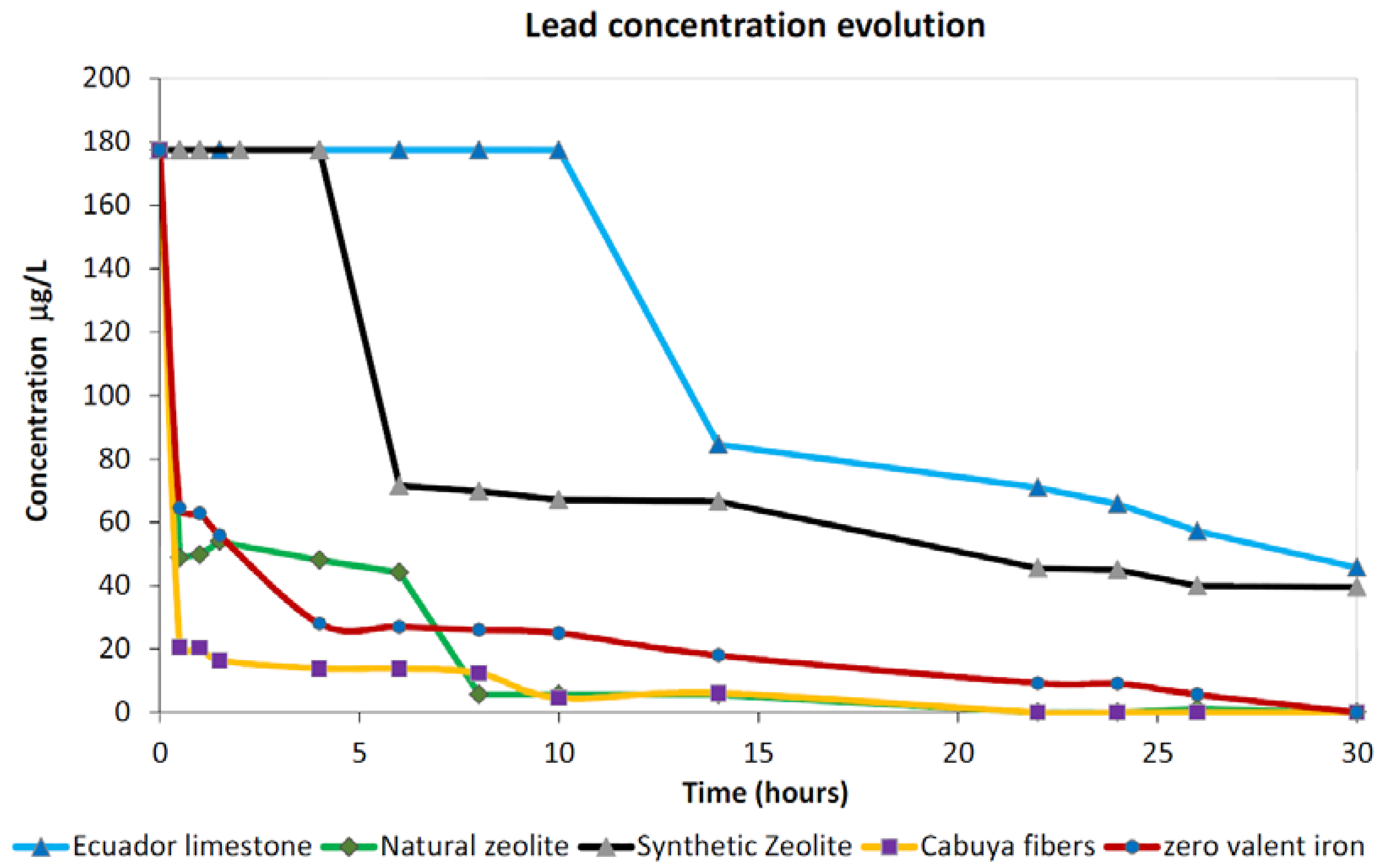
3.1.4. Lead
3.2. Heavy Metals Removal Comparison
3.3. Analysis of the Main Removal Mechanisms
4. Conclusions
- Large adsorption percentages (>90%) can be observed by employing, for the remediation of Cu- and Zn-contaminated slurry, all materials considered in the present study. Important removal performances can be observed after about 12 h of contact time in batch conditions. Cd removal by employing reactants whose main mechanism is surface adsorption, such as those considered in the present study, can require up to 25 h or more of contact time, in batch conditions, before useful removal percentages are observed, especially in the case of zeolites, both natural and synthetic, which can result in quite ineffective performances for the adsorption of this metal. High-adsorption performances for Pb can be observed for ZVI, cabuya fibers and natural zeolite, while results that are not competitive can be observed in the cases of limestone and synthetic zeolite.
- Cabuya fibers and natural zeolite can exhibit a neutral adsorption behavior at pH close to 7, while Ecuador limestone and ZVI could not exhibit adsorption abilities for pH values close to 8. The absence of adsorption can be observed for synthetic zeolite in alkaline conditions (pH = 9.6).
- Due to field conditions where several factors (e.g., rainwater recharge of groundwater reservoirs, redox and pH changes, organic matter occurrence, flux conditions, water-table oscillations and so on) can play a key role in the dynamics of dissolved species, our findings, obtained under controlled conditions, cannot be directly transferred to field applications. At the same time, our study demonstrates that all tested materials, both natural and synthetic, can be very effective at heavy metals removal, exhibiting a large potential for adsorption of these types of contaminant. Removal percentages stemming from our findings observed comparable and sometimes larger values than ZVI, which is the reactant usually employed for PRB remediation, highlighting the fact that these alternative media, after further field test investigations, can be considered as very competitive for the treatment of either groundwater or wastewater.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNESCO. Estrategia Regional para la Evaluación y Gestión de los Sistemas Acuíferos Transfronterizos en las Américas; Programa Hidrológico Internacional para América Latina y el Caribe (PHI-LAC): Montevideo, Uruguay, 2015. [Google Scholar]
- National Research Council (NRC). Contaminants in the Subsurface: Source Zone Assessment and Remediation; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Di Molfetta, A.; Sethi, R. Metodologie di bonifica di siti contaminati. Siti Contam. 2000, 1, 16–24. [Google Scholar]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Rivett, M.O.; Petts, J.; Butler, B.; Martin, I. Remediation of contaminated land and groundwater: Experience in England and Wales. J. Environ. Manag. 2002, 65, 251–268. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Choong, T.S.Y.; Chuaha, T.G.; Robiaha, Y.; Koaya, F.L.G.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Malik, A.H.; Khan, Z.M.; Mahmood, Q.; Nasreen, S.; Bhatti, Z.A. Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J. Hazard. Mater. 2009, 168, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Di Molfetta, A.; Sethi, R. Criteri di Progettazione di Barriere Permeabili Reattive a Ferro Zero Valente; Dipartimento di Georisorse e Territorio, Politecnico di Torino: Torino, Italy, 2001. [Google Scholar]
- Pattanayak, J.; Mondal, K.; Mathew, S.; Lalvani, S.B. A parametric evaluation of the removal of As(V) and As(III) by carbon-based adsorbents. Carbon 2000, 38, 589–596. [Google Scholar] [CrossRef]
- Chen, J.P.; Wang, X. Removal of copper, zinc and lead ion by activated carbon in pre-treated fixed bed columns. Sep. Purif. Technol. 2000, 19, 157–167. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; Alok, M.; Rathore, M.; Gupta, V. Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin. Water Treat. 2015, 54, 2883–2890. [Google Scholar] [CrossRef]
- Naushad, M.; AL-Othman, Z.A.; Islam, M. Adsorption of cadmium ion using a new composite cationexchanger polyaniline Sn(IV) silicate: Kinetics, thermodynamic and isotherm studies. Int. J. Environ. Sci. Technol. 2013, 10, 567–578. [Google Scholar] [CrossRef]
- Naushad, M.; AL-Othman, Z.A.; Mohammad, M.A.; Rabiul, A.; Gaber, E.E.; Mahamudur, S. Synthesis of sodium dodecyl sulfate-supported nanocomposite cation exchanger: Removal and recovery of Cu2+ from synthetic, pharmaceutical and alloy samples. J. Iran. Chem. Soc. 2015, 12, 1677–1686. [Google Scholar] [CrossRef]
- Naushad, M.; AL-Othman, Z.A.; Rabiul Awual, M.; Mohammad, M.A.; Eldesoky, G.E. Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics 2015, 21, 2237–2245. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.B.; Benner, S.G.; Blowes, D.W. Solid phase iron sulfur geochemistry of a reactive barrier for treatment of mine drainage. Appl. Geochem. 2000, 15, 1331–1343. [Google Scholar] [CrossRef]
- Korte, N.E. Zero-Valent Iron Permeable Reactive Barriers: A Review of Performance; Environmental Sciences Division Publication No. 5056; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2001.
- USEPA. Long-Term Performance of Permeable Reactive Barriers Using Zero-Valent Iron: An Evaluation at Two Sites; National Risk Management Research Lab ADA: Cincinnati, OH, USA, 2002.
- Gavaskar, A.; Tatar, L.; Condit, W. Cost and Performance Report: Nanoscale Zero-Valent Iron Technologies for Source Remediation; Contract Report CR-05-007-ENV; Naval Facilities Engineering Command, Engineering Service Center: Port Hueneme, CA, USA, 2005; pp. 93043–94370. [Google Scholar]
- Rangsivek, R.; Jekel, M.R. Removal of dissolved metals by zero-valent iron (ZVI): Kinetics, equilibria, processes and implications for storm water runoff treatment. Water Res. 2005, 39, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, D.; Simon, Y.; Knöller, K.; Kassahun, A. Immobilization of uranium and arsenic by injectible iron and hydrogen stimulated autotrophic sulphate reduction. J. Contam. Hydrol. 2007, 94, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Interstate Technology & Regulatory Council (ITRC). Permeable Reactive Barriers: Lessons Learned/New Directions; PRB Technology Update Team: Washington, DC, USA, 2005; p. 101. [Google Scholar]
- Perić, J.; Trgo, M.; Vukojević Medvidović, N. Removal of zinc, copper and lead by natural zeolite-a comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, F.; Fytas, K.; McConchie, D. Efficiency of Bauxsol™ in permeable reactive barriers to treat acid rock drainage. Mine Water Environ. 2006, 25, 37–44. [Google Scholar] [CrossRef]
- Alok, M.; Naushad, M.; Gaurav Sharma, Z.A.; ALothman, S.M.; Wabaidur, A.; Manawwer, A. Fabrication of MWCNTs/ThO2 nanocomposite and its adsorption behavior for the removal of Pb(II) metal from aqueous medium. Desalin. Water Treat. 2016, 57, 21863–21869. [Google Scholar]
- Naushad, M.; Ahamad, T.; Alothman, Z.A.; Shar, M.A.; AlHokbany, N.S.; Alshehri, S.M. Synthesis, characterization and application of curcumin formaldehyde resin for the removal of Cd2+ from wastewater: Kinetics, isotherms and thermodynamic studies. J. Ind. Eng. Chem. 2015, 29, 78–86. [Google Scholar] [CrossRef]
- Zhigang, J.; Lulu, Y.; Jianhong, L.; Qiuze, W.; Rongsun, Z. Preparation of magnetic carbon spheres derived form 8-quinoliolato Fe(III) complexe and its application in water treatment. J. Ind. Eng. Chem. 2015, 21, 111–117. [Google Scholar]
- Marrazzo, M. Applicazione Delle Barriere Permeabili Reattive e Dell’ossidazione Chimica in Situ Alla Bonifica Delle Acque Sotterranee; Istituto Superiore per la Ricerca Ambientale: Roma, Italy, 2009. [Google Scholar]
- Delvasto, S.; Toro, E.F.; Mejía de Gutierrez, R. An appropriate technology for manufacture of corrugated fique fiber reinforced cementitious sheets. Constr. Build. Mater. 2010, 24, 187–192. [Google Scholar] [CrossRef]
- NIIR Board of Consultants & Engineers. Natural Fibers Handbook with Cultivation & Uses; National Institute of Industrial Research: New Delhi, India, 2005; p. 560. ISBN 8186623981. [Google Scholar]
- Navacerrada, M.A.; Díaz, C.; Fernández, P. Characterization of a material based on short natural fique fibers. BioResources 2014, 9, 3480–3496. [Google Scholar] [CrossRef]
- Gómez Hoyos, C.; Vázquez, A. Flexural properties loss of unidirectional epoxy/fique composites immersed in water and alkaline medium for construction application. Compos. Part B 2012, 43, 3120–3130. [Google Scholar] [CrossRef]
- Gómez, C.; Torres, F.G.; Nakamatsu, J.; Arroyo, O.H. Thermal and Structural Analysis of Natural Fiber Reinforced Starch-Based Biocomposites. Int. J. Polym. Mater. Polym. Biomater. 2006, 55, 893–907. [Google Scholar] [CrossRef]
- Aziz, H.A.; Othman, N.; Yusuff, M.S.; Basri, D.; Ashaari, F.; Adlan, M.N.; Othman, F.; Johari, M.; Perwira, M. Removal of copper from water using limestone filtration technique—Determination of mechanism of removal. Environ. Int. 2001, 26, 395–399. [Google Scholar] [CrossRef]
- Aziz, H.A.; Yusoff, M.S.; Adlan, M.N.; Adnan, N.H.; Alias, S. Physico-chemical removal of iron from semi-aerobic landfill leachate by limestone filter. Waste Manag. 2004, 24, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: Post treatment by high quality limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sikora, S.; Kim, H.; Bonzongo, J.C.; Rhue, D.; Townsend, T.G. Evaluation of mineral substrates for in situ iron removal from groundwater. Environ. Earth Sci. 2013, 69, 2247–2255. [Google Scholar] [CrossRef]
- Watten, B.J.; Sibrell, P.L.; Schwartz, M.F. Acid neutralization within limestone sand reactors receiving coal mine drainage. Environ. Pollut. 2003, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Sasowsky, I.D.; Foos, A.; Miller, C.M. Lithic controls on the removal of iron and remediation of acidic mine drainage. Water Res. 2002, 34, 2742–2746. [Google Scholar] [CrossRef]
- Kallo, D. Applications of natural zeolites in water and wastewater treatment. In Natural Zeolites: Occurrence, Properties, Applications; Bish, D.L., Ming, D.W., Eds.; Chemical Research Center Institute for Chemistry Hungarian Academy of Sciences: Budapest, Hungary, 2001; pp. 519–550. [Google Scholar]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Fenglian, F.; Dionysios, D.D.; Hong, L. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar]
- Morrison, S.J.; Metzler, D.R.; Dwyer, B.P. Removal of As, Mn, Mo, Se, U, V, and Zn from groundwater by zero valent iron in a passive treatment cell: Reaction progress modelling. J. Contam. Hydrol. 2002, 56, 99–116. [Google Scholar] [CrossRef]
- Shi, L.N.; Zhang, X.; Chen, Z.L. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011, 45, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Mitra, P.; Sarkar, D.; Chakrabarti, S.; Dutta, B.K. Reduction of hexa-valent chromium with zero-valent iron: Batch kinetic studies and rate model. Chem. Eng. J. 2011, 171, 54–60. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Moraci, N.; Suraci, P. Estimate of the optimum weight ratio in Zero-Valent Iron/Pumice granular mixtures used in permeable reactive barriers for the remediation of nickel contaminated groundwater. J. Hazard. Mater. 2012, 207–208, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yi, S.; He, H.; Long, C.; Li, A. Preparation of nanoscale zero-valent iron supported on chelating resin with nitrogen donor atoms for simultaneous reduction of Pb2+ and NO3−. Chem. Eng. J. 2013, 230, 166–171. [Google Scholar] [CrossRef]
- Xiao, S.L.; Ma, H.; Shen, M.W.; Wang, S.Y.; Huang, Q.G.; Shi, X.Y. Excellent copper(II) removal using zero-valent iron nanoparticle-immobilized hybrid electrospun polymer nanofibrous mats. Colloid Surf. A 2011, 381, 48–54. [Google Scholar] [CrossRef]
- Kishimoto, N.; Iwano, S.; Narazaki, Y. Mechanistic consideration of zinc ion removal by zero-valent iron. Water Air Soil Pollut. 2011, 221, 183–189. [Google Scholar] [CrossRef]
- Wilkin, R.T.; McNeil, M.S. Laboratory evaluation of zero-valent iron to treat water impacted by acid mine drainage. Chemosphere 2003, 53, 715–725. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation; Dordrecht Kluwer Academic: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Kusin, F.M. A review of the importance of hydraulic residence time on improved design of mine water treatment systems. World Appl. Sci. J. 2013, 26, 1316–1322. [Google Scholar]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibers, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276/277, 1–24. [Google Scholar] [CrossRef]
- Lee, B.G.; Rowell, R.M. Removal of heavy metal ions from aqueous solutions using lignocellulosic fibers. J. Nat. Fibers 2004, 1, 97–108. [Google Scholar] [CrossRef]
- Fallico, C.; Troisi, S.; Molinari, A.; Rivera, M.F. Characterization of broom fibers for PRB in the remediation of aquifers contaminated by heavy metals. Biogeosci. J. 2010, 7, 2545–2556. [Google Scholar] [CrossRef]
- Arias, F.; Beneduci, A.; Chidichimo, F.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gañán, P.; Mondragón, I. Surface modification of fique fibers: Effects on their physico-mechanical properties. Polym. Composite 2002, 23, 383–394. [Google Scholar] [CrossRef]
- Kozlowski, R.M.; Mackiewicz-Talarczyk, M.; Barriga-Bedoya, J. Natural Fibers Production, Processing, and Application: Inventory and Future Prospects. Contemp. Sci. Polym. Mater. 2010, 1061, 41–51. [Google Scholar] [CrossRef]
- Angelini, L.G.; Lazzeri, A.; Levita, G.; Fontanelli, D.; Bozzi, C. Ramie (Boehmeria nivea (L.) Gaud.) and Spanish Broom (Spartium junceum L.) fibres for composite materials: Agronomical aspects, morphology and mechanical properties. Ind. Crop. Prod. 2000, 11, 145–161. [Google Scholar] [CrossRef]
- Mayacela Rojas, C.M.; Rivera Velásquez, M.F.; Tavolaro, A.; Molinari, A.; Fallico, C. Use of Vegetable Fibers for PRB to Remove Heavy Metals from Contaminated Aquifers—Comparisons among Cabuya Fibers, Broom Fibers and ZVI. Int. J. Environ. Res. Public Health 2017, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, R.; Kozlowska, J.; Rawluk, M.; Barriga, J. Potential of lignocellulosic fibrous raw materials, their properties and diversified applications. Nonlinear Opt. Quantum Opt. 2004, 31, 61–89. [Google Scholar]
- Kicińska-Jakubowska, A.; Bogacz, E.; Zimniewska, M. Review of Natural Fibers. Part I-Vegetable Fibers. J. Nat. Fibers 2012, 9, 150–167. [Google Scholar] [CrossRef]
- Tursi, A.; Beneduci, A.; Chidichimo, F.; De Vietro, N.; Chidichimo, G. Remediation of hydrocarbons polluted water by hydrophobic functionalized cellulose. Chemosphere 2018, 201, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Cerchiara, T.; Salerno, G.; Chidichimo, G.; Vetere, M.V.; Alampi, C.; Gallucci, M.C.; Conidi, C.; Cassano, A. A new physical-chemical process for the efficient production of cellulose fibers from Spanish broom (Spartium junceum L.). Bioresour. Technol. 2010, 101, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Okano, T. Localization of Iα and Iβ phases in algal cellulose revealed by acid treatments. Cellulose 2001, 8, 183–188. [Google Scholar] [CrossRef]
- Kubo, S.; Yasumitsu, U.; Yoshihiro, S. Catalytic graphitization of hardwood acetic acid lignin with nickel acetate. J. Wood Sci. 2003, 49, 188–192. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G. Green hydrotropic extraction technology for delignification of sugarcane bagasse by using alkybenzene sulfonates as hydrotropes. Chem. Eng. Sci. 2013, 115, 157–166. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lin, L.; Ko, F.K. X-Ray Diffraction Analysis of Kraft Lignins and Lignin-Derived Carbon Nanofibers. ASME. J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Allende, K.L.; Fletcher, T.D.; Sun, G. The effect of substrate media on the removal of arsenic, boron and iron from an acidic wastewater in planted column reactors. Chem. Eng. J. 2012, 179, 119–130. [Google Scholar] [CrossRef]
- Tavolaro, P.; Tavolaro, A.; Martino, G. Influence of zeolite PZC and pH on the immobilization of cytochrome c: A preliminary study regarding the preparation of new biomaterials. Colloid Surf. B 2009, 70, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Konczewicz, W.; Kozłowski, R. Application of osmotic pressure for evaluation of quality and quantity of fibre in flax and hemp. In Textiles for Sustainable Development; Nova Science Publishers: New York, NY, USA, 2007; pp. 95–102. [Google Scholar]
- Guo, X.; Zhang, S.; Shan, X.-Q. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Luo, L.; Zhang, J.; Christie, P.; Zhang, S. Adsorption of mercury on lignin: Combined surface complexation modeling and X-ray absorption spectroscopy studies. Environ. Pollut. 2012, 162, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Removal of heavy metals from urban storm water runoff using different filter materials. J. Environ. Chem. Eng. 2014, 2, 282–292. [Google Scholar] [CrossRef]
- Toran, L.; White, W.B. Variation in nitrate and calcium as indicators of recharge pathways in Nolte Spring, PA. Environ. Geol. 2005, 48, 854–860. [Google Scholar] [CrossRef]
- Dierberg, F.E.; DeBusk, T.A.; Jackson, S.D.; Chimney, M.J.; Pietro, K. Submerged aquatic vegetation-based treatment wetlands for removing phosphorus from agricultural runoff: Response to hydraulic and nutrient loading. Water Res. 2002, 36, 1409–1422. [Google Scholar] [CrossRef]
- Genc-Fuhrman, H.; Mikkelsen, P.S.; Ledin, A. Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater: Experimental comparison of 11 different sorbents. Water Res. 2007, 41, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.A.; Williams, C.D.; Roberts, C.L. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, Y.S. Simultaneous removal of coexistent heavy metals from simulated urban stormwater using four sorbents: A porous iron sorbent and its mixtures with zeolite and crystal gravel. J. Hazard. Mater 2009, 168, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Sponer, J.E.; Sobalik, Z.; Leszczynski, J.; Wicthterlova, B. Effect of metal coordination on the charge distribution over the cation binding sites of zeolites. A combined experimental and theoretical study. J. Phys. Chem. B 2001, 105, 8285–8290. [Google Scholar] [CrossRef]
- Trgo, M.; Peric, J.; Medvidovic, N.V. A comparative study of ion exchange kinetics in zinc/lead-modified zeolite-clinoptilolite systems. J. Hazard. Mater. 2006, 136, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar] [CrossRef] [PubMed]



| Tested Materials | ||||||
|---|---|---|---|---|---|---|
| Cabuya Fibers | Ecuador Limestone | Natural Zeolite | Zero Valent Iron (ZVI) | Synthetic Zeolite | ||
| Cu | Removal % | 99.24 | 96.33 | 99.89 | 93.42 | 98.98 |
| Contact time (hours) | 14 | 10 | 24 | 24 | 30 | |
| Optimum pH | 5.75 | 6.18 | 6.58 | 5.04 | 7 | |
| Zn | Removal % | 90.14 | 89.52 | 99.9 | 94.17 | 91.65 |
| Contact time (hours) | 24 | 10 | 10 | 14 | 24 | |
| Optimum pH | 6.85 | 6.67 | 6.81 | 5.87 | 7.19 | |
| Cd | Removal % | 96.67 | 73.43 | 49.09 | 87.83 | 52.66 |
| Contact time (hours) | 30 | 30 | 26 | 30 | 30 | |
| Optimum pH | 7.26 | 7.46 | 7.75 | 4.87 | 8.01 | |
| Pb | Removal % | 100 | 74.23 | 100 | 100 | 77.67 |
| Contact time (hours) | 22 | 30 | 22 | 30 | 30 | |
| Optimum pH | 6.45 | 7.04 | 6.78 | 4.06 | 8.16 | |
| Tested Material | pH |
|---|---|
| Cabuya fibers | 6.79 |
| Natural zeolite | 6.81 |
| ZVI | 8.00 |
| Ecuador limestone | 8.10 |
| Synthetic zeolite | 9.57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, A.; Mayacela Rojas, C.M.; Beneduci, A.; Tavolaro, A.; Rivera Velasquez, M.F.; Fallico, C. Adsorption Performance Analysis of Alternative Reactive Media for Remediation of Aquifers Affected by Heavy Metal Contamination. Int. J. Environ. Res. Public Health 2018, 15, 980. https://doi.org/10.3390/ijerph15050980
Molinari A, Mayacela Rojas CM, Beneduci A, Tavolaro A, Rivera Velasquez MF, Fallico C. Adsorption Performance Analysis of Alternative Reactive Media for Remediation of Aquifers Affected by Heavy Metal Contamination. International Journal of Environmental Research and Public Health. 2018; 15(5):980. https://doi.org/10.3390/ijerph15050980
Chicago/Turabian StyleMolinari, Antonio, Celia Margarita Mayacela Rojas, Amerigo Beneduci, Adalgisa Tavolaro, Maria Fernanda Rivera Velasquez, and Carmine Fallico. 2018. "Adsorption Performance Analysis of Alternative Reactive Media for Remediation of Aquifers Affected by Heavy Metal Contamination" International Journal of Environmental Research and Public Health 15, no. 5: 980. https://doi.org/10.3390/ijerph15050980
APA StyleMolinari, A., Mayacela Rojas, C. M., Beneduci, A., Tavolaro, A., Rivera Velasquez, M. F., & Fallico, C. (2018). Adsorption Performance Analysis of Alternative Reactive Media for Remediation of Aquifers Affected by Heavy Metal Contamination. International Journal of Environmental Research and Public Health, 15(5), 980. https://doi.org/10.3390/ijerph15050980