1. Introduction
Air pollution was recognized as a health risk factor by World Health Organization (WHO) already in 1958 [
1], but lack of data and related methodologies prevented development of specific recommendations at that time. The first air quality guidelines for Europe were published in 1987 [
2] and updated in 2000 [
3], when fine particles (PM
2.5) was given an exposure–response relationship without a threshold. So far, the latest air quality guideline “The global update 2005”, focused on four pollutants PM, O
3, NO
2, and sulfur dioxide (SO
2), and for the first time, set a guideline level for PM
2.5 [
4].
Due to adverse health effects of PM
2.5, WHO set a guideline to annual mean of 10 µg/m
3. This guideline is based on evidence of total cardiopulmonary and lung cancer mortality increases, with more than 95% confidence, due to long-term exposure to PM
2.5 concentrations higher than 10 µg/m
3. Since then there has been a growing evidence of adverse health impacts also at lower concentrations than 10 µg/m
3 (e.g., [
5,
6]). Consequently, the WHO working group, which reviewed the latest scientific evidence for health effects of air pollution [
7], recommended updating the WHO guidelines for air quality [
8].
Quantification of the health impacts helps to compare different risks and to prioritize policy actions. Health impact assessments can also be used to find out the most effective emission mitigation measures to gain the biggest health improvement [
9]. In the health impact assessment exposure is combined with population and health data using concentration–response (CR) functions. Ambient air concentrations are often used as proxies for exposure [
10].
The health risks of air pollution in Europe (HRAPIE) working group [
11] gave recommendations for quantifying the adverse health effects of PM
2.5, respirable particles (PM
10), O
3, and NO
2. In the pollutant–outcomes pairs, there are few newcomers. Chronic mortality was used instead of acute mortality for O
3. Chronic mortality impacts of NO
2 were recommended to be calculated for concentrations above 20 µg/m
3. However, the working group also acknowledged that this level might be too high [
12].
European Environmental Agency (EEA) has been developing and estimating the exposures and health impacts of ambient air pollution in European counties for over a decade. They use population exposure assessment at 10 km resolution. The assessment combines the European air quality monitoring network data (via AirBase) with spatial interpolation methods, PM
10/PM
2.5 modelling to extend the smaller number of PM
2.5 monitoring sites, and the European Monitoring and Evaluation Programme (EMEP) air quality model at 50 km resolution [
13,
14,
15].
EEA’s first health impact estimates were for PM
2.5 and PM
10 for 2005 [
16]. In the following air quality reports, they started using recommendations of the HRAPIE working group for health impact estimates, and included O
3 and NO
2 as well (
Table 1). The exposure estimation for NO
2 was improved for 2014. In the earlier exposure estimates, only background monitoring stations were included, but now also traffic locations were added [
17]. This change did not have a large impact on the population-weighted averages, but it affected the exposure distributions. Adding of the traffic stations led to increase in the population at the highest exposure group. Since NO
2 health impacts were calculated for exposure levels above 20 µg/m
3, this change had a big impact on the results.
Early application of air quality models for characterization of health effects was applied for mortality and separating long-range transported particles from the national sources [
21,
22,
23,
24]. Long-range transport was estimated by early implementation of the chemical transport model, applied in Finland at 5 km resolution [
23]. Unit emission source receptor matrices were developed for simplified estimation of exposures from national sources at 1 km resolution [
24]. Mortality related to PM
2.5 exposure was estimated using relative risks from expert elicitation (1.006–1.01 per 1 µg/m
3) [
25,
26], and morbidity outcomes or years of life lost were not estimated.
Fine particles have been estimated to be the leading environmental risk factor in Finland (e.g., [
27,
28]). National health impact estimates, accounting also for morbidity, were developed based on methods used in the Clean Air for Europe (CAFE) program [
29] in 2010–2011 in [
30,
31]. The health impacts were discounted and age weighted, giving less weight on health impacts happening far in the future and on very young or old populations. A constant discount rate of 3% was used in the background health data. This means that the health impacts happening in future were valued 3% less each year. In European Perspectives on Environmental Burden of Disease (EBoDE) study, the health impacts were calculated with and without discounting. Removing discounting more than doubled the disease burden attributable to PM
2.5 (11,000 to 24,000 DALY/a) in Finland [
31].
In this work, we estimate the health impacts of ambient air pollution in Finland in 2015. According to the WHO recommendations, we add NO2 to the estimated pollutants, and for the first time apply spatially resolved exposure data at 1 km resolution together with burden of disease methodology. Specifically, we (i) estimate the exposures to PM2.5, PM10, NO2, and O3; (ii) quantify and compare health impacts by indicator pollutant and by health endpoint; (iii) assess the respective age and gender distributions; (iv) conduct parametric uncertainty and sensitivity analysis for exposure estimation and relative risks; and (v) discuss the sensitivity of NO2 estimates to the choice of a cut-off level.
4. Discussion
In the recent Global Burden of Disease study, air pollution was evaluated as one of the most important environmental health risk factors [
53]. In our work, we focused on the health impacts of four ambient air pollutants. We estimated the exposure to air pollution in Finland in 2015 using a state of art chemical transport model with high spatial resolution. The predicted concentrations were further adjusted for the difference of the observed and predicted concentrations at nationally available monitoring stations. The impacts of adjustment were small (ca. +5%) for PM
2.5 concentrations, but for the other components (NO
2, PM
10, SOMO35) much more substantial (
Table 3), and therefore, here, deemed essential.
We estimated 1,500 deaths were related to PM
2.5 exposure in 2015. This is of the same magnitude with the number of deaths attributable to PM
2.5 estimated by EEA (
Table 1). The number of deaths estimated by EEA, available for the period 2005–2014, range from 1700 to 2500, highlighting the variability of exposures between consecutive years. The current and ISTE estimates for years of life lost are based on the WHO Global Frontier 2050 projections for life expectancy, which is roughly 10 years higher than the current national one. This leads to substantial difference in comparison with YLL figures of EEA.
In comparison to earlier national estimates for PM
2.5, the differences reflect (i) temporal trends in exposures from 2000 to 2015; (ii) differences and developments in exposure estimation methods (including chemical transport modelling, source receptor matrices, and use of monitoring network data); and (iii) differences in the concentration–response modelling (
Table 6).
EEA has recognized the problematic nature of cut-off values and has calculated NO
2 estimates with cut-off values of 10 and 20 µg/m
3, showing that the lowering of the cut-off value had an 11-fold increase of the number of estimated deaths in Finland [
20]. Problems arise e.g., when averaging exposures between areas that are partly below and partly over the cut-off level, leading to a “no effects” estimate.
We evaluated NO
2 mortality using concentration–response function without a threshold (RR = 1.0027 (
Table 2)). WHO recommendations [
12], though, included also a significantly higher relative risk (1.055) for NO
2 related mortality, based on a cut-off level of 20 µg/m
3. This level was given due to larger uncertainties in the relation below 20 µg/m
3. However, in the later recommendations, they state that this might be too conservative an approach, since there are studies which show a relationship between mortality and NO
2 exposure at lower levels as well [
12].
We tested the sensitivity of the NO
2 mortality (RR = 1.055) using three cut-off levels (
Table 7). We found that the differences between shown estimates are huge (e.g., 23 vs. 1200 deaths; 370 vs. 20,000 YLL), concretely showing that this scientifically quite uncertain response model parameter has an utmost importance for the numerical results.
The fractional bias of SOMO35 was expectedly large. This is a result of the low stability of all threshold-based indexes, which was pointed out by [
54]. It explains the disproportional reaction of the index to the SILAM underestimation of ozone, of which traces remain, even after the simple bias correction. Secondly, our SOMO35 index is lower than that computed by EEA because the latter is based on monitoring data, all but three are located in background locations, and thus, show higher levels than those prevailing in the urban populated areas. It is actually likely that the monitoring station-based EEA exposure estimates may be too high. Here, the chemical transport model, in principle, accounts for the degradation of ozone in urban areas, and may, thus, contain information missing from the EEA model.
In our calculations, we used observed concentrations from all monitoring stations (
n = 21) as in previous estimates (i.e., [
55]). The majority of the stations are background stations which have higher ozone levels than urban ones, leading to a risk of overestimation. We did sensitivity analysis for the SOMO35 concentrations in different station types. The population lives mainly in urban and suburban areas, so we calculated the PWC using stations located in those areas (
n = 7). We also calculated the concentration on rural background stations (
n = 11) to find out how much it differs from the urban/suburban stations. The adjusted population-weighted concentrations for urban/suburban areas and for rural background stations were 740 µg/m
3 and 1400 µg/m
3, respectively, while the PWC for all stations was 1040 µg/m
3. Concentrations from the all stations might overestimate the exposure up to 41%.
The SILAM model is known to underestimate O3 concentrations during warm seasons, and therefore, the seasonal peculiarities need to be further examined. Here, the adjustment to observed concentrations should, though, correct this underestimation.
Recent updates in the disease burden methodology, especially dropping off discounting and age weighting by the global assessment community, as well as projected changes in life expectancy, have increased the disease burden estimates. This increases the estimates in comparison to those evaluated earlier using WHO GBD 2004 disease burden data. Current work uses WHO Global Frontier 2050 life expectancies (92 years at birth), which is substantially larger than national life expectancy in Finland (84 years for females and 79 years for males in 2015) [
56].
In the current approach, relative risks are assumed to be same for male and female [
12]. We also assumed same exposure over different age groups and genders. This is a clear weakness of our analysis. However, we did some primary analysis related to differences in PM
2.5 exposure between age groups and genders, and found differences less than 2% of the mean PM
2.5 exposure on the population level. Although, it is possible that regionally larger differences exist. We will investigate these in more detail in a geospatial follow-up study, while in the current work, the same exposures were used for both genders and ages.
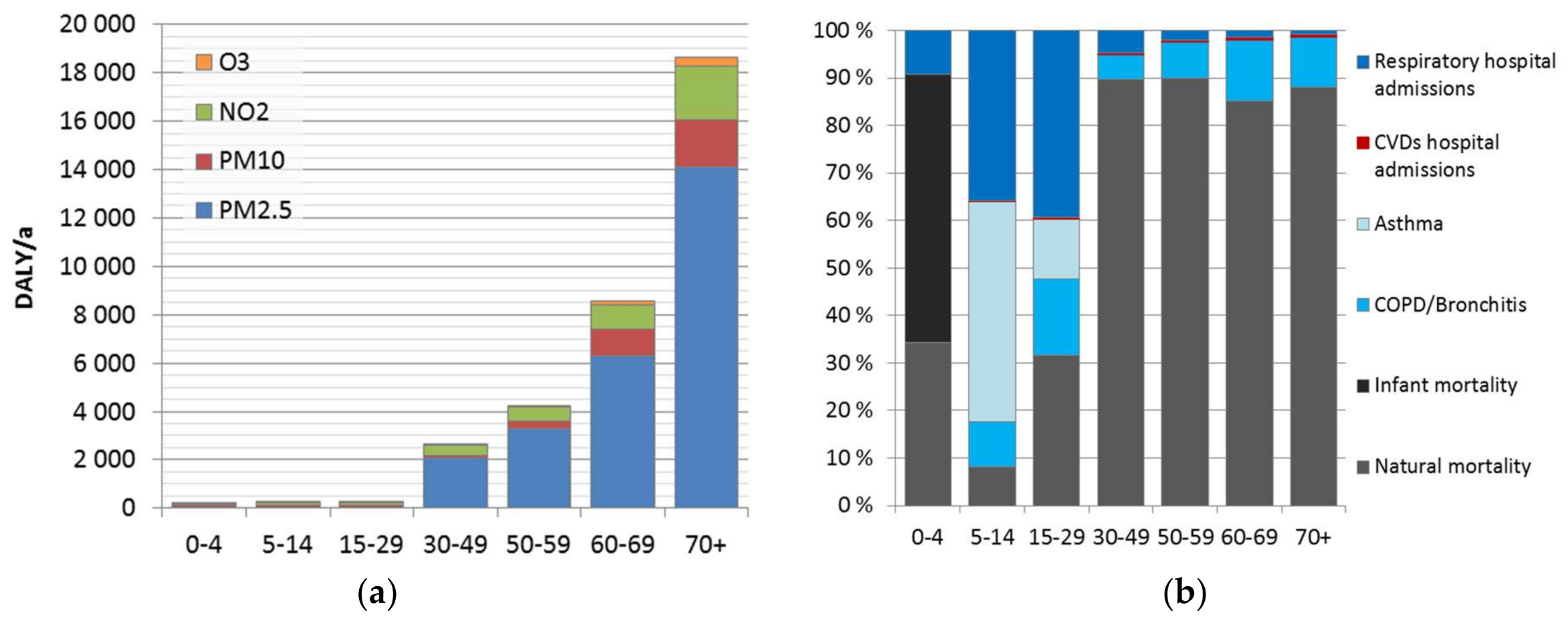
We found that the minor differences in the attributable burden between the genders are caused by the background disease burden. Much larger differences were found between population age groups, caused by age-dependence of the background disease burden, and included pollutant–outcome pairs. Overall, currently estimated impacts of air pollution increase heavily on population over 30 years, representing 98% of the total attributable burden. This is mainly due to mortality impacts. Use of natural mortality as an endpoint does not include the corresponding morbidity. The evaluation is lacking other effects, such as learning/academic performance of children and students, or productivity at work. These effects should be elaborated in more detail, to form a more comprehensive picture of the overall effects of air pollution.
The use of point value for exposure leads to numerical differences in the results when using non-linear exposure–response relationships, such as the traditional log-linear function (e.g., [
27]) or the integrated exposure response (IER) function (e.g., [
57]). The non-linear IER functions have been used in the recent global disease burden assessments of air pollution [
57,
58]. As part of the sensitivity analyses, we quantified these using an assumed normal distribution with variance estimated from the adjusted SILAM data, showing that in the current work, the use of point value leads to an underestimation by 3.8% when log-linear functions are used. Difference can be higher, though, in cases where a cut-off is used, and the exposure is close to the cut-off level. The IER functions include a cut-off level. Use of IER functions without taking into account the exposure distribution in areas where the concentrations are low can lead to substantially lower health impact estimates than when log-linear functions are used.
Another commonly used approach to handle exposure variability is related to spatial modelling (e.g., [
57,
59]), where population is allocated to grid cells and respective exposure levels. Such an approach may underestimate exposure variance due to deterministic point value modelling approaches, or due to coarse spatial resolution. Comparison of the benefits from spatial variation versus model error and embedded underestimation of variance needs to be further elaborated, but tentative interpretation of the current results suggest that the probability distribution approach works well.
Ambient air contains a mixture of contaminants, and therefore, we are exposed to different air pollutants at the same time. PM, NO2, and O3 correlate with each other to some extent, and their health impacts can overlap. WHO recommended different health endpoints for PM2.5 and PM10, which helps to avoid double-counting. Some of the NO2 long term effects were thought to overlap with particles up to 33%, which was taken into account in our analysis.
Air pollution has been estimated as the leading environmental risk factor in Finland, and particles as the most important air pollutant [
27,
60]. The latter is well confirmed in the current results. Especially the use of PM
2.5 and PM
10 exposures in the assessment highlights their indicator role—it cannot be interpreted that PM
10 effects would be the sum of fine and coarse particles. PM
10 is merely used as an alternative—often less reliable, but more widely available—exposure indicator.
5. Conclusions
In this work, concentrations of PM2.5, PM10, O3, and NO2 were estimated for Finland for 2015 using chemical transport model (SILAM) with adjustment to monitoring data. The concentrations were population weighted at 1 km resolution and used as exposure estimates. Resulting exposure levels were slightly smaller than averages of the air quality monitoring stations (e.g., −0.5 µg/m3 for PM2.5).
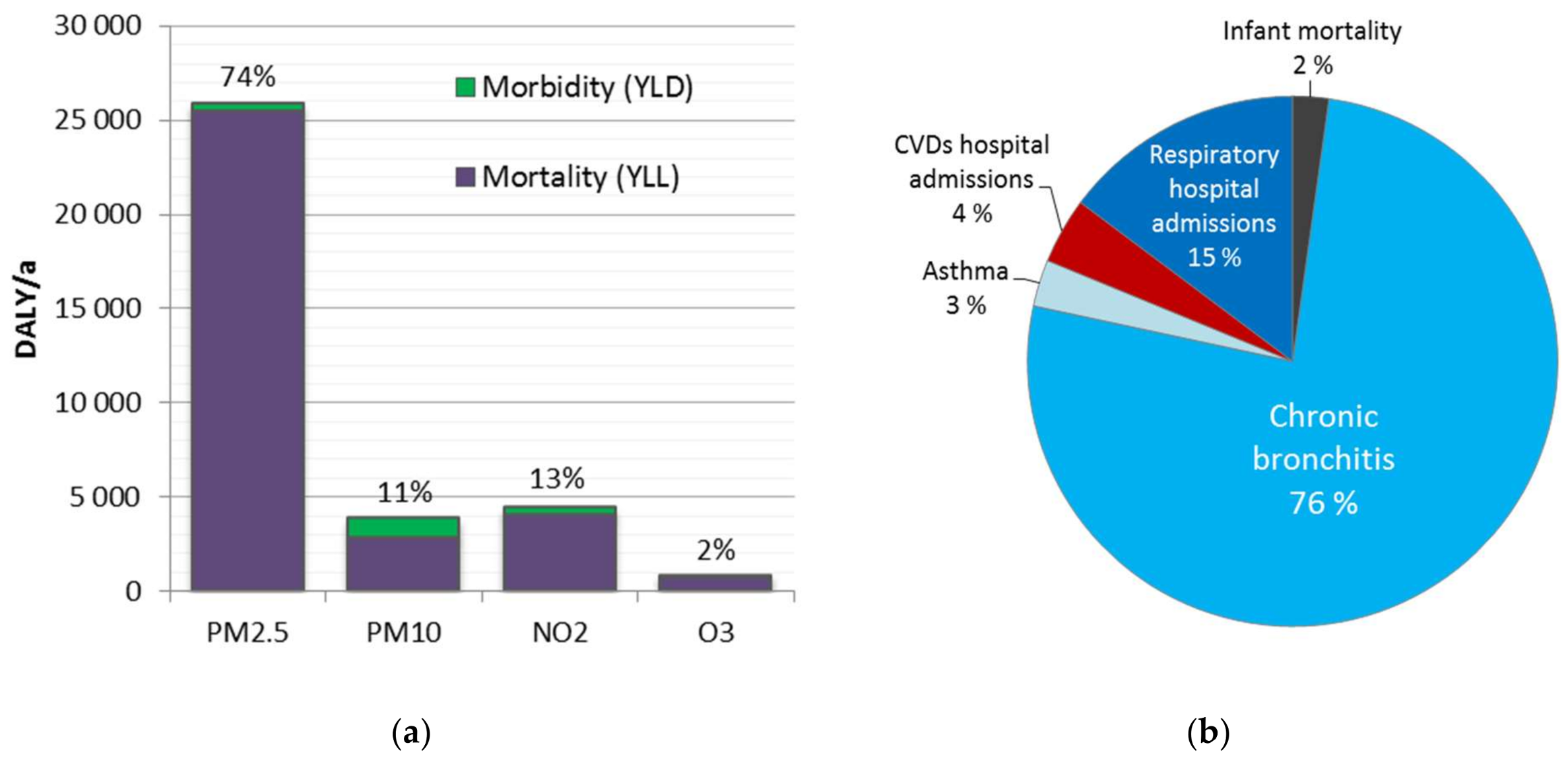
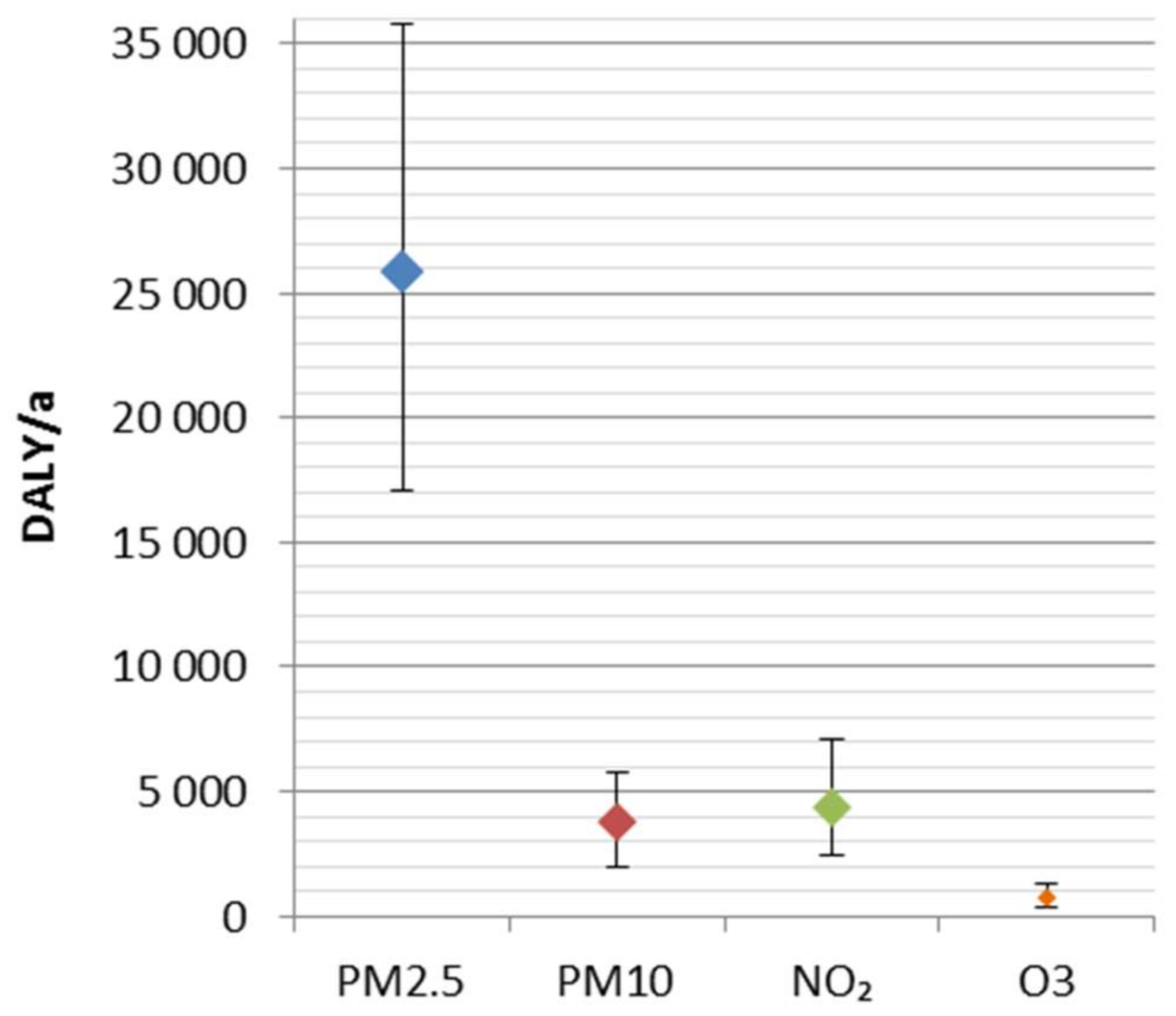
The disease burden attributable to the four ambient air pollutants was 34,800 DALY (25,400–45,500 95% CI). It represents ca. 2% of the total national burden of disease, and includes 2000 (1400–2600 95% CI) premature deaths. A major share (74%) of the disease burden was attributed to fine particles (PM2.5). The results are consistent with the previous comparative risk assessments, which have suggested fine particles as the leading environmental risk factor in Finland.
Disease burden was significantly higher at adult age groups, especially after 30 years old (98%). This is strongly related to the dominant role of mortality, which increases as a function of age. Before 30 years old, morbidity was 60% of the disease burden, while for age groups above 30 years, it was only 4.5%. However, morbidity impacts might be underestimated. It may be useful to improve the estimation and investigate also impacts on learning, academic performance, and productivity.
The uncertainties related to concentration–response functions were larger than ones related to the exposure. Overall, in the ranking of particles, NO2 and O3 seems robust, regardless of the uncertainties. In order to improve the estimation of health impacts, the shape of concentration–response functions, especially at current low exposure levels, should be studied in more detail, e.g., using health register-based epidemiological methods.