Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Culture
2.2. Nanoparticles
2.3. Algal Inhibition Assay
2.4. Lipid Peroxidation Assay
2.5. Transmission Electron Microscopy Imaging of Algae and TiO2 Nanoparticle Samples
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanoparticles
3.2. Algal Inhibition
3.3. Lipid Peroxidation: MDA Formation
3.4. TEM Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, F.; Cheng, Y.B.; Caruso, R.A. Mesoporous Anatase TiO2 beads with high surface areas and controllable pore sizes: A superior candidate for high-performance dye-sensitized solar cells. Adv. Mater. 2009, 21, 2206–2210. [Google Scholar] [CrossRef]
- Wang, B.; Jing, L.Q.; Qu, Y.C.; Li, S.D.; Jiang, B.J.; Yang, L.B.; Xin, B.F.; Fu, H.G. Enhancement of the photocatalytic activity of TiO2 nanoparticles by surface-capping DBS groups. Appl. Surf. Sci. 2006, 252, 2817–2825. [Google Scholar]
- More, B. Physical sunscreens: On the comeback trail. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, A.; Schneider, Y.J. Engineered nanomaterials in food: Implications for food safety and consumer health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef] [PubMed]
- Esterkin, C.; Negro, A.C.; Alfano, O.M.; Cassano, A.E. Air pollution remediation in a fixed bed photocatalytic reactor coated with TiO2. AIChE J. 2005, 51, 2298–2310. [Google Scholar] [CrossRef]
- Ferguson, M.; Hoffmann, M.R.; Hering, J.G. TiO2-photocatalyzed As(III) oxidation in aqueous suspensions: Reaction kinetics and effects of adsorption. Environ. Sci. Technol. 2005, 39, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, C.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of upper bounds and trends in nano-TiO2 production as a basis for exposure assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [PubMed]
- OECD. List of Manufactured Nanomaterials and List of Endpoints for Phase One of the Sponsorship Programme for the Testing on Manufactured Nanomaterials: Revision. In Series on the Safety of Manufactured Nanomaterials, ENV/JM/MONO(2010)46; Organisation for Economic Co-Operation and Development: Paris, France, 2010. [Google Scholar]
- Oomen, A.G.; Bleeker, E.A.J.; Bos, P.M.J.; van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.A.; Marcomini, A.; et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.M.J.; Gottardo, S.; Scott-Fordsmand, J.J.; van Tongeren, M.; Semenzin, E.; Fernandes, T.F.; Hristozov, D.; Hunt, N.; Irfan, M.A.; Landsiedel, R. The MARINA risk assessment strategy: A flexible strategy for efficient information collection and risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 15007–15021. [Google Scholar] [CrossRef] [PubMed]
- Teske, S.S.; Detweiler, C.S. The biomechanisms of metal and metal-oxide nanoparticles’ interactions with cells. Int. J. Environ. Res. Public Health 2015, 12, 1112–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leu, Y.R.; Aitken, R.J.; Riediker, M. Inventory of engineered nanoparticle-containing consumer products available in the Singapore retail market and likelihood of release into the aquatic environment. Int. J. Environ. Res. Public Health 2015, 12, 8717–8743. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Chen, Y.; Sommerfeld, M.; Hu, Q. Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii. Chemosphere 2008, 73, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Metzler, D.; Li, M.; Erdem, A.; Huang, C.P. Responses of algae to photocatalytic nano-TiO2 particles with an emphasis on the effect of particle size. Chem. Eng. J. 2011, 170, 538–546. [Google Scholar] [CrossRef]
- Metzler, D.; Erdem, A.; Tseng, Y.H.; Huang, C.P. Responses of algal cells to engineered nanoparticles measured as algal cell population, chlorophyll a, and lipid peroxidation: Effect of particle size and type. J. Nanotechnol. 2012, 2012, 12. [Google Scholar] [CrossRef]
- Sadiq, I.; Dalai, S.; Chandrasekaran, N.; Mukherjee, A. Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp. Ecotoxicol. Environ. Saf. 2011, 74, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Curtis, T.P.; Egerton, T.A.; Kosa, S.A.M.; Tinlin, J.R. Photoelectrocatalytic and photocatalytic disinfection of E. coli suspensions by titanium dioxide. Appl. Catal. B 2003, 41, 376–386. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Kelly, S.A.; Havrilla, C.M.; Brady, T.C.; Abramo, K.H.; Levin, E.D. Oxidative stress in toxicology: Established mammalian and emerging piscine model systems. Environ. Health Perspect. 1998, 106, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Klaine, S.J.; Cho, J.; Kim, S.H.; Kim, S.D. Oxidative stress responses of Daphnia magna exposed to TiO2 nanoparticles according to size fraction. Sci. Total Environ. 2010, 408, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium Dioxide (P25) produces reactive oxygen species in immortalized Brain Microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.; Lyon, D.Y.; McIntosh, A.; Alvarez, P.J.J. Comparative toxicity of nano-scale TiO2, SiO2 and ZnO water suspensions. Water Sci. Technol. 2006, 54, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Metzler, D.; Cha, D.; Huang, C.P. Inhibition of bacteria by photocatalytic nano-TiO2 particles in the absence of light. Int. J. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef][Green Version]
- Lee, W.; An, Y.J. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 2013, 91, 536–544. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for Testing Chemicals, Test no. 201 Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Publishing: Paris, France, 2011. [Google Scholar]
- USEPA. Short Term Methods for Estimating the Choronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, EPA-821-R-02-013.2002; U.S. Enviromental Protection Agency, Office of Water: Washington, DC, USA, 2002.
- Kato, M.; Suzuki, M.; Fujita, K.; Horie, M.; Endoh, S.; Yoshida, Y.; Iwahashi, H.; Takahashi, K.; Nakamura, A.; Kinugasa, S. Reliable size determination of nanoparticles using dynamic light scattering method for in vitro toxicology assessment. Toxicol. In Vitro 2009, 23, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Fujita, K.; Horie, M.; Suzuki, M.; Nakamura, A.; Endoh, S.; Yoshida, Y.; Iwahashi, H.; Takahashi, K.; Kinugasa, S. Dispersion characteristics of various metal oxide secondary nanoparticles in culture medium for in vitro toxicology assessment. Toxicol. In Vitro 2010, 24, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Yoon, M.; Song, N.W. Photochemical Properties and Cytotoxicity of TiO2 Nanoparticles Depending on the Degree of Agglomeration. In Proceedings of the XX IMEKO World Congress, Metrology for Green Growth, Busan, Korea, 9–14 September 2012; IMEKO: Busan, Korea, 2012. [Google Scholar]
- Othman, S.H.; Rashid, S.A.; Ghazi, T.I.M.; Abdullah, N. Dispersion and stabilization of photocatalytic TiO2 nanoparticles in aqueous suspension for coatings applications. J. Nanomater. 2012, 2012, 2. [Google Scholar] [CrossRef]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Leroy, P.; Tornassat, C.; Bizi, M. Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles. J. Colloid Interface Sci. 2011, 356, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M.; Lead, J.R. Nanoparticle dispersity in toxicology. Nat. Nanotechnol. 2013, 8, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Nur, Y.; Lead, J.R.; Baalousha, M. Evaluation of charge and agglomeration behavior of TiO2 nanoparticles in ecotoxicological media. Sci. Total Environ. 2015, 535, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, W.X. Modification of metal bioaccumulation and toxicity in Daphnia magna by titanium dioxide nanoparticles. Environ. Pollut. 2014, 186, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Thio, B.; Lee, J.H.; Meredith, J.C.; Keller, A.A. Measuring the influence of solution chemistry on the adhesion of Au nanoparticles to mica using colloid probe atomic force microscopy. Langmuir 2010, 26, 13995–14003. [Google Scholar] [CrossRef] [PubMed]
- Clement, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants-Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Gustafsson, J.; Cronholm, P.; Moller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Fekete-Kertész, I.; Maros, G.; Gruiz, K.; Molnár, M. The effect of TiO2 nanoparticles on the aquatic ecosystem: A comparative ecotoxicity study with test organisms of different trophic levels. Periodica Polytech. Chem. Eng. 2016, 60, 231–243. [Google Scholar] [CrossRef]
- Manier, N.; Le Manach, S.; Bado-Nilles, A.; Pandard, P. Effect of two TiO2 nanoparticles on the growth of unicellular green algae using the OECD 201 test guideline: Influence of the exposure system. J. Toxicol. Environ. Chem. 2015, 98, 860–876. [Google Scholar]
- Li, M.; Hu, C.; Zhu, Q.; Chen, L.; Kong, A.; Liu, Z. Copper and zinc induction of lipid peroxidation and efects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 2006, 62, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Soto, P.; Gaete, H.; Hidalgo, M.E. Assessment of catalase activity, lipid peroxidation, chlorophyll-a, and growth rate in the freshwater green algae Pseudokirchneriella subcapitata exposed to copper and zinc. Lat. Am. J. Aquat. Res. 2011, 39, 280–285. [Google Scholar] [CrossRef]
- Li, X.; Zhou, S.; Fan, W. Effect of nano-Al2O3 on the toxicity and oxidative stress of copper towards Scenedesmus obliquus. Int. J. Environ. Res. Public Health 2016, 13, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.S.; Mishra, R.D.; Tripathi, S.; Dwivedi, D.; Gupta, K. Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla certicillata (L.F.) Royle. Aquat. Toxicol. 2006, 80, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, L.; Liu, Y.; Deng, S.; Wu, H.; Wang, G. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012, 84, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.J.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Intracellular uptake: A possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS ONE 2010, 5, e15196. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, C.; Chernenko, T.; Diem, M.; Gu, A.Z. Impact of nano titanium dioxide exposure on cellular structure of Anabaena variabilis and evidence of internalization. Environ. Toxicol. Chem. 2011, 30, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand-Hammarström, B.; Akfur, C.M.; Andersson, P.O.; Lejon, C.; Österlund, L.; Bucht, A. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology 2012, 6, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Moreno-Garrido, I.; Yeste, M.P.; Gatica, J.M.; Blasco, J. Toxicity of TiO2, in nanoparticle or bulk form to freshwater and marine microalgae under visible light and UV-A radiation. Environ. Pollut. 2017, 227, 39–48. [Google Scholar] [CrossRef] [PubMed]
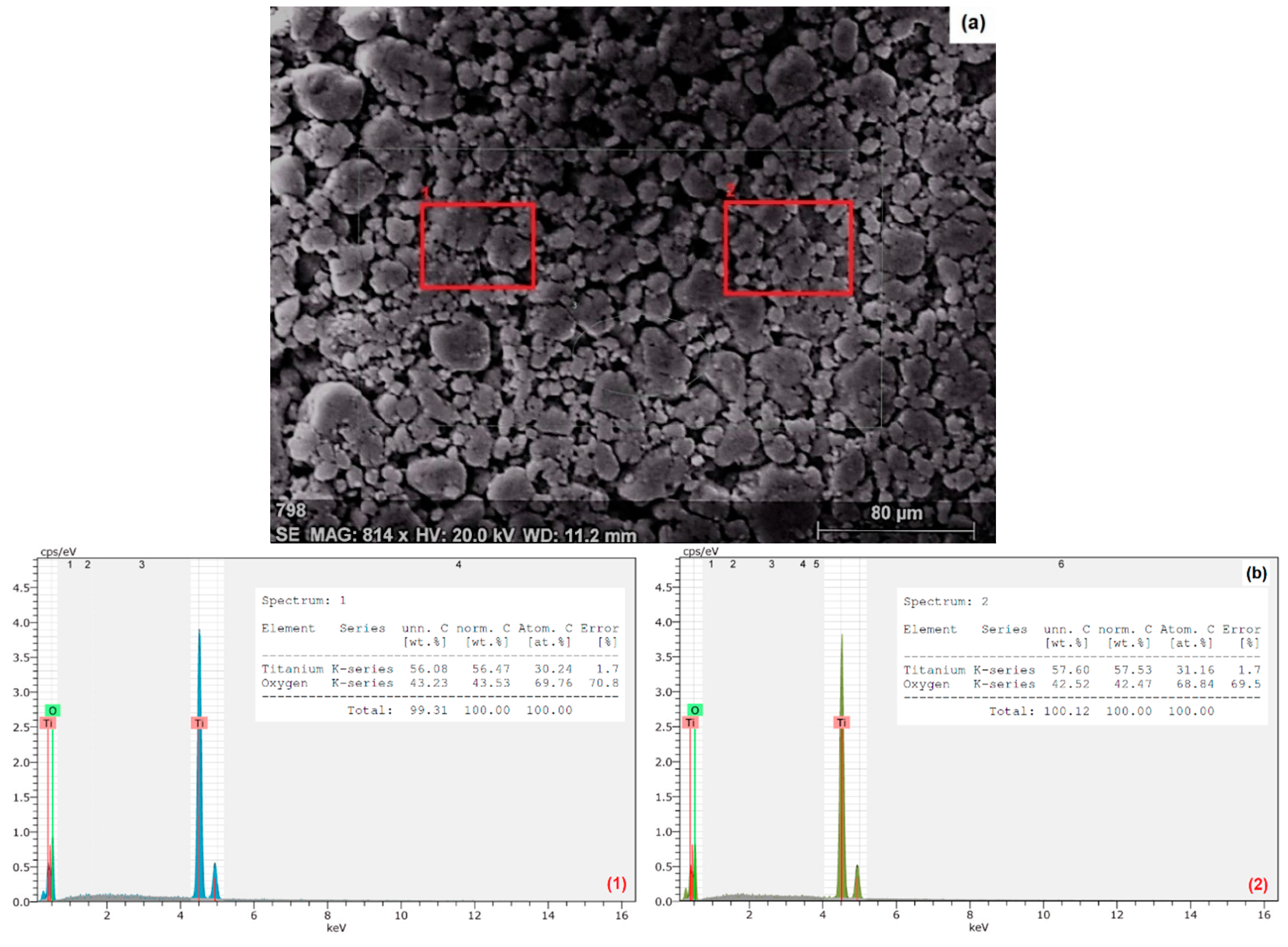

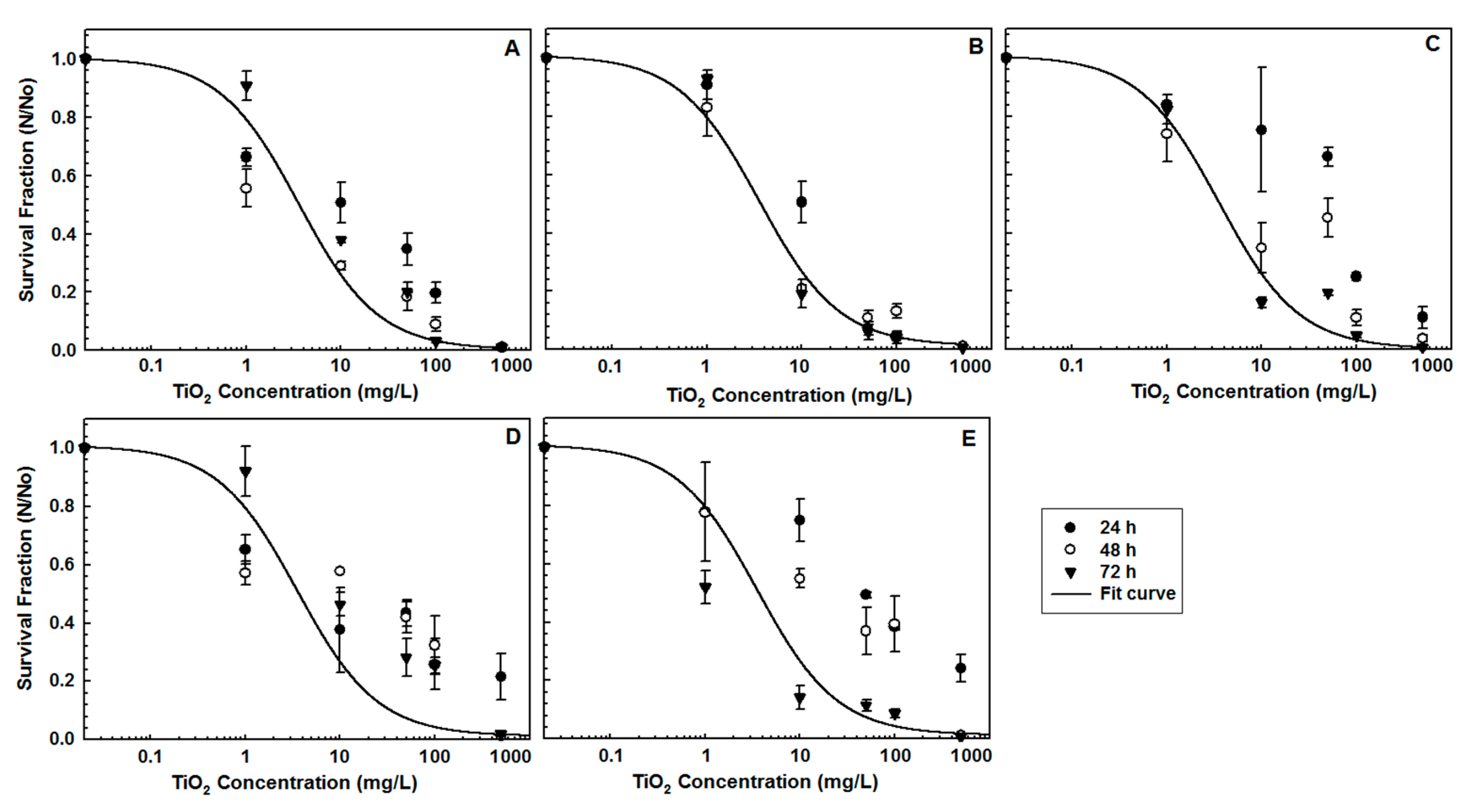
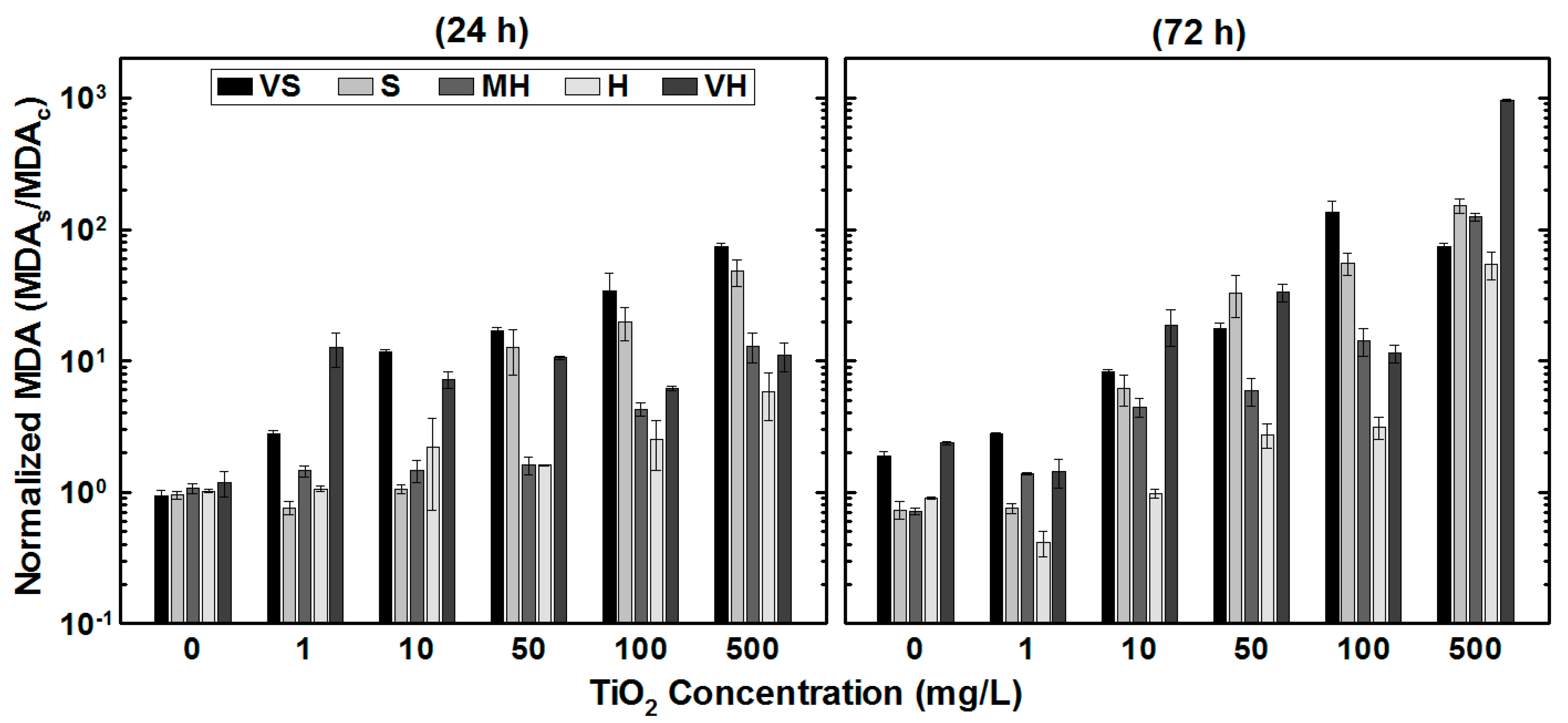
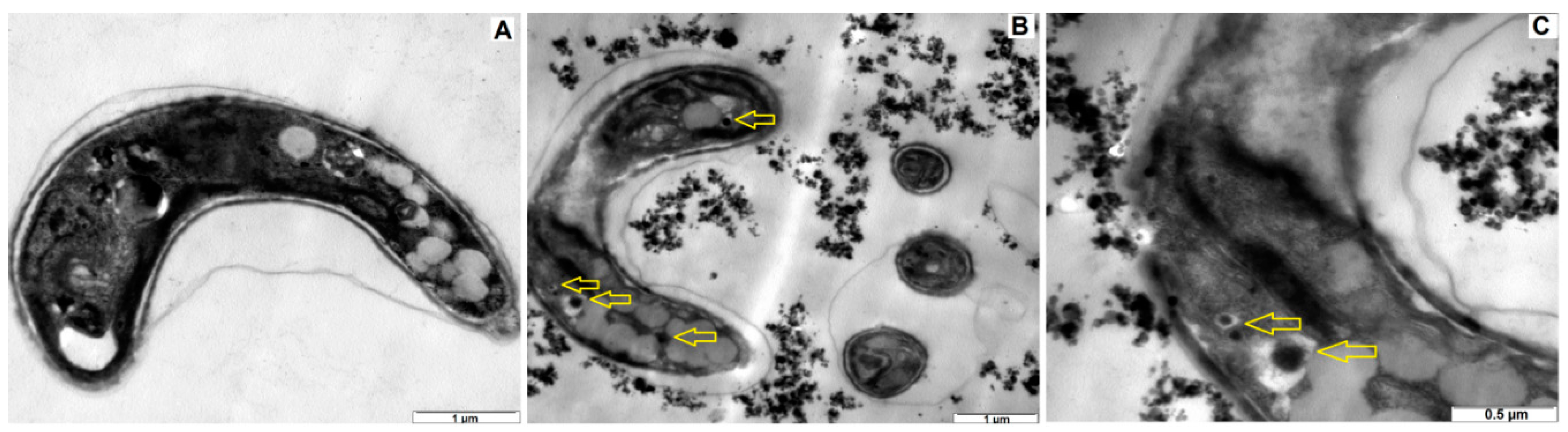
| Water Type | Reagents Added to Deionized Water (mg/L) | Approximate Final Water Quality | |||||
|---|---|---|---|---|---|---|---|
| NaHCO3 | CaSO4·H2O | MgSO4 | KCl | pH 1 | Hardness 2 | Alkalinity 2 | |
| Very soft | 12.0 | 7.5 | 7.5 | 0.5 | 6.4–6.8 | 10–13 | 10–13 |
| Soft | 48.0 | 30.0 | 30.0 | 2.0 | 7.2–7.6 | 40–48 | 30–35 |
| Moderately hard | 96.0 | 60.0 | 60.0 | 4.0 | 7.4–7.8 | 80–100 | 57–64 |
| Hard | 192.0 | 120.0 | 120.0 | 8.0 | 7.6–7.8 | 160–180 | 110–120 |
| Very hard | 384.0 | 240.0 | 240.0 | 16.0 | 8.0–8.4 | 280–320 | 225–245 |
| Synthetic Freshwater Solution Type | Ionic Strength (mM) | pH | Nanoparticle Size (nm) | Zeta Potential (mV) | |||
|---|---|---|---|---|---|---|---|
| 0 | 72 h | 0 | 72 h | 0 | 72 h | ||
| Very soft | 0.5 | 6.5 ± 0.3 | 7.0 ± 0.2 | 20 ± 4 | 212 ± 19 | −23.0 | −18.4 |
| Soft | 1 | 7.3 ± 0.2 | 7.5 ± 0.1 | 44 ± 7 | 287 ± 25 | −20.5 | −17.6 |
| Moderately hard | 2 | 7.6 ± 0.1 | 7.8 ± 0.2 | 61 ± 22 | 546 ± 71 | −16.0 | −15.7 |
| Very hard | 8 | 8.2 ± 0.2 | 8.0 ± 0.2 | 100 ± 49 | 1428 ± 202 | −6.9 | −13.4 |
| Synthetic Freshwater Solution Type | Mass Concentration (mg/L) | Number Concentration (np/nc) | ||
|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | |
| Very soft | 18.12 ± 1.3 | 4.16 ± 0.05 | 1698 ± 214 | 381 ± 13.4 |
| Soft | 17.96 ± 2.8 | 3.58 ± 0.16 | 645 ± 98 | 324 ± 9.5 |
| Moderately hard | 56.4 ± 9.9 | 9.32 ± 0.11 | 60.3 ± 24 | 54.5 ± 5.2 |
| Very hard | 98.3 ± 12.4 | 12.14 ± 0.09 | 16.2 ± 3.5 | 5.99 ± 0.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkaleli, M.; Erdem, A. Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation. Int. J. Environ. Res. Public Health 2018, 15, 416. https://doi.org/10.3390/ijerph15030416
Ozkaleli M, Erdem A. Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation. International Journal of Environmental Research and Public Health. 2018; 15(3):416. https://doi.org/10.3390/ijerph15030416
Chicago/Turabian StyleOzkaleli, Merve, and Ayca Erdem. 2018. "Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation" International Journal of Environmental Research and Public Health 15, no. 3: 416. https://doi.org/10.3390/ijerph15030416
APA StyleOzkaleli, M., & Erdem, A. (2018). Biotoxicity of TiO2 Nanoparticles on Raphidocelis subcapitata Microalgae Exemplified by Membrane Deformation. International Journal of Environmental Research and Public Health, 15(3), 416. https://doi.org/10.3390/ijerph15030416




