Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary Diseases in Adults and Effect Modification by Demographic and Lifestyle Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Spirometry
2.3. Exposure Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, Y.; Liu, S.; Chen, X.; Zou, W.; Zhao, D.; Li, X.; Pu, J.; Huang, L.; Chen, J.; et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: Results from a cross-sectional study in China. Thorax 2017, 72, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Gold, D.R.; Schwartz, J.D.; Koutrakis, P.; Washko, G.R.; O’Connor, G.T.; Mittleman, M.A. Short-term exposure to air pollution and lung function in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2013, 188, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Dorans, K.S.; Gold, D.R.; Schwartz, J.; Koutrakis, P.; Washko, G.R.; O’Connor, G.T.; Mittleman, M.A. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am. J. Respir. Crit. Care Med. 2015, 191, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Paulina, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.; Hui, D.S. Air pollution and chronic obstructive pulmonary disease. Respirology 2012, 17, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kubesch, N.J.; de Nazelle, A.; Westerdahl, D.; Martinez, D.; Carrasco-Turigas, G.; Bouso, L.; Guerra, S.; Nieuwenhuijsen, M.J. Respiratory and inflammatory responses to short-term exposure to traffic-related air pollution with and without moderate physical activity. Occup. Environ. Med. 2015, 72, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Schikowski, T.; Carsin, A.E.; Cai, Y.; Jacquemin, B.; Sanchez, M.; Vierkötter, A.; Marcon, A.; Keidel, D.; Sugiri, D.; et al. Adult lung function and long-term air pollution exposure. ESCAPE: A multicentre cohort study and meta-analysis. Eur. Respir. J. 2015, 45, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Paulose-Ram, R.; Tilert, T.; Dillon, C.F.; Brody, D.J. Cigarette smoking and lung obstruction among adults aged 40–79: United States; 2007–2012. NCHS Data Brief. 2015, 181, 1–8. [Google Scholar]
- Medbø, A.; Melbye, H. Lung function testing in the elderly—Can we still use FEV1/FVC<70% as a criterion of COPD? Respir. Med. 2007, 101, 1097–1105. [Google Scholar]
- Forbes, L.J.; Kapetanakis, V.; Rudnicka, A.R.; Cook, D.G.; Bush, T.; Stedman, J.; Whincup, P.; Strachan, D.P.; Anderson, H.R. Chronic exposure to outdoor air pollution and lung function in adults. Thorax 2009, 64, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.B.B.; Biersteker, K. Effects of indoor exposure to nitrogen dioxide on pulmonary function of women living in urban and rural areas. Environ. Int. 1989, 15, 375–381. [Google Scholar] [CrossRef]
- Kesavachandran, C.N.; Bihari, V.; Pangtey, B.S.; Kamal, R.; Singh, A.; Srivastava, A.K. Gender disparity in lung function abnormalities among a population exposed to particulate matter concentration in ambient air in the National Capital Region, India. J. Health Pollut. 2015, 5, 47–60. [Google Scholar] [CrossRef]
- Schikowski, T.; Sugiri, D.; Ranft, U.; Gehring, U.; Heinrich, J.; Wichmann, H.E.; Krämer, U. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir. Res. 2005, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Nuvolone, D.; della Maggiore, R.; Maio, S.; Fresco, R.; Baldacci, S.; Carrozzi, L.; Pistelli, F.; Viegi, G. Geographical information system and environmental epidemiology: A cross-sectional spatial analysis of the effects of traffic-related air pollution on population respiratory health. Environ. Health 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Pujades-Rodríguez, M.; McKeever, T.; Lewis, S.; Whyatt, D.; Britton, J.; Venn, A. Effect of traffic pollution on respiratory and allergic disease in adults: Cross-sectional and longitudinal analyses. BMC Pulm. Med. 2009, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Pujades-Rodríguez, M.; Lewis, S.; McKeever, T.; Britton, J.; Venn, A. Effect of living close to a main road on asthma, allergy, lung function and chronic obstructive pulmonary disease. Occup. Environ. Med. 2009, 66, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J. Lung function effects of chronic exposure to air pollution. Thorax 2009, 64, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Götschi, T.; Sunyer, J.; Chinn, S.; de Marco, R.; Forsberg, B.; Gauderman, J.W.; Garcia-Esteban, R.; Heinrich, J.; Jacquemin, B.; Jarvis, D.; et al. Air pollution and lung function in the European Community Respiratory Health Survey. Int. J. Epidemiol. 2008, 37, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Ranft, U.; Sugiri, D.; Vierkötter, A.; Brüning, T.; Harth, V.; Krämer, U. Decline in air pollution and change in prevalence in respiratory symptoms and chronic obstructive pulmonary disease in elderly women. Respir. Res. 2010, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Sugiri, D.; Reimann, V.; Pesch, B.; Ranft, U.; Krämer, U. Contribution of smoking and air pollution exposure in urban areas to social differences in respiratory health. BMC Public Health 2008, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Mills, I.C.; Anderson, H.R.; Cohen, A.; Hansell, A.; Kauffmann, F.; Krämer, U.; Marcon, A.; Perez, L.; Sunyer, J.; et al. Ambient air pollution: A cause of COPD? Eur. Respir. J. 2014, 43, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Adam, M.; Marcon, A.; Cai, Y.; Vierkötter, A.; Carsin, A.E.; Jacquemin, B.; Al Kanani, Z.; Beelen, R.; Birk, M.; et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur. Respir. J. 2014, 44, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Götschi, T.; Heinrich, J.; Sunyer, J.; Künzli, N. Long-term effects of ambient air pollution on lung function: A review. Epidemiology 2008, 19, 690–701. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society Statement. Standardization of spirometry; 1994 update. Am. J. Respir. Crit. Care Med. 1995, 152, 1107–1136. [Google Scholar]
- Lee, J.Y.; Leem, J.H.; Kim, H.C.; Hwang, S.S.; Jung, D.Y.; Park, M.S.; Kim, J.; Lee, J.J.; Park, N.W.; Kang, S.C. Land use regression model for assessing exposure and impacts of air pollutants in school children. J. Korean Soc. Atmos. Environ. 2012, 28, 571–580. (In Korean) [Google Scholar] [CrossRef]
- Lamichhane, D.K.; Kim, H.C.; Choi, C.M.; Shin, M.H.; Shim, Y.M.; Leem, J.H.; Ryu, J.S.; Nam, H.S.; Park, S.M. Lung cancer risk and residential exposure to air pollution: A Korean population-based case-control study. Yonsei Med. J. 2017, 58, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Heiss, G.; Rose, K.M.; Whitsel, E.; Lurmann, F.; London, S.J. Traffic exposure and lung function in adults: the Atherosclerosis Risk in Communities study. Thorax 2007, 62, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Santos, U.P.; Garcia, M.L.S.B.; Braga, A.L.F.; Pereira, L.A.A.; Lin, C.A.; de André, P.A.; de André, C.D.S.; da Motta Singer, J.; Saldiva, P.H.N. Association between traffic air pollution and reduced forced vital capacity: A study using personal monitors for outdoor workers. PLoS ONE 2016, 11, e0163225. [Google Scholar] [CrossRef] [PubMed]
- Probst-Hensch, N.M.; Curjuric, I.; Pierre-Olivier, B. Longitudinal change of prebronchodilator spirometric obstruction and health outcomes: Results from the SAPALDIA cohort. Thorax 2010, 65, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, S.; Hebbern, C.; Cakmak, J.D.; Vanos, J. The modifying effect of socioeconomic status on the relationship between traffic, air pollution and respiratory health in elementary schoolchildren. J. Environ. Manage. 2016, 177, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Song, Y.; Xie, J.; Cui, X.; Zhang, B.; Shi, T.; Yuan, J.; Chen, W. Short-term effects of outdoor air pollution on lung function among female non-smokers in China. Sci. Rep. 2016, 6, 34947. [Google Scholar] [CrossRef] [PubMed]
- Eckel, S.P.; Louis, T.A.; Chaves, P.H.; Fried, L.P.; Margolis, A.H.G. Modification of the association between ambient air pollution and lung function by frailty status among older adults in the Cardiovascular Health Study. Am. J. Epidemiol. 2012, 176, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Viegi, G.; Maio, S.; Simoni, M.; Baldacci, S.; Annesi-Maesano, I. The Epidemiological Link between Ageing and Respiratory Diseases. In European Respiratory Monograph 43: Respiratory Diseases in the Elderly; Bellia, V., Incalzi, R.A., Eds.; European Respiratory Society: Plymouth, UK, 2009; pp. 1–17. [Google Scholar]
- Wang, J.M.; Ueng, T.H.; Lin, J.K. Biochemical and morphological alterations in the lungs and livers of mice following exposure to polluted air in a traffic tunnel. Proc. Natl. Sci. Counc. Repub. China B 1992, 16, 77–83. [Google Scholar] [PubMed]
- Hatzis, C.; Godleski, J.J.; González-Flecha, B.; Wolfson, J.M.; Koutrakis, P. Ambient particulate matter exhibits direct inhibitory effects on oxidative stress enzymes. Environ. Sci. Technol. 2006, 40, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, M.; Fehrenbach, A.; Heimann, S.; Fehrenbach, H.; Renz, H.; Garn, H.; Herz, U. NO2-induced airway inflammation is associated with progressive airflow limitation and development of emphysema-like lesions in C57bl/6 mice. Exp. Toxicol. Pathol. 2005, 56, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ciencewicki, J.; Trivedi, S.; Kleeberger, S.R. Oxidants and the pathogenesis of lung diseases. J. Allergy Clin. Immunol. 2008, 122, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Mannino, D.M.; Sonia Buist, A.; Vollmer, W.M. Chronic obstructive pulmonary disease in the older adult: What defines abnormal lung function? Thorax 2007, 62, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Güder, G.; Brenner, S.; Angermann, C.E.; Ertl, G.; Held, M.; Sachs, A.P.; Lammers, J.W.; Zanen, P.; Hoes, A.W.; Störk, S.; et al. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir. Res. 2012, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Hardie, J.A.; Buist, A.S.; Vollmer, W.M.; Ellingsen, I.; Bakke, P.S.; Mørkve, O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur. Respir. J. 2002, 20, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Hangaard, S.; Helle, T.; Nielsen, C.; Hejlesen, O.K. Causes of misdiagnosis of chronic obstructive pulmonary disease: A systematic scoping review. Respir. Med. 2017, 129, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease. 2016. Available online: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/ (accessed on 18 February 2018).
- Schermer, T.R.; Robberts, B.; Crockett, A.J.; Thoonen, B.P.; Lucas, A.; Grootens, J.; Smeele, I.J.; Thamrin, C.; Reddel, H.K. Should the diagnosis of COPD be based on a single spirometry test? NPJ Prim. Care. Respir. Med. 2016, 26, 16059. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Beelen, R.; De Hoogh, K.; Vienneau, D.; Gulliver, J.; Fischer, P.; Briggs, D. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos. Environ. 2008, 42, 7561–7578. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sheppard, L.; Bergen, S.; Szpiro, A.A.; Sampson, P.D.; Kaufman, J.D.; Vedal, S. Prediction of fine particulate matter chemical components with a spatio-temporal model for the Multi-Ethnic Study of Atherosclerosis cohort. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 520–528. [Google Scholar] [CrossRef] [PubMed]
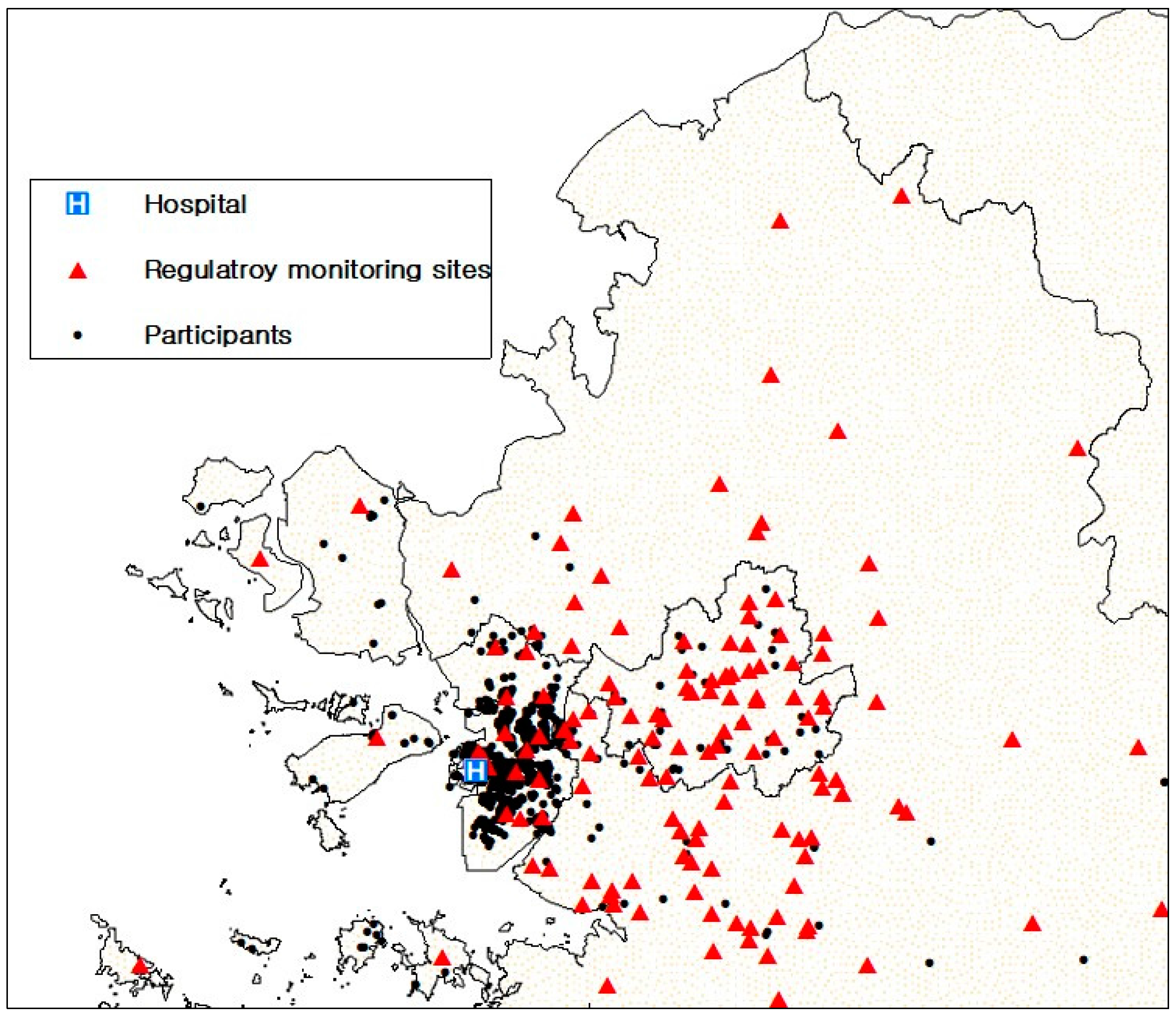
| Variable | n (%) or mean ± SD | COPD (n) | Prevalence (%) | Adjusted * OR (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Female | 683 (54.03) | 30 | 4.39 | Reference |
| Male | 581 (45.97) | 64 | 11.02 | 1.20 (0.53–2.74) |
| Age, years | ||||
| <50 | 225 (17.80) | 12 | 5.33 | Reference |
| 50–59 | 527 (41.69) | 31 | 5.88 | 1.36 (0.66–2.78) |
| 60–69 | 380 (30.06) | 37 | 9.74 | 2.69 (1.25–5.78) |
| ≥70 | 132 (10.44) | 14 | 10.61 | 3.14 (1.25–7.91) |
| Education | ||||
| <High school | 341 (26.98) | 22 | 6.45 | Reference |
| High school | 503 (39.79) | 43 | 8.55 | 1.39 (0.77–2.50) |
| >High school | 420 (33.23) | 29 | 6.90 | 1.07 (0.56–2.02) |
| BMI | ||||
| <18.5 | 17 (1.34) | 1 | 5.88 | Reference |
| 18.5–25 | 799 (63.21) | 69 | 8.64 | 0.81 (0.10–6.51) |
| >25 | 448 (35.44) | 24 | 5.36 | 0.42 (0.05–3.53) |
| Smoking | ||||
| Non-smoker | 796 (62.97) | 34 | 4.27 | Reference |
| Former smoker | 291 (23.02) | 34 | 11.68 | 2.52 (1.09–5.81) |
| Current smoker | 177 (14.00) | 26 | 14.69 | 4.33 (1.84–10.22) |
| Drinking | ||||
| No | 672 (53.16) | 40 | 5.95 | Reference |
| Yes | 592 (46.84) | 54 | 9.12 | 1.01 (0.59–1.72) |
| Exercise | ||||
| Yes | 528 (41.77) | 33 | 6.25 | Reference |
| No | 736 (58.23) | 61 | 8.29 | 1.19 (0.74–1.91) |
| Hypertension | ||||
| No | 872 (68.99) | 64 | 7.34 | Reference |
| Yes | 392 (31.01) | 30 | 7.65 | 0.77 (0.46–1.29) |
| Diabetes mellitus | ||||
| No | 1119 (88.53) | 78 | 6.97 | Reference |
| Yes | 145 (11.47) | 16 | 11.03 | 1.42 (0.76–2.65) |
| Hyperlipidemia | ||||
| No | 954 (75.47) | 66 | 6.92 | Reference |
| Yes | 310 (24.53) | 28 | 9.03 | 1.37 (0.81–2.30) |
| History of stroke | ||||
| No | 1239 (98.02) | 92 | 7.43 | Reference |
| Yes | 25 (1.98) | 2 | 8.00 | 0.87 (0.19–4.02) |
| Family history of COPD | ||||
| No | 1255 (99.29) | 92 | 7.33 | Reference |
| Yes | 9 (0.71) | 2 | 22.22 | 3.97 (0.70–22.51) |
| Family history of asthma | ||||
| No | 1237 (97.86) | 94 | 7.60 | |
| Yes | 27 (2.14) | 0 | 0 | NS |
| History of angina pectoris | ||||
| No | 1211 (95.81) | 89 | 7.35 | Reference |
| Yes | 53 (4.19) | 5 | 9.43 | 0.80 (0.29–2.20) |
| FVC, L (meanness) | 86.69 ± 12.69 | |||
| FEV1, L (mean ± SD) | 90.40 ± 14.17 | |||
| % FEV1/FVC | 80.63 ± 8.27 | |||
| COPD (FEV1/FVC) | ||||
| <0.7 | 94 (7.44) | |||
| ≥0.7 | 1170 (92.56) |
| Length of exposure | Pollutant (μg/m3) | Mean (SD) | Median | IQR | Range |
|---|---|---|---|---|---|
| 5 years | PM2.5 | 35.82 (5.49) | 37.07 | 6.64 | 14.94–46.5 |
| PM10 | 53.56 (4.40) | 52.81 | 5.53 | 39.18–69.21 | |
| NO2 | 45.63 (17.68) | 49.83 | 18.38 | 3.34–118.94 | |
| 3 years | PM2.5 | 33.83 (5.62) | 35.22 | 7.05 | 14.49–46.24 |
| PM10 | 50.98 (4.30) | 50.17 | 5.44 | 38.87–67.32 | |
| NO2 | 44.64 (17.65) | 48.6 | 18.87 | 3.34–135.87 | |
| 1 year | PM2.5 | 33.39 (6.05) | 34.97 | 7.26 | 14.52–48.08 |
| PM10 | 50.68 (4.57) | 49.53 | 6.39 | 39.56–73.34 | |
| NO2 | 44.81 (17.75) | 49.17 | 18.05 | 3.50–132.97 |
| Air Pollutants * | COPD | |
|---|---|---|
| Crude | Adjusted † | |
| OR (95% CI) | OR (95% CI) | |
| NO2 (μg/m3) | ||
| Lowest tertile (<41.0) | Reference | Reference |
| Medium tertile (≥41.0 and <53.8) | 1.28 (0.73–2.25) | 1.39 (0.77–2.52) |
| Highest tertile (≥53.8) | 1.93 (1.14–3.27) | 1.83 (1.04–3.21) |
| p for trend | 0.012 | |
| Per 10 μg/m3 increase | 1.13 (0.99–1.27) | 1.14 (1.00–1.30) |
| PM10 (μg/m3) | ||
| Lowest tertile (<48.5) | Reference | Reference |
| Medium tertile (≥48.5 and <52.2) | 1.17 (0.68–2.01) | 1.23 (0.70–2.15) |
| Highest tertile (≥52.2) | 1.51 (0.90–2.54) | 1.57 (0.92–2.69) |
| p for trend | 0.113 | |
| Per 10 μg/m3 increase | 1.38 (0.87–2.21) | 1.39 (0.85–2.25) |
| PM2.5 (μg/m3) | ||
| Lowest tertile (<32.7) | Reference | Reference |
| Medium tertile (≥32.7 and <37.1) | 1.43 (0.82–2.48) | 1.54 (0.86–2.75) |
| Highest tertile (≥37.1) | 1.77 (1.04–3.02) | 1.79 (1.02–3.13) |
| p for trend | 0.035 | |
| Per 10 μg/m3 increase | 1.32 (0.88–1.97) | 1.34 (0.89–2.02) |
| Parameter | Length of exposure | Air Pollutant (μg/m3) | Crude | Adjusted * |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | |||
| FVC (l) | 5 years | NO2 | 0.35 (−0.05, 0.75) | 0.18 (−0.22, 0.59) |
| PM10 | −1.38 (2.97, 0.21) | −1.52 (−3.10, 0.05) | ||
| PM2.5 | −0.61 (−1.89, 0.66) | −0.62 (−1.89, 0.65) | ||
| 3 years | NO2 | 0.33 (−0.06, 0.73) | 0.18 (−0.22, 0.58) | |
| PM10 | −1.52 (−3.14, 0.11) | −1.73 (−3.35, −0.12) | ||
| PM2.5 | −0.83 (−2.08, 0.41) | −0.85 (−2.10, 0.39) | ||
| 1 year | NO2 | 0.27 (−0.13, 0.66) | 0.11 (−0.28, 0.52) | |
| PM10 | −1.46 (−2.99, 0.07) | −1.77 (−3.29, −0.25) | ||
| PM2.5 | −0.80 (−1.96, 0.36) | −0.85 (−2.01, 0.30) | ||
| FEV1 (l) | 5 years | NO2 | 0.19 (−0.25, 0.63) | 0.11 (−0.33, 0.56) |
| PM10 | −1.58 (−3.36, 0.19) | −1.73 (−3.50, 0.03) | ||
| PM2.5 | −0.81 (−2.24, 0.61) | −0.86 (−2.28, 0.56) | ||
| 3 years | NO2 | 0.19 (−0.25, 0.64) | 0.13 (−0.32, 0.58) | |
| PM10 | −1.62 (−3.44, 0.19) | −1.85 (−3.65, −0.05) | ||
| PM2.5 | −0.83 (−2.22, 0.57) | −0.94 (−2.33, 0.45) | ||
| 1 year | NO2 | 0.15 (−0.29, 0.59) | 0.09 (−0.36, 0.53) | |
| PM10 | −1.13 (−2.84, 0.58) | −1.46 (−3.16, 0.24) | ||
| PM2.5 | −0.78 (−2.07, 0.51) | −0.96 (−2.25, 0.32) | ||
| FEV1/FVC | 5 years | NO2 | −0.07 (−0.32, 0.19) | −0.05 (−0.29, 0.19) |
| PM10 | 0.05 (−0.99, 1.08) | 0.09 (−0.93, 1.10) | ||
| PM2.5 | 0.00 (−0.83, 0.84) | −0.09 (−1.03, 0.85) | ||
| 3 years | NO2 | −0.04 (−0.30, 0.22) | −0.03 (−0.28, 0.21) | |
| PM10 | 0.11 (−0.95, 1.17) | 0.16 (−0.89, 1.21) | ||
| PM2.5 | 0.08 (−0.73, 0.90) | 0.02 (−0.89, 0.93) | ||
| 1 year | NO2 | −0.04 (−0.30, 0.22) | −0.03 (−0.27, 0.21) | |
| PM10 | 0.28 (−0.72, 1.28) | 0.40 (−0.57, 1.37) | ||
| PM2.5 | 0.00 (−0.75, 0.76) | −0.00 (−0.85, 0.85) |
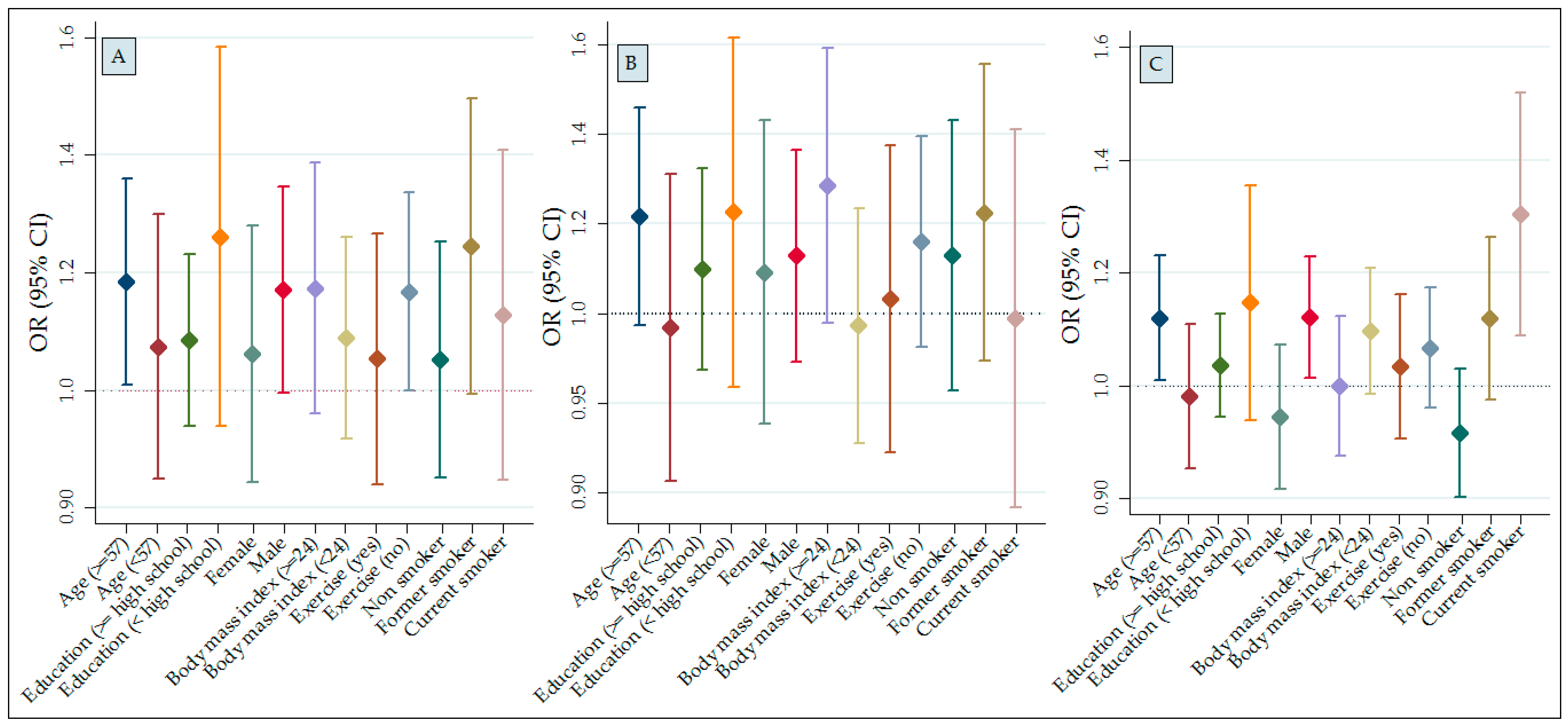
| Parameter | Stratified Characteristics | Air Pollutants | ||
|---|---|---|---|---|
| NO2 | PM10 | PM2.5 | ||
| β (95% CI) | β (95% CI) | β (95% CI) | ||
| FVC | Age | |||
| <57 | −0.31 (−0.87, 0.24) | −0.97 (−3.19, 1.25) | −1.63 (3.33, 0.01) | |
| ≥57 | 0.48 (−0.05, 1.01) | −2.39 (−4.67, −0.11) | −0.57 (2.41, 1.28) | |
| p for interaction | 0.545 | 0.089 | 0.252 | |
| Sex | ||||
| Male | 0.21 (−0.36, 0.79) | −2.69 (−4.92, −0.46) | −0.85 (−2.72, 1.02) | |
| Female | 0.14 (−0.36, 0.63) | −0.98 (−3.27, 1.32) | −1.04 (−2.75, 0.68) | |
| p for interaction | 0.530 | 0.403 | 0.787 | |
| Body mass index | ||||
| <24 | −0.03 (−0.64, 0.58) | −2.25 (−4.64, 0.15) | −0.66 (−2.52, 1.20) | |
| ≥24 | 0.29 (−0.23, 0.82) | −1.33 (−3.48, 0.83) | −1.35 (−2.99, 0.29) | |
| p for interaction | 0.943 | 0.993 | 0.434 | |
| Exercise | ||||
| Yes | 0.19 (−0.39, 0.76) | −1.68 (−4.35, 0.99) | −0.11 (−2.01, 1.79) | |
| No | 0.19 (−0.34, 0.72) | −1.75 (−3.83, 0.34) | −1.76 (−3.49, −0.04) | |
| p for interaction | 0.653 | 0.796 | 0.171 | |
| Education | ||||
| <High school | 0.59 (−0.19, 0.14) | −4.26 (−7.27, −1.26) | −0.87 (−3.73, 1.99) | |
| ≥High school | 0.03 (−0.42, 0.49) | −0.77 (−2.62, 1.09) | −0.82 (−2.22, 0.58) | |
| p for interaction | 0.193 | 0.065 | 0.939 | |
| Smoking | ||||
| Current smoker | 0.31 (−0.76, 1.39) | −2.53 (−6.95, 1.92) | −0.15 (−3.23, 2.93) | |
| Former smoker | 0.00 (−0.82, 0.82) | −3.61 (−6.93, −0.29) | −0.31 (−3.27, 2.65) | |
| Non-smoker | 0.27 (−0.23, 0.77) | −1.43 (−3.45, 0.58) | −1.57 (−3.16, 0.02) | |
| p for interaction | 0.935 | 0.815 | 0.412 | |
| Age | ||||
| <57 | −0.27 (−0.80, 0.26) | 0.18 (−1.99, 2.35) | −0.21 (−1.97, 1.54) | |
| ≥57 | 0.33 (−0.25, 0.90) | −3.42 (−6.12, −0.72) | −1.89 (−4.03, 0.25) | |
| p for interaction | 0.870 | 0.007 | 0.273 | |
| Sex | ||||
| Male | 0.18 (−0.50, 0.86) | −2.64 (−5.20, −0.01) | −0.64 (−2.69, 1.41) | |
| Female | 0.05 (−0.51, 0.61) | −1.07 (−3.68, 1.53) | −1.41 (−3.34, 0.53) | |
| P for interaction | 0.564 | 0.482 | 0.581 | |
| Body mass index | ||||
| <24 | −0.08 (−0.73, 0.58) | −1.34 (−3.94, 1.26) | −1.28 (−3.29, 0.72) | |
| ≥24 | 0.23 (−0.38, 0.84) | −2.49 (−4.99, 0.01) | −1.11 (−3.02, 0.80) | |
| p for interaction | 0.902 | 0.276 | 0.853 | |
| Exercise | ||||
| Yes | 0.04 (−0.57, 0.65) | −1.95 (−4.87, 0.98) | 0.24 (−1.79, 0.23) | |
| No | 0.19 (−0.40, 0.79) | −1.57 (−3.89, 0.76) | −2.03 (−3.95, −0.10) | |
| p for interaction | 0.976 | 0.971 | 0.107 | |
| Education | ||||
| <High school | 0.63 (−0.33, 1.59) | −5.99 (−9.66, −2.32) | −3.03 (−5.98, −0.09) | |
| ≥High school | −0.03 (−0.54, 0.47) | −0.23 (−2.29, 0.18) | −0.03 (−1.59, 0.15) | |
| p for interaction | 0.201 | 0.008 | 0.092 | |
| Smoking | ||||
| Current smoker | −0.01 (−1.25, 1.14) | −1.87 (−6.78, 3.04) | −2.51 (−5.89, 0.87) | |
| Former smoker | 0.16 (−0.65, 0.97) | −3.51 (−7.48, 0.46) | −0.45 (−3.54, 2.65) | |
| Non-smoker | 0.21 (−3.00, 0.72) | −1.60 (−3.88, 0.68) | −1.02 (−2.81, 0.78) | |
| p for interaction | 0.709 | 0.839 | 0.660 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, D.K.; Leem, J.H.; Kim, H.C. Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary Diseases in Adults and Effect Modification by Demographic and Lifestyle Factors. Int. J. Environ. Res. Public Health 2018, 15, 363. https://doi.org/10.3390/ijerph15020363
Lamichhane DK, Leem JH, Kim HC. Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary Diseases in Adults and Effect Modification by Demographic and Lifestyle Factors. International Journal of Environmental Research and Public Health. 2018; 15(2):363. https://doi.org/10.3390/ijerph15020363
Chicago/Turabian StyleLamichhane, Dirga Kumar, Jong Han Leem, and Hwan Cheol Kim. 2018. "Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary Diseases in Adults and Effect Modification by Demographic and Lifestyle Factors" International Journal of Environmental Research and Public Health 15, no. 2: 363. https://doi.org/10.3390/ijerph15020363
APA StyleLamichhane, D. K., Leem, J. H., & Kim, H. C. (2018). Associations between Ambient Particulate Matter and Nitrogen Dioxide and Chronic Obstructive Pulmonary Diseases in Adults and Effect Modification by Demographic and Lifestyle Factors. International Journal of Environmental Research and Public Health, 15(2), 363. https://doi.org/10.3390/ijerph15020363






