A Simple Alternative Method for Preservation of 2-Methylisoborneol in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Analysis Methods
3. Results and Discussion
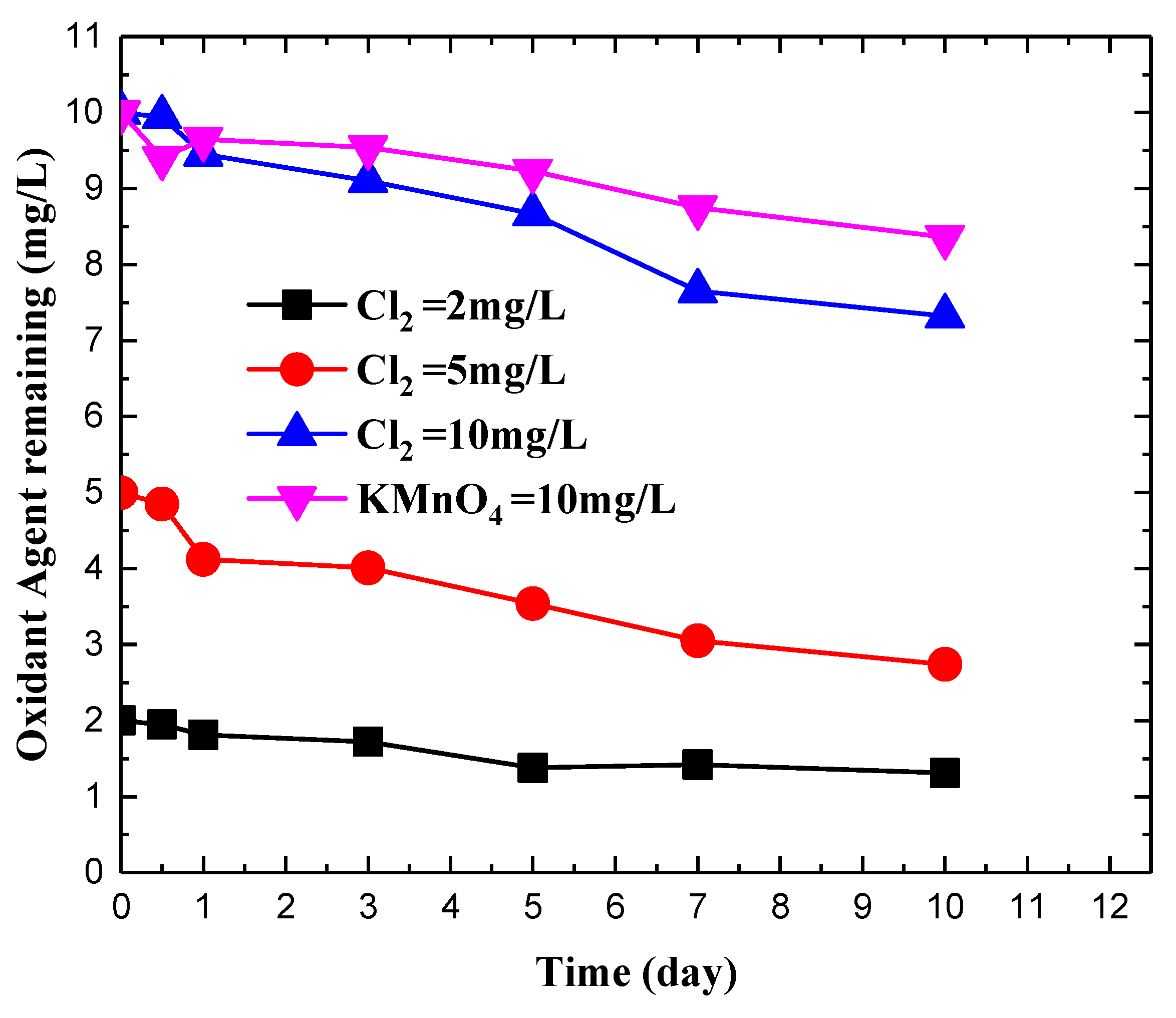
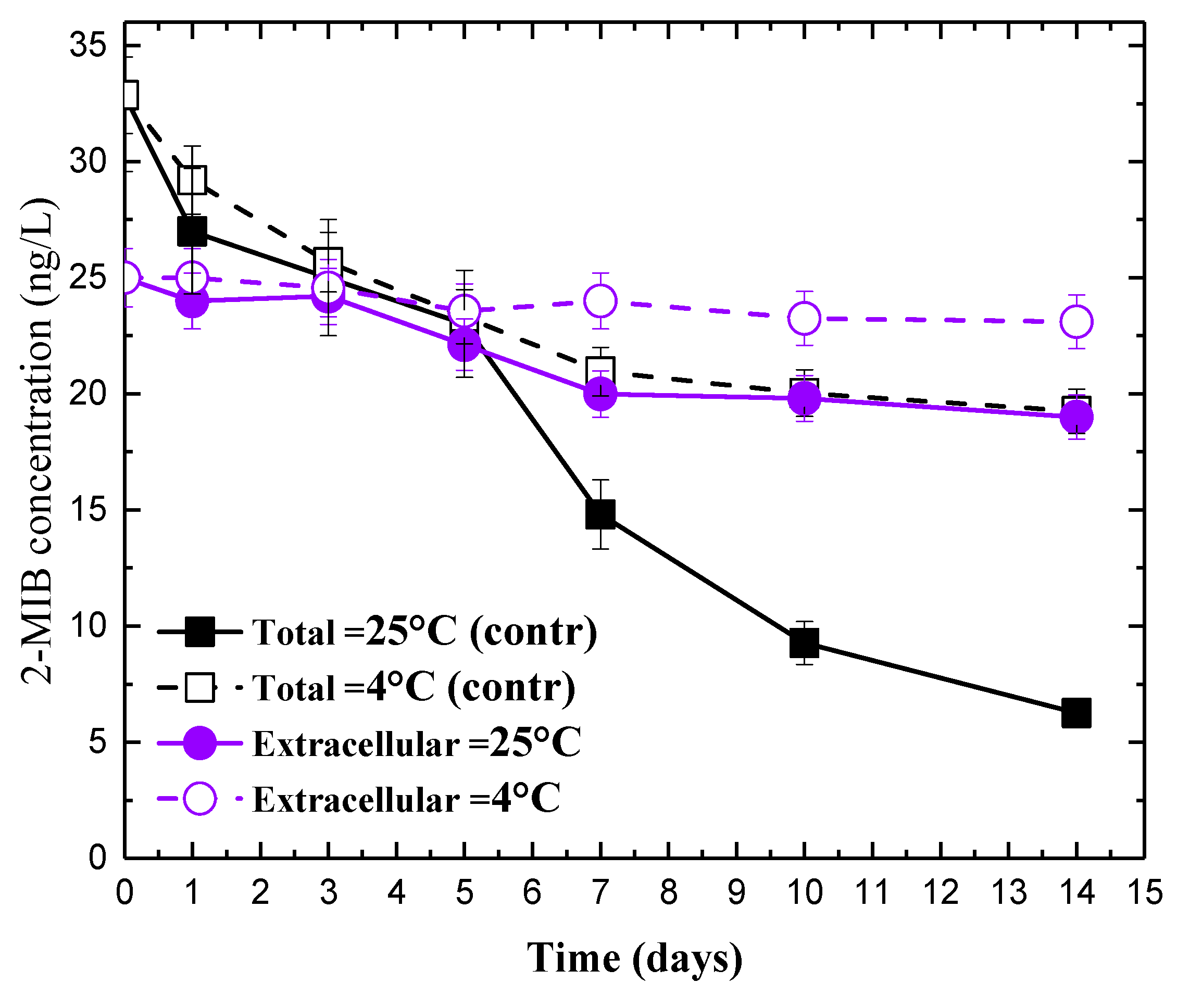
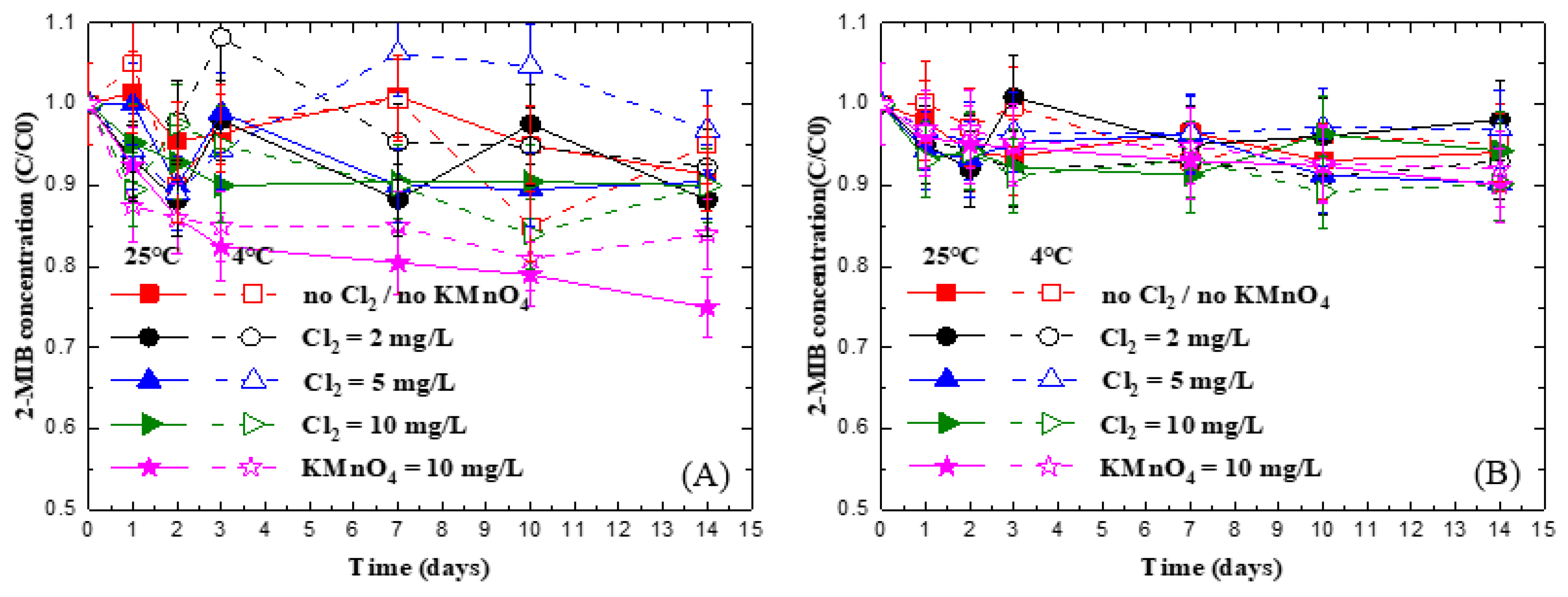
3.1. Impact of Chlorine on 2-MIB Concentration in Deionized Water
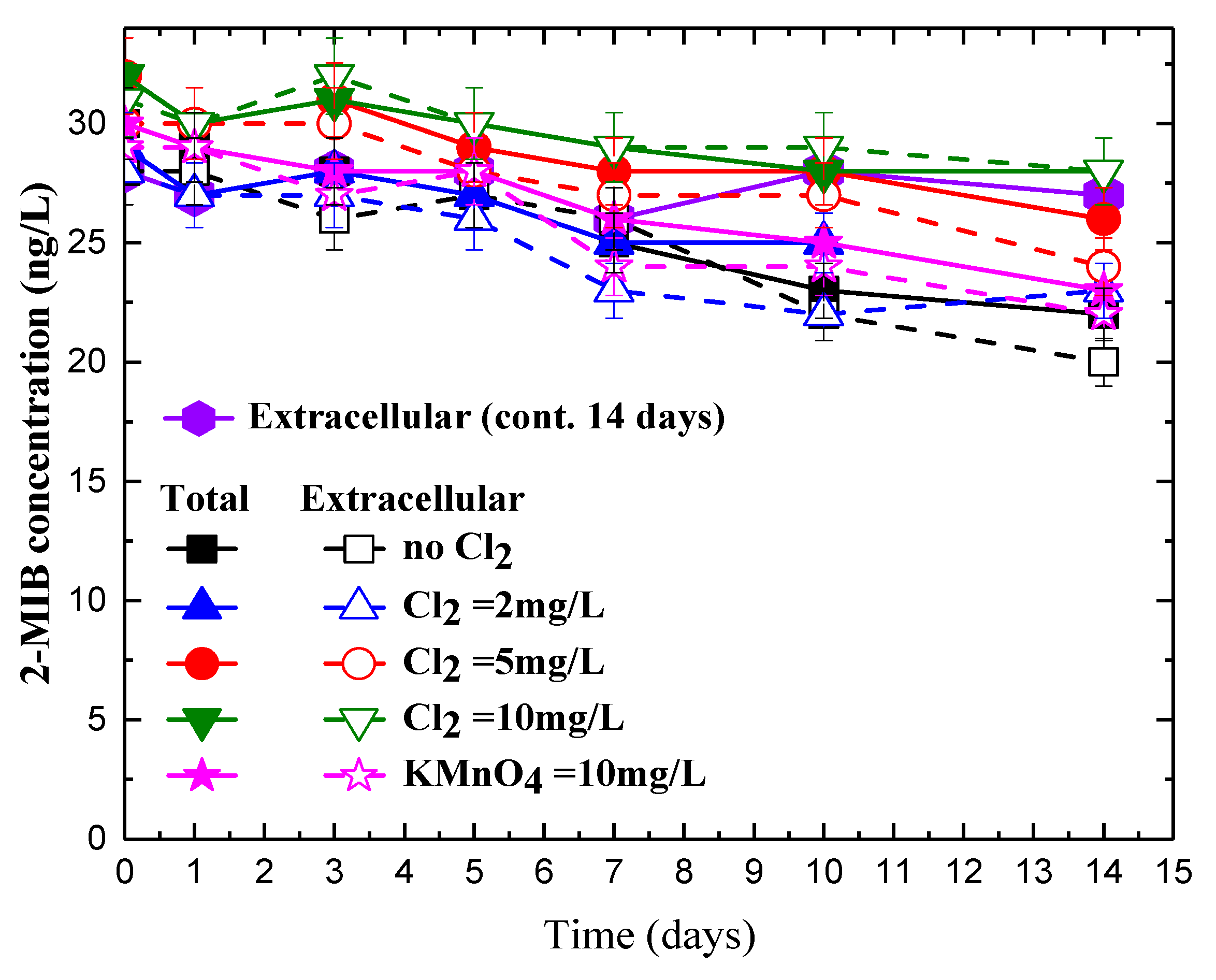
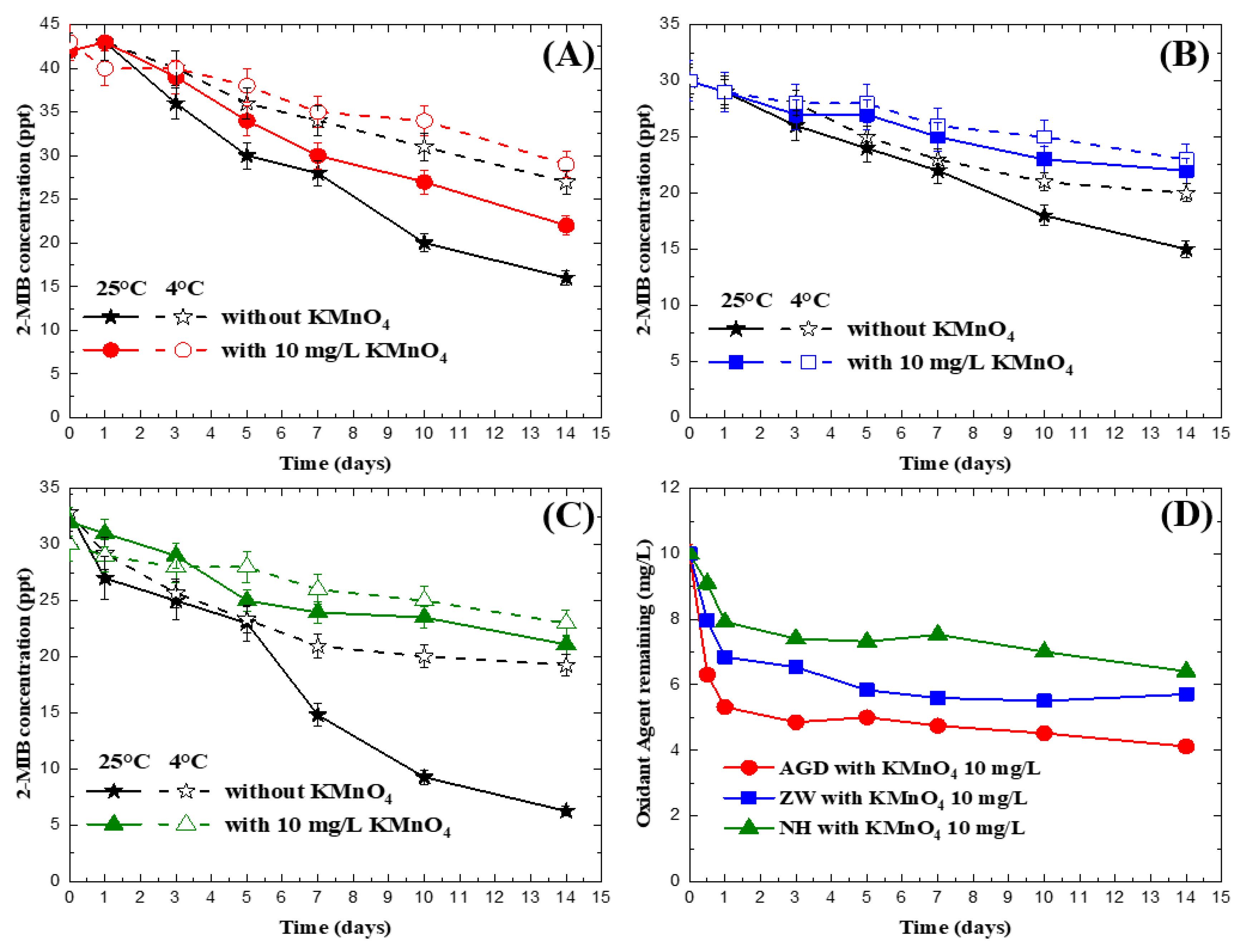
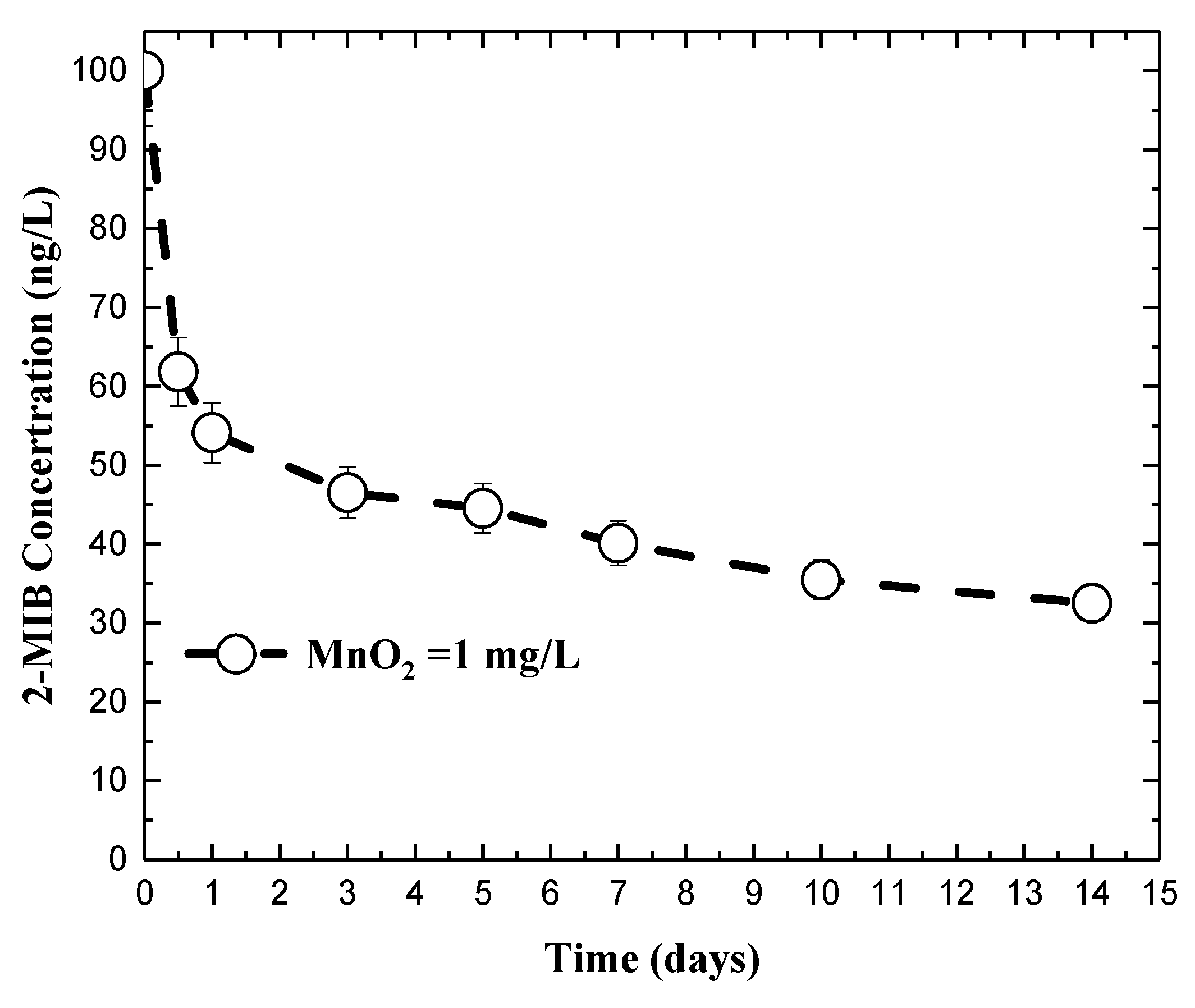
3.2. Impact of Permanganate on 2-MIB Concentration in Natural Water
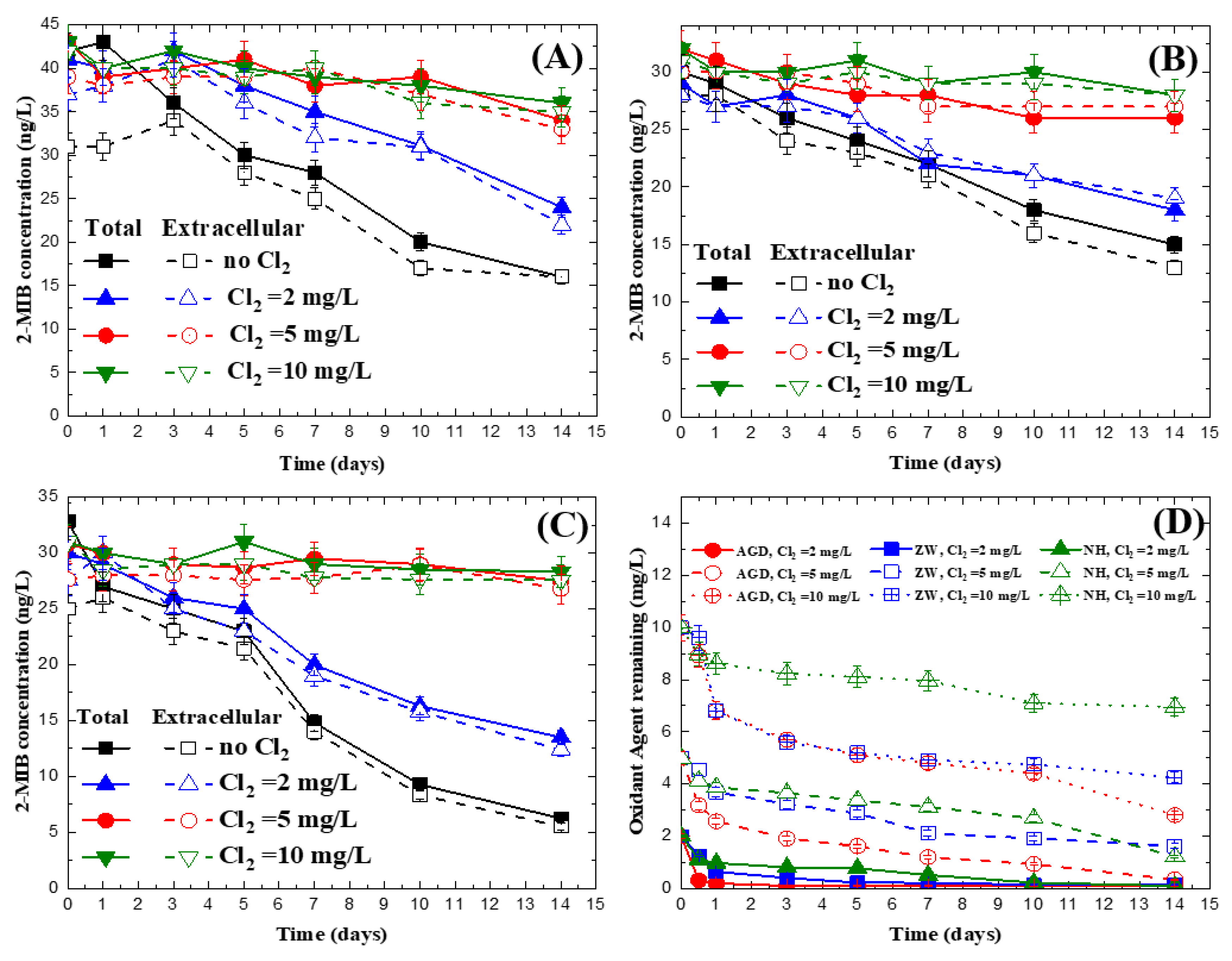
3.3. Impact of Chlorine on 2-MIB Concentration in Natural Water
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
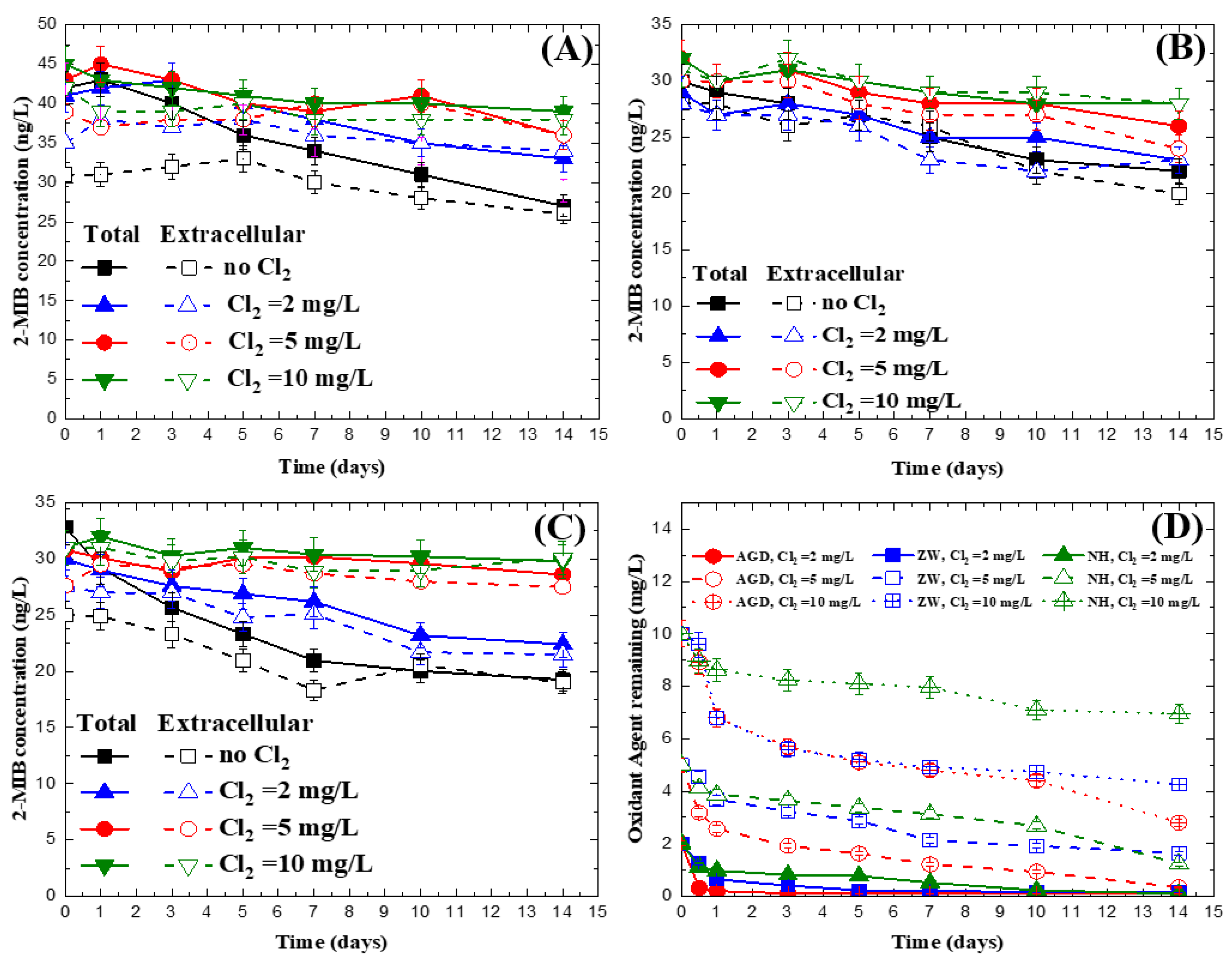
| Without Chlorine | Chlorine = 2 mg/L | Chlorine = 5 mg/L | Chlorine = 10 mg/L | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Extracellular | Total | Extracellular | Total | Extracellular | Total | Extracellular | |
| AGD | 7.36 × 10−2 | 5.29 × 10−2 | 3.78 × 10−2 | 3.52 × 10−2 | 1.21 × 10−2 | 9.61 × 10−3 | 1.10 × 10−2 | 1.20 × 10−2 |
| (R2 = 0.98) | (R2 = 0.83) | (R2 = 0.90) | (R2 = 0.75) | (R2 = 0.69) | (R2 = 0.56) | (R2 = 0.86) | (R2 = 0.86) | |
| ZW | 5.04 × 10−2 | 4.52 × 10−2 | 3.42 × 10−2 | 2.89 × 10−2 | 1.95 × 10−2 | 1.88 × 10−2 | 6.65 × 10−3 | 5.65 × 10−3 |
| (R2 = 0.99) | (R2 = 0.98) | (R2 = 0.94) | (R2 = 0.96) | (R2 = 0.94) | (R2 = 0.88) | (R2 = 0.60) | (R2 = 0.74) | |
| NH | 1.21 × 10−1 | 1.18 × 10−1 | 5.99 × 10−2 | 6.27 × 10−2 | 6.04 × 10−3 | 9.79 × 10−4 | 5.66 × 10−3 | 6.49 × 10−3 |
| (R2 = 0.97) | (R2 = 0.95) | (R2 = 0.98) | (R2 = 0.98) | (R2 = 0.72) | (R2 = 0.05) | (R2 = 0.57) | (R2 = 0.62) | |
References
- Zhou, X.; Zhang, K.; Zhang, T.; Li, C.; Mao, X. An ignored and potential source of taste and odor (T&O) issues-biofilms in drinking water distribution system (DWDS). Appl. Microbiol. Biotechnol. 2017, 101, 3537–3550. [Google Scholar] [PubMed]
- Jűttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.B.; Ridal, J.; Zaitlin, B.; Lo, A. Odours from pulp mill effluent treatment ponds: The origin of significant levels of geosmin and 2-methylisoborneol (MIB). Chemosphere 2003, 51, 765–773. [Google Scholar] [CrossRef]
- Suffet, I.H.M.; Corado, A.; Chou, D.; McGuire, M.J.; Butterworth, S. Awwa taste and odor survey. Am. Water Works Assoc. (AWWA) Water Environ. 1996, 88, 168–180. [Google Scholar] [CrossRef]
- Newcombe, G.; Morrison, J.; Hepplewhite, C. Simultaneous adsorption of MIB and NOM onto activated carbon. I. Characterisation of the system and NOM adsorption. Carbon 2002, 40, 2135–2146. [Google Scholar] [CrossRef]
- Lin, T.F.; Liu, C.L.; Yang, F.C.; Hung, H.W. Effect of residual chlorine on the analysis of geosmin, 2-MIB and MTBE in drinking water using the spme technique. Water Res. 2003, 37, 21–26. [Google Scholar] [CrossRef]
- Bruchet, A.; Duguet, J.P.; Suffe, I.H. Role of oxidants and disinfectants on the removal, masking and generation of tastes and odours. Rev. Environ. Sci. Biotechnol. 2004, 3, 33–41. [Google Scholar] [CrossRef]
- Dionigi, C.P.; Lawlor, T.E.; McFarland, J.E.; Johnsen, P.B. Evaluation of geosmin and 2-methylisoborneol on the histidine dependence of TA98 and TA100 salmonella typhimurium tester strains. Water Res. 1993, 27, 1615–1618. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- Wu, J.-T.; Jűttner, F. Differential partitioning of geosmin and 2-methylisoborneol between cellular constituents in oscillatoria tenuis. Arch. Microbiol. 1988, 150, 580–583. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Schöller, C.E.G.; Gűrtler, H.; Pedersen, R.; Molin, S.; Wilkins, K. Volatile metabolites from actinomycetes. J. Agric. Food Chem. 2002, 50, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Börjesson, T.S.; Stöllman, U.M.; Schnűrer, J.L. Off-odorous compounds produced by molds on oatmeal agar: Identification and relation to other growth characteristics. J. Agric. Food Chem. 1993, 41, 2104–2111. [Google Scholar] [CrossRef]
- Dickschat, J.S.; Nawrath, T.; Thiel, V.; Kunze, B.; Müller, R.; Schulz, S. Biosynthesis of the off-flavor 2-methylisoborneol by the myxobacterium nannocystis exedens. Angew. Chem. Int. Ed. 2007, 46, 8287–8290. [Google Scholar] [CrossRef] [PubMed]
- Giglio, S.; Chou, W.K.W.; Ikeda, H.; Cane, D.E.; Monis, P.T. Biosynthesis of 2-methylisoborneol in cyanobacteria. Environ. Sci. Technol. 2011, 45, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, G.; Taylor, W.D. A guide to geosmin- and MIB-producing cyanobacteria in the united states. Water Sci. Technol. 2004, 49, 19–24. [Google Scholar] [PubMed]
- Kakimoto, M.; Ishikawa, T.; Miyagi, A.; Saito, K.; Miyazaki, M.; Asaeda, T.; Yamaguchi, M.; Uchimiya, H.; Kawai-Yamada, M. Culture temperature affects gene expression and metabolic pathways in the 2-methylisoborneol-producing cyanobacterium pseudanabaena galeata. J. Plant Physiol. 2014, 171, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yu, J.; An, W.; Yang, M.; Chen, G.; Zhang, S. Identification of causative compounds and microorganisms for musty odor occurrence in the huangpu river, china. J. Environ. Sci. 2013, 25, 460–465. [Google Scholar] [CrossRef]
- Suurnäkki, S.; Gomez-Saez, G.V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D.P.; Sivonen, K. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Res. 2015, 68, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Newcombe, G.; Sztajnbok, P. The application of powdered activated carbon for MIB and geosmin removal: Predicting pac doses in four raw waters. Water Res. 2001, 35, 1325–1333. [Google Scholar] [CrossRef]
- Hsieh, W.-H.; Chang, D.-W.; Lin, T.-F. Occurrence and removal of earthy-musty odorants in two waterworks in kinmen island, taiwan. J. Hazard. Toxic Radioact. Waste 2014, 18, 04014012. [Google Scholar] [CrossRef]
- Lalezary, S.; Pirbazari, M.; McGuire, M.J. Oxidation of five earthy-musty taste and odor compounds. J. Am. Water Works Assoc. 1986, 78, 62–69. [Google Scholar] [CrossRef]
- Nerenberg, R.; Rittmann, B.E.; Soucie, W.J. Ozone/biofiltration for removing MIB and geosmin. J. Am. Water Works Assoc. 2000, 92, 85–95. [Google Scholar] [CrossRef]
- Eaton, R.W.; Sandusky, P. Biotransformations of 2-methylisoborneol by camphor-degrading bacteria. Appl. Environ. Microbiol. 2009, 75, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, G.; Wolfe, R.L.; Means, E.G. Degradation of 2-methylisoborneol by aquatic bacteria. Appl. Environ. Microbiol. 1988, 54, 2424–2431. [Google Scholar] [PubMed]
- Lauderdale, C.V.; Aldrich, H.C.; Lindner, A.S. Isolation and characterization of a bacterium capable of removing taste- and odor-causing 2-methylisoborneol from water. Water Res. 2004, 38, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Hoefel, D.; Bock, F.; Saint, C.P.; Newcombe, G. Biodegradation rates of 2-methylisoborneol (MIB) and geosmin through sand filters and in bioreactors. Chemosphere 2007, 66, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, E.; Shimizu, A.; Ishibashi, Y. 2-methylisoborneol degradation by the cam operon from pseudomonas putida ppg1. Water Sci. Technol. 1995, 31, 79–86. [Google Scholar]
- Kim, T.-K.; Moon, B.-R.; Kim, T.; Kim, M.-K.; Zoh, K.-D. Degradation mechanisms of geosmin and 2-MIB during UV photolysis and UV/chlorine reactions. Chemosphere 2016, 162, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.C.; Lin, T.F.; Liu, C.L.; Lai, S.D. The effect of oxidants on 2-MIB concentration with the presence of cyanobacteria. Water Sci. Technol. 2004, 49, 281–288. [Google Scholar] [PubMed]
- Hung, H.-W.; Lin, T.-F.; Chiu, C.-H.; Chang, Y.-C.; Hsieh, T.-Y. Trace analysis of n-nitrosamines in water using solid-phase microextraction coupled with gas chromatograph–tandem mass spectrometry. Water Air Soil Pollut. 2010, 213, 459–469. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Hobson, P.; Burch, M.D.; Lin, T.-F. In situ measurement of odor compound production by benthic cyanobacteria. J. Environ. Monit. 2010, 12, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; Han, X.K.; Huang, Z.H. Experimental study on combing chlorine and potassium permanganate to inactivate bacteria. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–3. [Google Scholar]
- Tucker, C.S.; Boyd, C.E. Relationships between potassium permanganate treatment and water quality. Trans. Am. Fish. Soc. 1977, 106, 481–488. [Google Scholar] [CrossRef]
- Freitas, R.M.; Perilli, T.A.G.; Ladeira, A.C.Q. Oxidative precipitation of manganese from acid mine drainage by potassium permanganate. J. Chem. 2013, 2013, 287257. [Google Scholar] [CrossRef]
- Moyers, B.; Wu, J.S. Removal of organic precursors by permanganate oxidation and alum coagulation. Water Res. 1985, 19, 309–314. [Google Scholar] [CrossRef]
- Zawacki, J. KMnO4 contributes to least cost treatment solution. Water Eng. Manag. 1992, 139, 18–19. [Google Scholar]
- Rand, J.L.; Gagnon, G.A.; Knowles, A. Establishing minimum free chlorine residual concentration for microbial control in a municipal drinking water distribution system. Water Pract. Technol. 2014, 9, 491–501. [Google Scholar] [CrossRef]
- Kaleli, H.A.; Islam, M.R. Effect of temperature on the growth of wastewater bacteria. Toxicol. Environ. Chem. 1997, 59, 111–123. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.-C.; Chiu, Y.-T.; Lin, T.-F. A Simple Alternative Method for Preservation of 2-Methylisoborneol in Water Samples. Int. J. Environ. Res. Public Health 2018, 15, 1015. https://doi.org/10.3390/ijerph15051015
Fan C-C, Chiu Y-T, Lin T-F. A Simple Alternative Method for Preservation of 2-Methylisoborneol in Water Samples. International Journal of Environmental Research and Public Health. 2018; 15(5):1015. https://doi.org/10.3390/ijerph15051015
Chicago/Turabian StyleFan, Chun-Cheng, Yi-Ting Chiu, and Tsair-Fuh Lin. 2018. "A Simple Alternative Method for Preservation of 2-Methylisoborneol in Water Samples" International Journal of Environmental Research and Public Health 15, no. 5: 1015. https://doi.org/10.3390/ijerph15051015
APA StyleFan, C.-C., Chiu, Y.-T., & Lin, T.-F. (2018). A Simple Alternative Method for Preservation of 2-Methylisoborneol in Water Samples. International Journal of Environmental Research and Public Health, 15(5), 1015. https://doi.org/10.3390/ijerph15051015





