Analysis of the Content of Chromium in Certain Parts of the Human Knee Joint
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef] [PubMed]
- Granadino, V.A.; Parra de Machado, L.; Romero, R.A. Determination of total chromium in whole blood, blood components, bone, and urine by fast furnace program electrothermal atomization AAS and using neither analyte isoformation nor background correction. Anal. Chem. 1994, 66, 3624–3631. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Allen, M.J.; Myer, B.J.; Millett, P.J.; Rushton, N. The effects of particulate cobalt, chromium and cobalt-chromium alloy on human osteoblast-like cells in vitro. J. Bone Jt. Surg. Br. 1997, 79, 475–482. [Google Scholar] [CrossRef]
- McCarty, M.F. Anabolic effects of insulin on bone suggest a role for chromium picolinate in preservation of bone density. Med. Hypotheses 1995, 45, 241–246. [Google Scholar] [CrossRef]
- Flores-Cano, J.V.; Leyva-Ramos, R.; Carrasco-Marin, F.; Aragón-Piña, A.; Salazar-Rabago, J.J.; Leyva-Ramos, S. Adsorption mechanism of chromium (III) from water solution on bone char: Effect of operating conditions. Adsorption 2016, 22, 297–308. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wicklund, B.H.; Gustilo, R.B.; Tsukayama, D.T. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials 1996, 17, 2233–2240. [Google Scholar] [CrossRef]
- Rakow, A.; Schoon, J.; Dienelt, A.; John, T.; Textor, M.; Duda, G.; Perka, C.; Schulze, F.; Ode, A. Influence of particulate and dissociated metal-on-metal hip endoprosthesis wear on mesenchymal stromal cells in vivo and in vitro. Biomaterials 2016, 98, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Davda, K.; Lali, F.V.; Sampson, B.; Skinner, J.A.; Hart, A.J. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J. Bone Jt. Surg. Br. 2011, 93, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, H.S.; Minski, M.J. Metal ion release after total hip replacement. Biomaterials 1980, 1, 193–198. [Google Scholar] [CrossRef]
- Luetzner, J.; Krummenauer, F.; Lengel, A.M.; Ziegler, J.; Witzleb, W.C. Serum metal ion exposure after total knee arthroplasty. Clin. Orthop. Relat. Res. 2007, 461, 136–142. [Google Scholar] [CrossRef] [PubMed]
- McPhee, I.B.; Swanson, C.E. Metal ion levels in patients with stainless steel spinal instrumentation. Spine 2007, 32, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Vidaud, C.; Bourgeois, D.; Meyer, D. Bone as target organ for metals: The case of f-elements. Chem. Res. Toxicol. 2012, 25, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.W.; Kuo, S.M.; Chou, C.H.; Lee, T. Determination of 14 elements in Taiwanese bones. Sci. Total Environ. 2000, 255, 45–55. [Google Scholar] [CrossRef]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Sobczyk, K.; Wiechuła, D. Distribution of magnesium, calcium, sodium and potassium in tissues of the hip joint. Magnes. Res. 2013, 26, 125–131. [Google Scholar] [PubMed]
- Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.I.; Kalisinska, E.; Sokolowski, S.; Kolodziej, L.; Budis, H.; Safranow, K.; Kot, K.; Ciosek, Z.; Tomska, N.; et al. Influence of environmental factors and relationships between vanadium, chromium, and calcium in human bone. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zioła-Frankowska, A.; Kubaszewski, Ł.; Dąbrowski, M.; Kowalski, A.; Rogala, P.; Strzyżewski, W.; Łabędź, W.; Uklejewski, R.; Novotny, K.; Kanicky, V.; et al. The content of the 14 metals in cancellous and cortical bone of the hip joint affected by osteoarthritis. Biomed. Res. Int. 2015, 2015, 815648. [Google Scholar] [CrossRef] [PubMed]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Kusz, D.; Gajda, Z.; Sobczyk, K. Interactions between concentrations of chemical elements in human femoral heads. Arch. Environ. Contam. Toxicol. 2009, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, H.; Hanihara, K.; Suzuki, T.; Himeno, S.I.; Kawabe, T.; Hongo, T.; Morita, M. Elemental composition of ancient Japanese bones. Sci. Total Environ. 1986, 52, 93–107. [Google Scholar] [CrossRef]
- Garcia, F.; Ortega, A.; Domingo, J.L.; Corbella, J. Accumulation of metals in autopsy tissues of subjects living in Tarragona county, Spain. J. Environ. Sci. Health 2001, 36, 1767–1786. [Google Scholar] [CrossRef]
- Yoshinaga, J.; Suzuki, T.; Morita, M.; Hayakawa, M. Trace elements in ribs of elderly people and elemental variation in the presence of chronic diseases. Sci. Total Environ. 1995, 162, 239–252. [Google Scholar] [CrossRef]
- Roczniak, W.; Brodziak-Dopierała, B.; Cipora, E.; Jakóbik-Kolon, A.; Kluczka, J.; Babuśka-Roczniak, M. Factors that affect the content of cadmium, nickel, copper and zinc in tissues of the knee joint. Biol. Trace Elem. Res. 2017, 178, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Roczniak, W.; Brodziak-Dopierała, B.; Cipora, E.; Mitko, K.; Jakóbik-Kolon, A.; Konieczny, M.; Babuśka-Roczniak, M. The content of structural and trace elements in the knee joint tissues. Int. J. Environ. Res. Public Health 2017, 14, 1441. [Google Scholar] [CrossRef] [PubMed]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Sobczyk, K.; Wiechuła, D. Chromium content in the human hip joint tissues. Biomed. Environ. Sci. 2015, 28, 89–96. [Google Scholar] [PubMed]
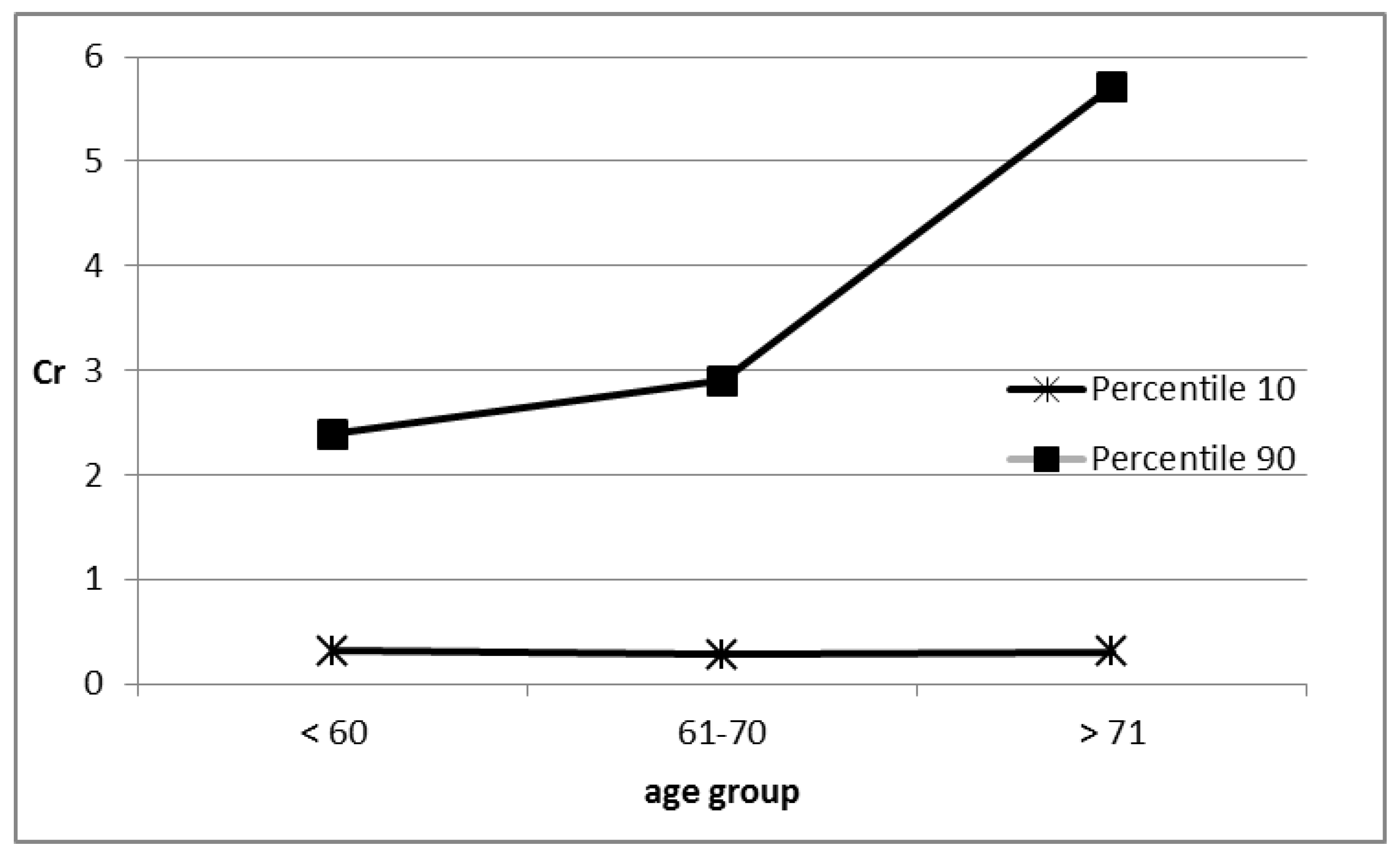
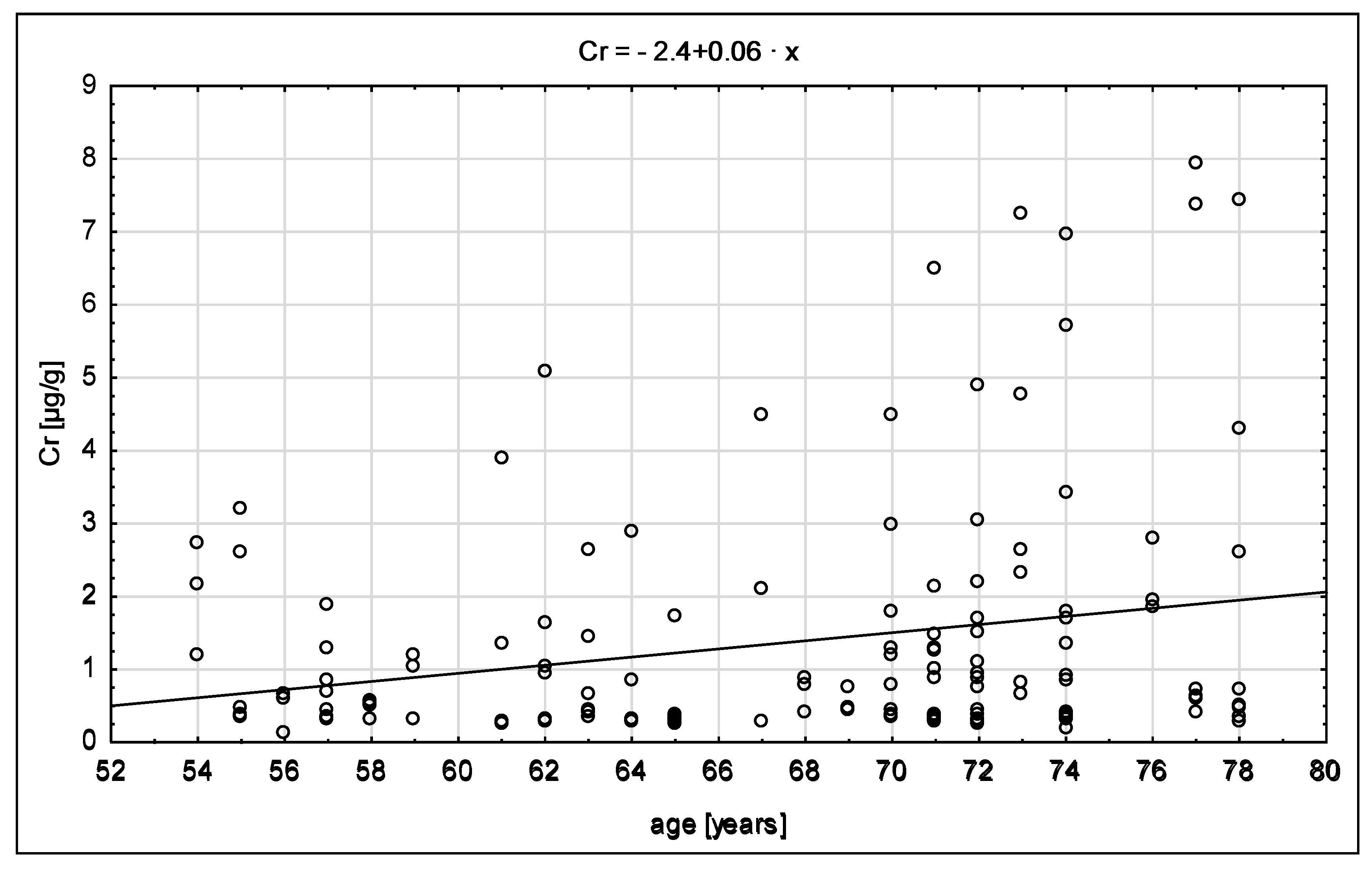
| Parameters | Whole Population n = 50 | Females n = 36 | Males n = 14 |
|---|---|---|---|
| Age (years) | |||
| AM ± SD | 67.46 ± 7.11 | 67.22 ± 7.09 | 68.07 ± 7.20 |
| range | 54–78 | 54–78 | 56–78 |
| Body weight (kg) | |||
| AM ± SD | 83.54 ± 14.56 | 81.45 ± 14.19 | 88.58 ± 14.56 |
| range | 54–115 | 54–115 | 66–108 |
| Height (cm) | |||
| AM ± SD | 164.37 ± 9.32 | 160.24 ± 6.14 | 174.33 ± 8.11 |
| range | 149–189 | 149–173 | 165–189 |
| Smokers (n, %) | |||
| - nonsmokers | 20 (40%) | 19 (38%) | 1 (2%) |
| - smokers | 21 (42%) | 10 (20%) | 11 (22%) |
| - smokers in the past | 9 (18%) | 5 (10%) | 4 (8%) |
| Place of residence (%) | |||
| Village | 11 (22%) | 7 (14%) | 4 (8%) |
| Town | 39 (78%) | 29 (58%) | 10 (20%) |
| Knee (%) | |||
| Left | 24 (48%) | 18 (36%) | 6 (12%) |
| Right | 26 (52%) | 18 (36%) | 8 (16%) |
| Beginning pain (years, %) | |||
| < 5 | 16 (32%) | 11 (22%) | 5 (10%) |
| < 10 | 21 (42%) | 15 (30%) | 6 (12%) |
| 10 | 13 (26%) | 10 (20%) | 3 (9%) |
| Earlier knee endoprosthesis (%) | |||
| Yes | 13 (26%) | 10 (20%) | 3 (6%) |
| No | 37 (74%) | 26 (52%) | 11 (22%) |
| Degenerative changes in the other knee (%) | |||
| Yes | 33 (66%) | 23 (46%) | 10 (20%) |
| No | 17 (34%) | 13 (26%) | 4 (8%) |
| Contact with chemicals in the workplace (factory polyvinyl chloride, zinc smelter) (%) | 3 (6%) | 1 (2%) | 2 (4%) |
| AM ± SD | Med | Range | Percentile 10 | Percentile 90 | CV | |
|---|---|---|---|---|---|---|
| MEN | ||||||
| Meniscus | 1.33 ± 1.11 | 0.80 | 0.13–3.00 | 0.34 | 2.90 | 84 |
| Tibia | 1.25 ± 1.65 | 0.53 | 0.28–6.49 | 0.30 | 2.21 | 133 |
| Femur | 1.57 ± 1.67 | 0.90 | 0.30–5.72 | 0.32 | 4.49 | 106 |
| WOMEN | ||||||
| Meniscus | 1.12 ± 1.38 | 0.65 | 0.28–6.97 | 0.32 | 2.09 | 123 |
| Tibia | 1.28 ± 1.56 | 0.67 | 0.25–7.25 | 0.30 | 3.89 | 122 |
| Femur | 1.67 ± 2.17 | 0.61 | 0.20–7.95 | 0.27 | 4.90 | 130 |
| TOTAL | ||||||
| Meniscus | 1.18 ± 1.30 | 0.67 | 0.13–6.97 | 0.32 | 2.84 | 110 |
| Tibia | 1.27 ± 1.57 | 0.60 | 0.25–7.25 | 0.30 | 3.66 | 124 |
| Femur | 1.64 ± 2.03 | 0.76 | 0.20–7.95 | 0.29 | 4.84 | 124 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roczniak, W.; Brodziak-Dopierała, B.; Cipora, E.; Jakóbik-Kolon, A.; Konieczny, M.; Babuśka-Roczniak, M. Analysis of the Content of Chromium in Certain Parts of the Human Knee Joint. Int. J. Environ. Res. Public Health 2018, 15, 1013. https://doi.org/10.3390/ijerph15051013
Roczniak W, Brodziak-Dopierała B, Cipora E, Jakóbik-Kolon A, Konieczny M, Babuśka-Roczniak M. Analysis of the Content of Chromium in Certain Parts of the Human Knee Joint. International Journal of Environmental Research and Public Health. 2018; 15(5):1013. https://doi.org/10.3390/ijerph15051013
Chicago/Turabian StyleRoczniak, Wojciech, Barbara Brodziak-Dopierała, Elżbieta Cipora, Agata Jakóbik-Kolon, Magdalena Konieczny, and Magdalena Babuśka-Roczniak. 2018. "Analysis of the Content of Chromium in Certain Parts of the Human Knee Joint" International Journal of Environmental Research and Public Health 15, no. 5: 1013. https://doi.org/10.3390/ijerph15051013
APA StyleRoczniak, W., Brodziak-Dopierała, B., Cipora, E., Jakóbik-Kolon, A., Konieczny, M., & Babuśka-Roczniak, M. (2018). Analysis of the Content of Chromium in Certain Parts of the Human Knee Joint. International Journal of Environmental Research and Public Health, 15(5), 1013. https://doi.org/10.3390/ijerph15051013







