Epigallocatechin-3 Gallate Inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLA-B and Reduces CD8 MKG2D Lymphocytes of Alopecia Areata Patients
Abstract
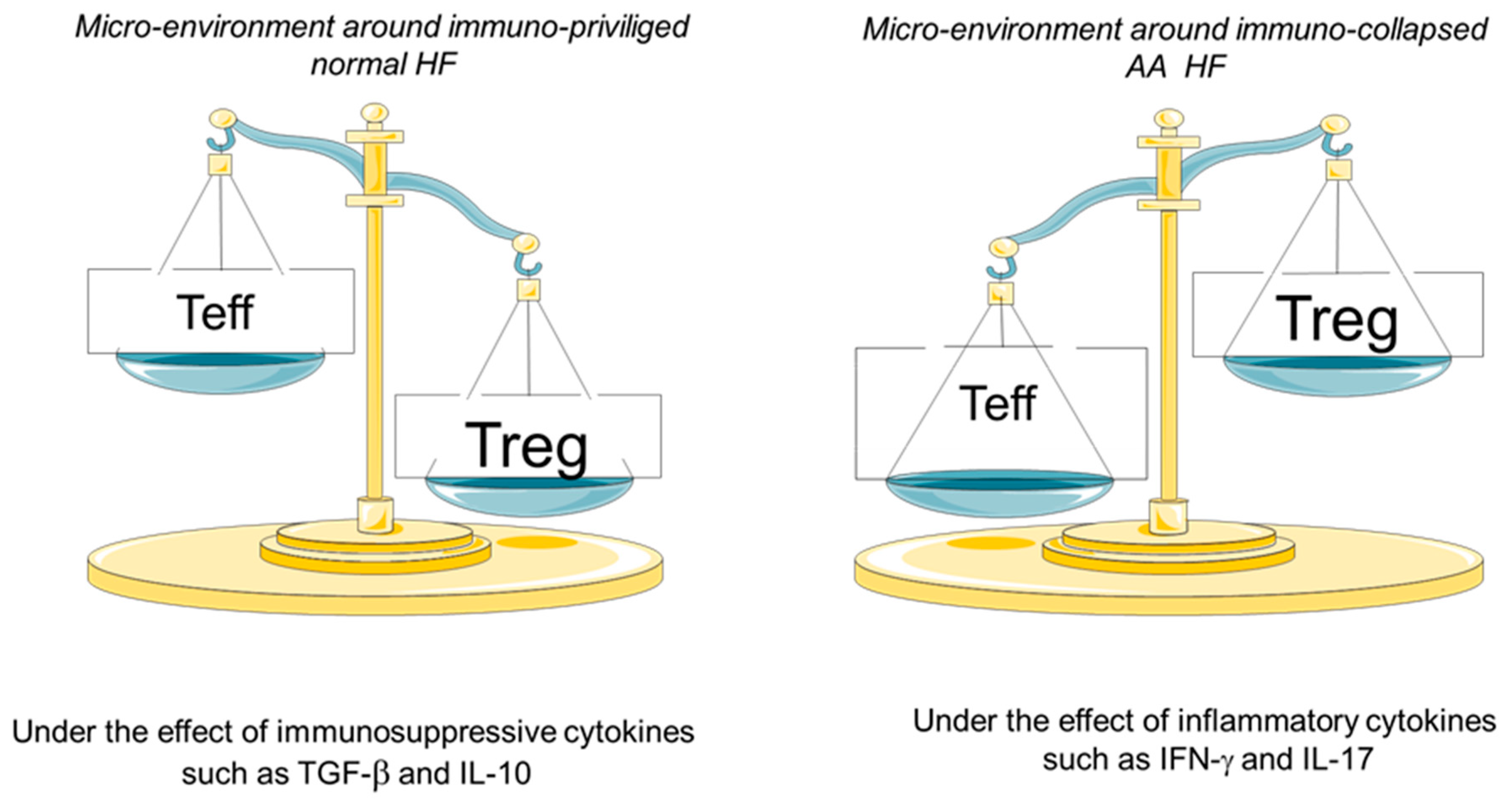
1. Introduction
2. Material and Methods
2.1. Blood Samples
2.2. Cell Lines
2.3. Treatment with EGCG
2.4. Peripheral Blood Mononuclear Cell Separation and FACS Analysis
2.5. Western Blotting
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. RNA Extraction and cDNA Synthesis
2.8. Q-PCR Analysis of Gene Expression
2.9. Statistical Analysis
3. Results
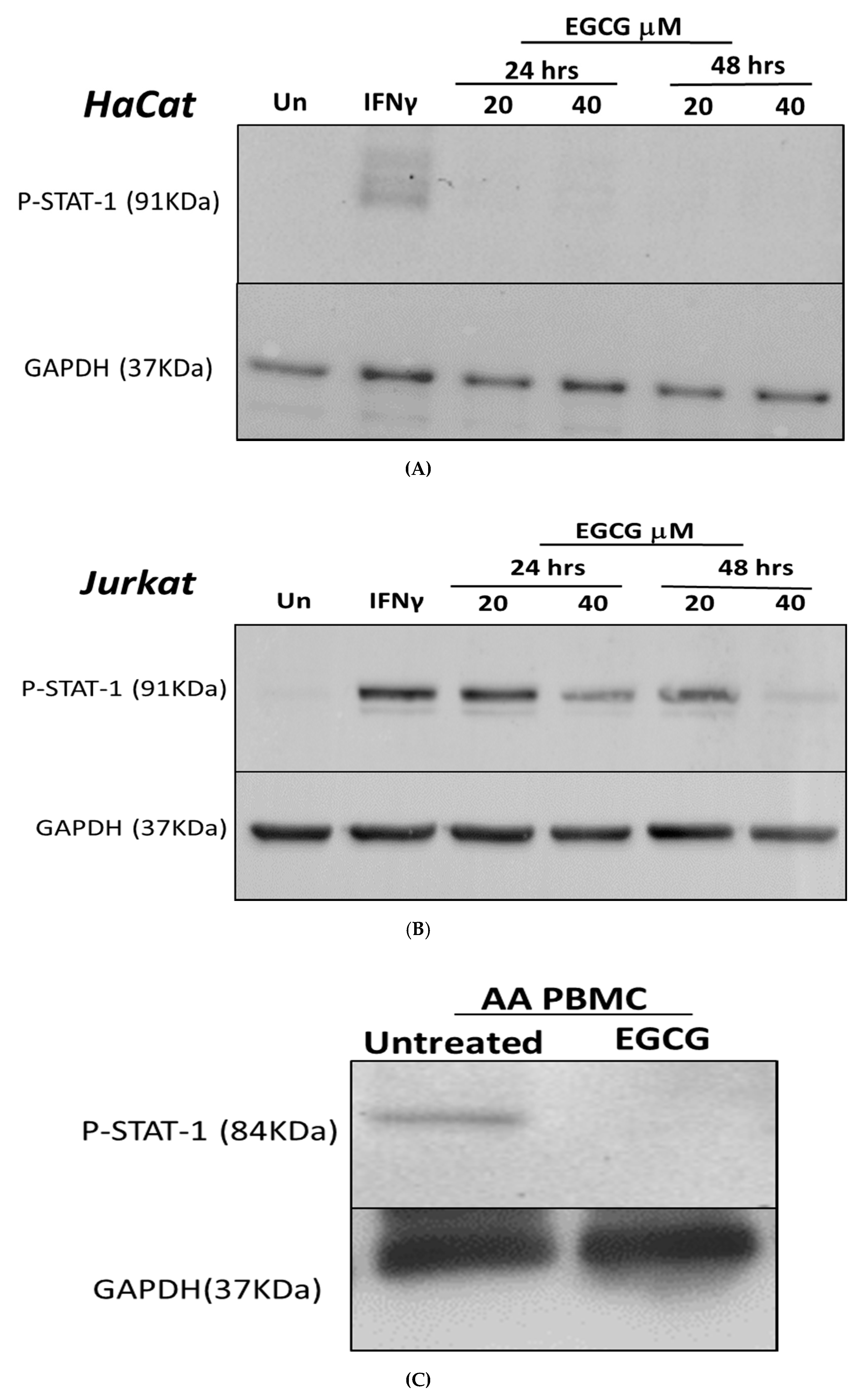
3.1. Inhibition of IFN-γ Signalling Pathway by EGCG
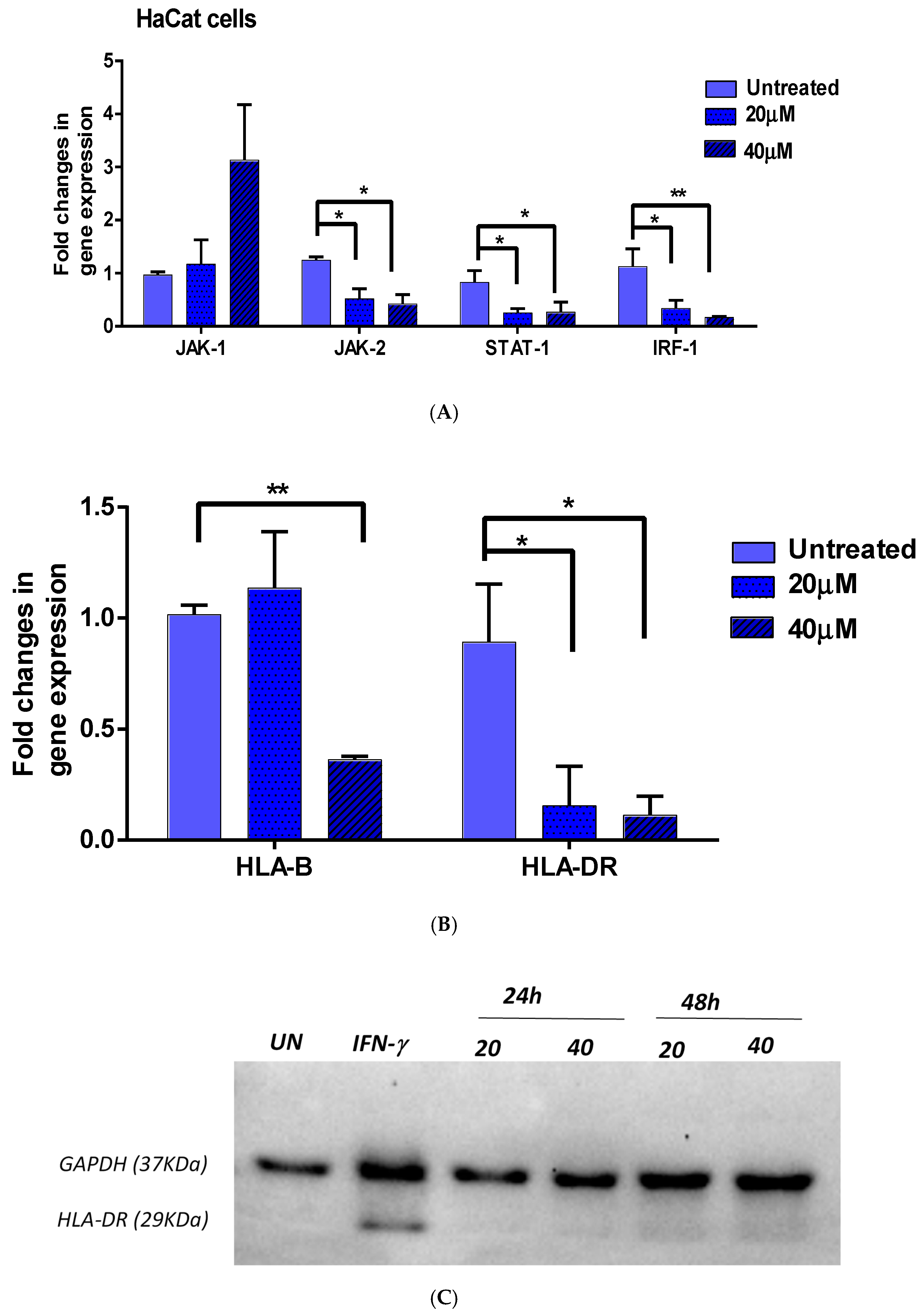
3.2. Effect of EGCG on IFN-γ Downstream Genes
3.2.1. JAK1/JAK2/STAT1 and IRF-1
3.2.2. HLA-DR and HLA-B
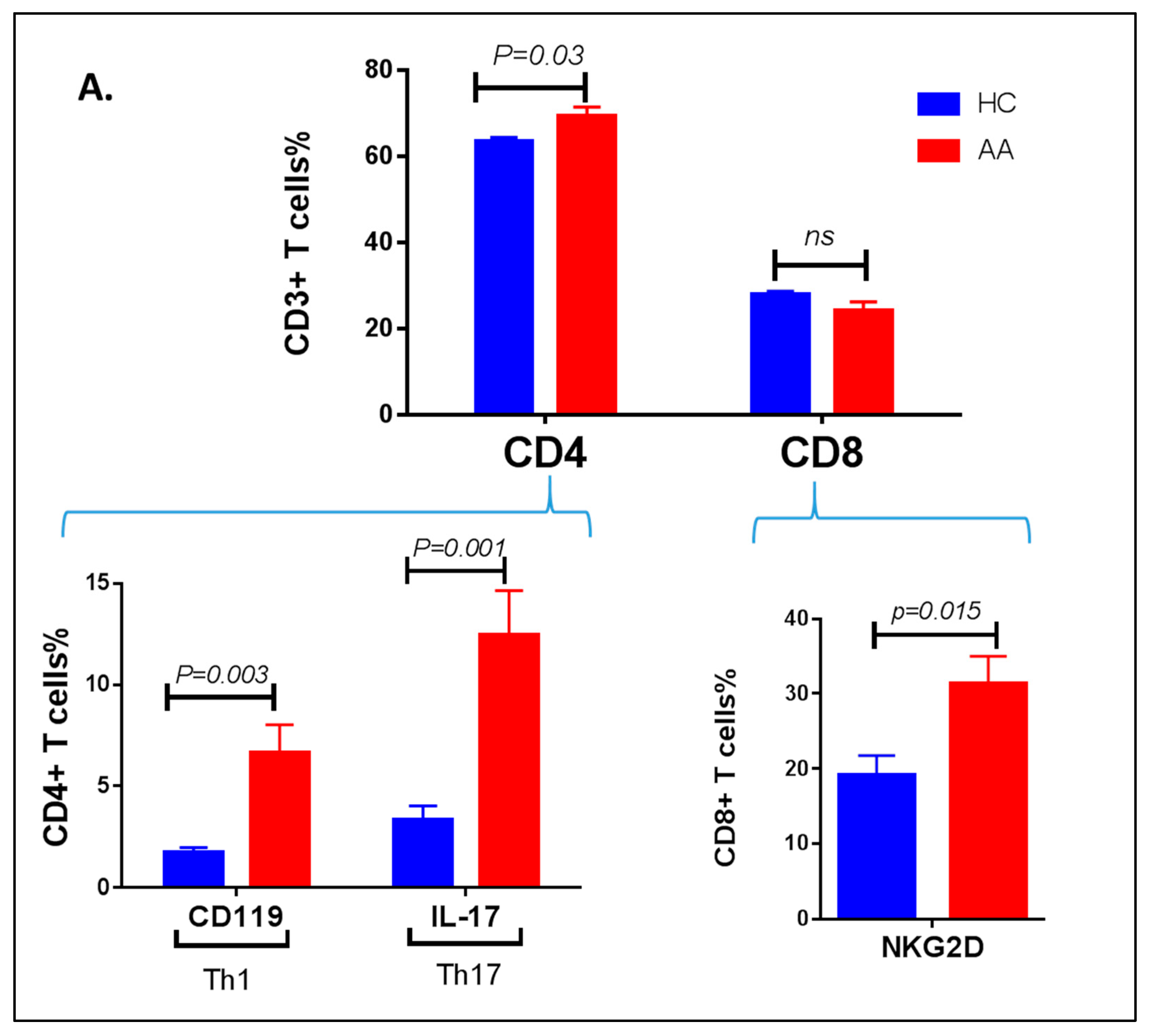
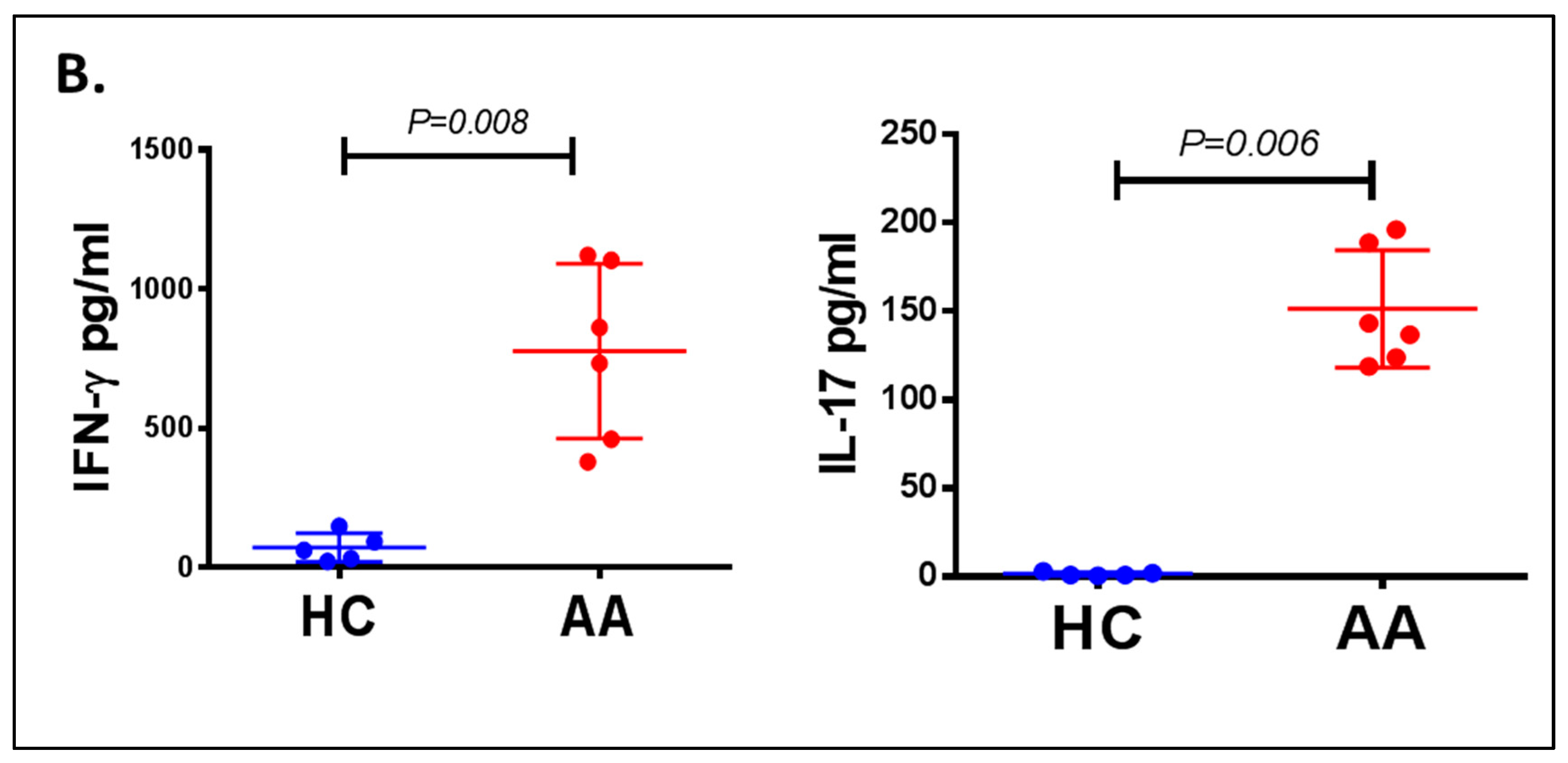
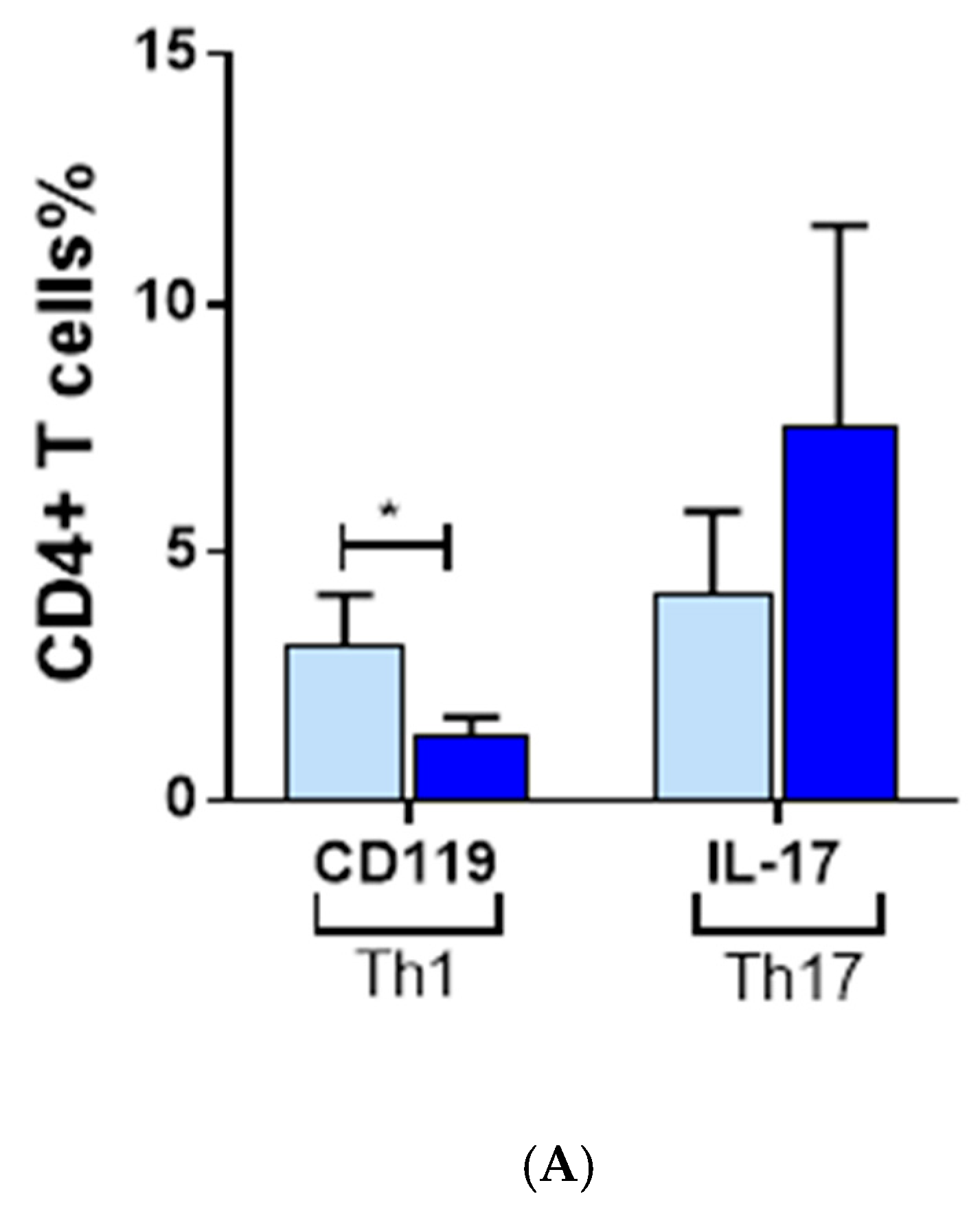
3.3. Inflammatory Cells Th1, NKG2D+ and Th17 and Their Cytokines in AA Patients
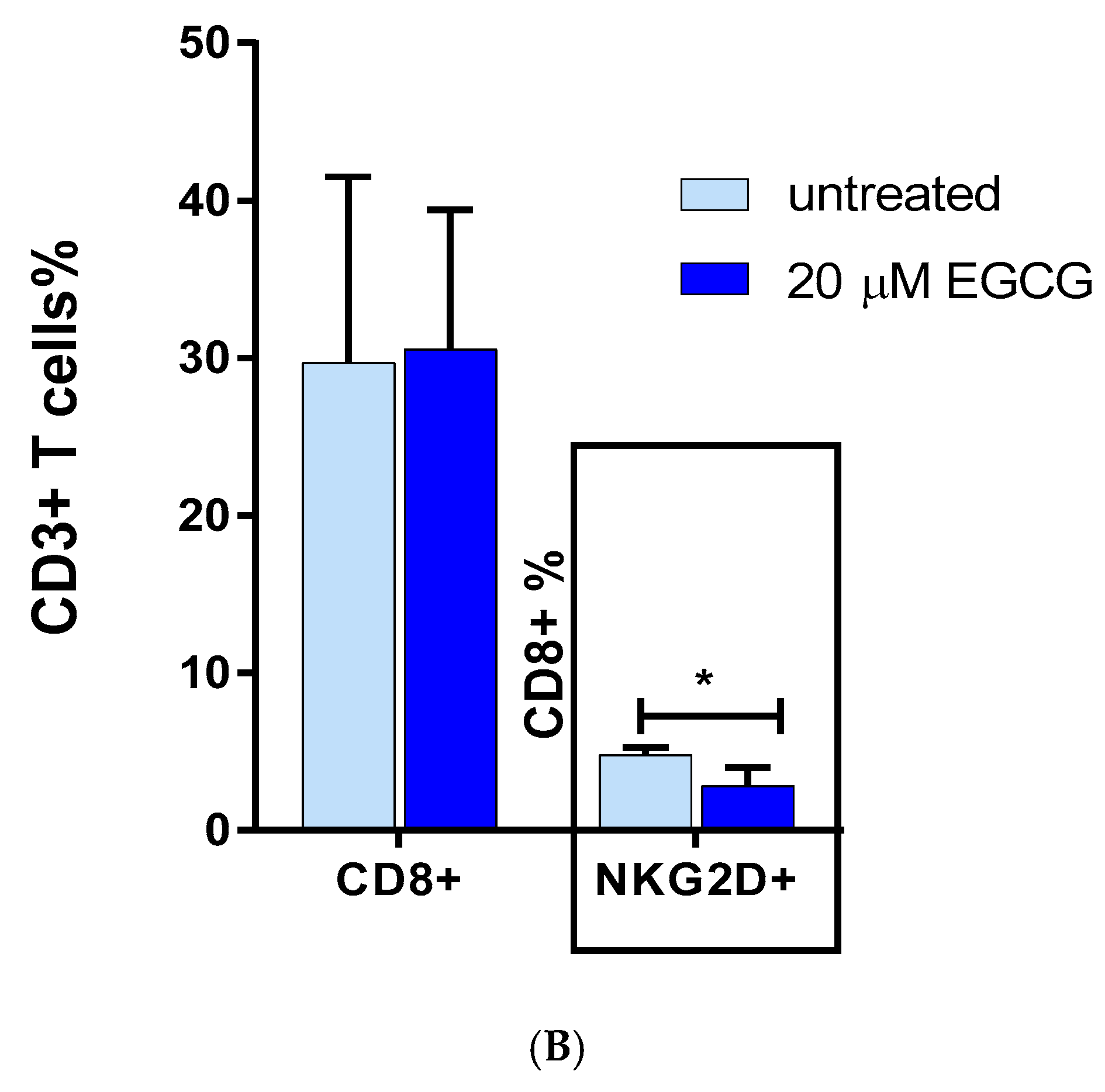
3.4. The Effect of EGCG on CD8 NKG2D Lymphocytes
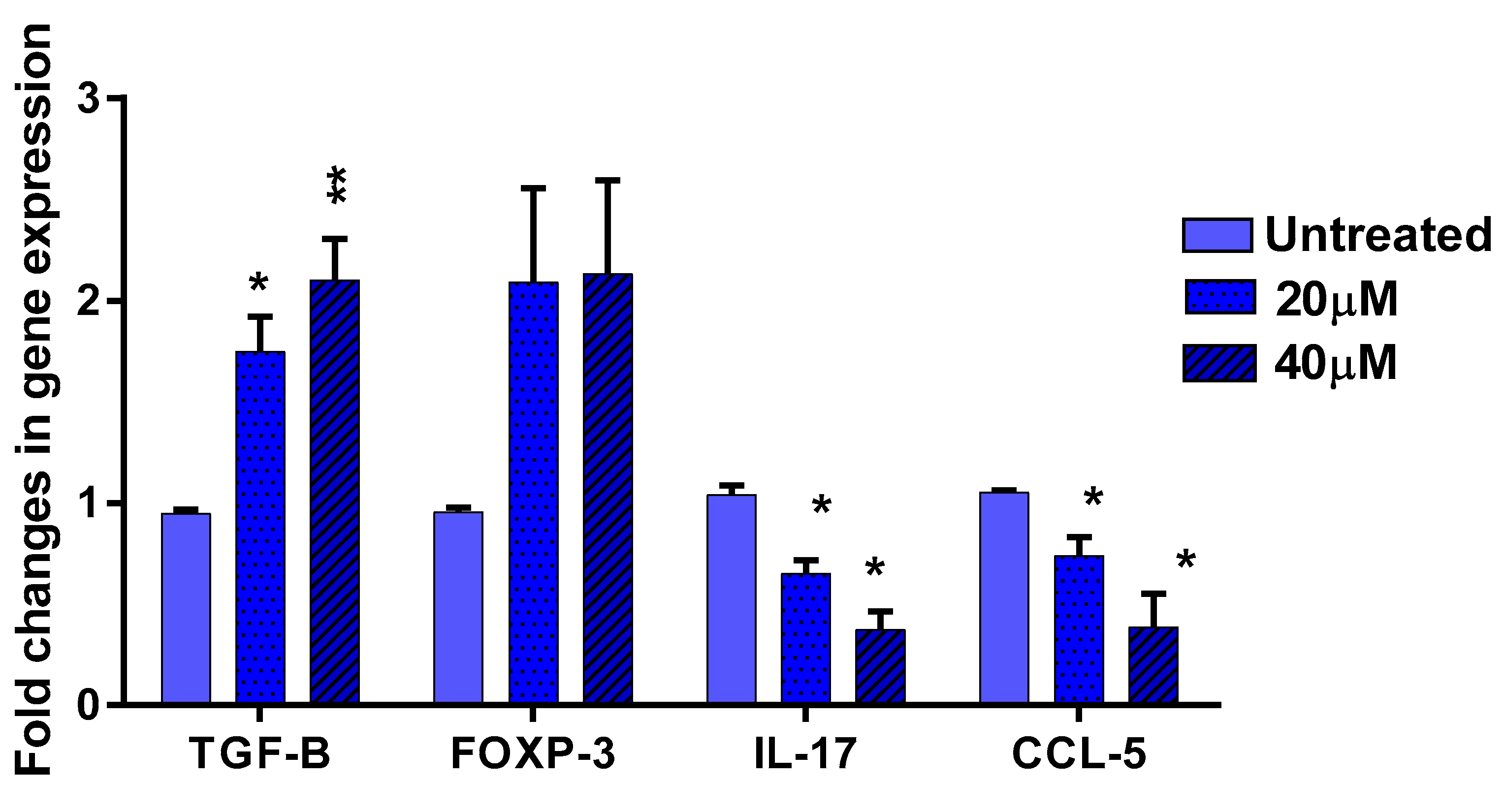
3.5. The Effect of EGCG on Pro- and Anti-Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- McDonagh, A.; Tazi-Ahnini, R. Epidemiology and genetics of alopecia areata. Clin. Exp. Dermatol. 2002, 27, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–117. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, M.; Jackow, C.M.; Dahm, N.; Hordinsky, M.; Reveille, J.D.; Duvic, M. Alopecia areata in families: Association with the HLA locus. J. Investig. Dermatol. Symp. Proc. 1999, 4, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Barahmani, N.; De Andrade, M.; Slusser, J.P.; Zhang, Q.; Duvic, M. Major histocompatibility complex class I chain-related gene A polymorphisms and extended haplotypes are associated with familial alopecia areata. J. Investig. Dermatol. 2006, 126, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Tazi-Ahnini, R.; Cork, M.; Wengraf, D.; Wilson, A.G.; Gawkrodger, D.J.; Birch, M.P.; Messenger, A.G.; McDonagh, A.J. Notch4, a non-HLA gene in the MHC is strongly associated with the most severe form of alopecia areata. Hum. Genet. 2003, 112, 400–403. [Google Scholar]
- Collins, S.; Dominguez, M.; Ilmarinen, T.; Costigan, C.; Irvine, A.D. Dermatological manifestations of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy syndrome. Br. J. Dermatol. 2006, 154, 1088–1093. [Google Scholar] [CrossRef]
- Tazi-Ahnini, R.; Cork, M.J.; Gawkrodger, D.J.; Birch, M.P.; Wengraf, D.; McDonagh, A.J.; Messenger, A.G. Role of the autoimmune regulator (AIRE) gene in alopecia areata: Strong association of a potentially functional AIRE polymorphism with alopecia universalis. Tissue Antigens 2002, 60, 489–495. [Google Scholar] [CrossRef]
- Wengraf, D.A.; McDonagh, A.J.; Lovewell, T.R.; Vasilopoulos, Y.; Macdonald-Hull, S.P.; Cork, M.J.; Messenger, A.G.; Tazi-Ahnini, R. Genetic analysis of autoimmune regulator haplotypes in alopecia areata. Tissue Antigens 2008, 71, 206–212. [Google Scholar] [CrossRef]
- Pforr, J.; Blaumeiser, B.; Becker, T.; Freudenberg-Hua, Y.; Hanneken, S.; Eigelshoven, S.; Cuyt, I.; De Weert, J.; Lambert, J.; Kruse, R.; et al. Investigation of the p.Ser278Arg polymorphism of the autoimmune regulator (AIRE) gene in alopecia areata. Tissue Antigens 2006, 68, 58–61. [Google Scholar] [CrossRef]
- Kemp, E.H.; McDonagh, A.J.; Wengraf, D.A.; Messenger, A.G.; Gawkrodger, D.J.; Cork, M.J.; Tazi-Ahnini, R. The non-synonymous C1858T substitution in the PTPN22 gene is associated with susceptibility to the severe forms of alopecia areata. Hum. Immunol. 2006, 67, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Betz, R.C.; König, K.; Flaquer, A.; Redler, S.; Eigelshoven, S.; Kortüm, A.K.; Hanneken, S.; Hillmer, A.; Tüting, T.; Lambert, J.; et al. The R620W polymorphism in PTPN22 confers general susceptibility for the development of alopecia areata. Br. J. Dermatol. 2008, 158, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Cork, M.J.; Tarlow, J.K.; Clay, F.E.; Crane, A.; Blakemore, A.I.; McDonagh, A.J.; Messenger, A.G.; Duff, G.W. An allele of the interleukin-1 receptor antagonist as a genetic severity factor in alopecia areata. J. Investig. Dermatol. 1995, 104, 15S–16S. [Google Scholar] [CrossRef] [PubMed]
- Tarlow, J.K.; Clay, F.E.; Cork, M.J.; Blakemore, A.I.; McDonagh, A.J.; Messenger, A.G.; Duff, G.W. Severity of alopecia areata is associated with a polymorphism in the interleukin-1 receptor antagonist gene. J. Investig. Dermatol. 1994, 103, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Barahamani, N.; De Andrade, M.; Slusser, J.; Zhang, Q.; Duvic, M. Interleukin-1 receptor antagonist allele 2 and familial alopecia areata. J. Investig. Dermatol. 2002, 118, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Tazi-Ahnini, R.; Cox, A.; McDonagh, A.J.; Nicklin, M.J.; di Giovine, F.S.; Timms, J.M.; Messenger, A.G.; Dimitropoulou, P.; Duff, G.W.; Cork, M.J. Genetic analysis of the interleukin-1 receptor antagonist and its homologue IL-1L1 in alopecia areata: Strong severity association and possible gene interaction. Eur. J. Immunogenet. 2002, 29, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tazi-Ahnini, R.; di Giovine, F.S.; McDonagh, A.J.; Messenger, A.G.; Amadou, C.; Cox, A.; Duff, G.W.; Cork, M.J. Structure and polymorphism of the human gene for the interferon-induced p78 protein (MX1): Evidence of association with alopecia areata in the Down syndrome region. Hum. Genet. 2000, 106, 639–645. [Google Scholar]
- Pan, F.; Yu, H.; Dang, E.V. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science 2009, 325, 1142–1146. [Google Scholar] [CrossRef]
- Akar, A.; Arca, E.; Erbil, H.; Akay, C.; Sayal, A.; Gür, A.R. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J. Dermatol. Sci. 2002, 29, 85–90. [Google Scholar] [CrossRef]
- Karasawa, R.; Ozaki, S.; Nishioka, K.; Kato, T. Autoantibodies to peroxiredoxin I and IV in patients with systemic autoimmune diseases. Microbiol. Immunol. 2005, 49, 57–65. [Google Scholar] [CrossRef]
- Pielberg, G.R.; Golovko, A.; Sundström, E.; Curik, I.; Lennartsson, J.; Seltenhammer, M.H.; Druml, T.; Binns, M.; Fitzsimmons, C.; Lindgren, G.; et al. A cis-acting regulatory mutation causes premature hair graying and susceptibility to melanoma in the horse. Nat. Genet. 2008, 40, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Singh, L.K.; Boucher, W.; Pang, X.; Letourneau, R.; Webster, E.; Chrousos, G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 1998, 139, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Liotiri, S.; Bodó, E.; Hagen, E.; Bíró, T.; Arck, P.C.; Paus, R. Probing the effects of stress mediators on the human hair follicle: Substance P holds central position. Am. J. Pathol. 2007, 171, 1872–1886. [Google Scholar] [CrossRef] [PubMed]
- Tal, M.; Liberman, R. Local injection of nerve growth factor (NGF) triggers degranulation of mast cells in rat paw. Neurosci. Lett. 1997, 221, 129–132. [Google Scholar] [CrossRef]
- Ansel, J.; Brown, J.; Payan, D.; Brown, M.A. Substance P selectively activates TNF-α gene expression in murine mast cells. J. Immunol. 1993, 150, 4478–4485. [Google Scholar] [PubMed]
- Westgate, G.E.; Craggs, R.I.; Gibson, W.T. Immune privilege in hair-growth. J. Investig. Dermatol. 1991, 97, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Christoph, T.; Muller-Rover, S.; Audring, H.; Tobin, D.J.; Hermes, B.; Cotsarelis, G.; Rückert, R.; Paus, R. The human hair follicle immune system: Cellular composition and immune privilege. Br. J. Dermatol. 2000, 142, 862–873. [Google Scholar] [CrossRef]
- Moresi, J.M.; Horn, T.D. Distribution of langerhans cells in human hair follicle. J. Cutan. Pathol. 1997, 24, 636–640. [Google Scholar] [CrossRef]
- Paus, R.; Cotsarelis, G.; Epstein, F.H. The biology of hair follicles. New Eng. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef]
- Gruschwitz, M.; Müller, P.U.; Sepp, N.; Hofer, E.; Fontana, A.; Wick, G. Transcription and expression of transforming growth factor type beta in the skin of progressive systemic sclerosis: A mediator of fibrosis? J. Investig. Dermatol. 1990, 94, 197–203. [Google Scholar] [CrossRef]
- Paus, R.; Slominski, A.; Czarnetzki, B.M. Is alopecia-areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class-I expression in the anagen hair bulb. Yale J. Biol. Med. 1993, 66, 541–554. [Google Scholar] [PubMed]
- Ito, T.; Ito, N.; Bettermann, A.; Tokura, Y.; Takigawa, M.; Paus, R. Collapse and restoration of MHC class-I-dependent immune privilege: Exploiting the human hair follicle as a model. Am. J. Pathol. 2004, 164, 623–634. [Google Scholar] [CrossRef]
- Messenger, A.G.; Bleehen, S.S. Expression of HLA-DR by anagen hair follicles in alopecia areata. J. Investig. Dermatol. 1985, 85, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Mc Donagh, A.J.; Snowden, J.A.; Stierle, C.; Elliott, K.; Messenger, A.G. HLA and ICAM-1 expression in alopecia areata in vivo and in vitro: The role of cytokines. Br. J. Dermatol. 1993, 129, 250–256. [Google Scholar] [CrossRef]
- Emilo, L.K.; Vera, H.P.; John, S.G. HLA-DR expression by hair follicle keratinocytes in alopecia areata: Evidence that it is secondary to the lymphoid infiltration. J. Investig. Dermatol. 1988, 90, 193. [Google Scholar]
- Taylor, A.W.; Streilein, J.W.; Cousins, S.W. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous-humor. Curr. Eye Res. 1992, 11, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Ito, N.; Takigawa, M.; Ito, T. The hair follicle and immune privilege. J. Investig. Dermatol. Symp. Proc. 2003, 8, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Paus, R.; Kalish, R.S. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J. Clin. Investig. 2007, 117, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Ungar, B.; Noda, S.; Shroff, A.; Mansouri, Y.; Fuentes-Duculan, J.; Czernik, A.; Zheng, X.; Estrada, Y.D.; Xu, H.; et al. Alopecia areata profiling shows Th1, Th2, and IL-23 cytokine activation without parallel Th17/Th22 skewing. J. Allergy. Clin. Immunol. 2015, 136, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Todes-Taylor, N.; Turner, R.; Wood, G.S. T cell subpopulations in alopecia areata. J. Am. Acad. Dermatol. 1984, 11, 216–223. [Google Scholar] [CrossRef]
- Perret, C.; Wiesner-Menzel, L.; Happle, R. Immunohistochemical analysis of T-cell subsets in the peribulbar and intrabulbar infiltrates of alopecia areata. Acta Derm. Venereol 1984, 64, 26–30. [Google Scholar] [PubMed]
- Zhu, J.F.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Romagnani, P.; Annunziato, F.; Piccinni, M.P.; Maggi, E.; Romagnani, S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur. Cytokine Netw. 2000, 11, 510–511. [Google Scholar] [PubMed]
- Noble, A.; Staynov, D.Z.; Kemeny, D.M. Generation of rat Th2-like cells in-vitro is interleukin-4-dependent and inhibited by interferon-gamma. Immunology 1993, 79, 562–567. [Google Scholar] [PubMed]
- Ghoreschi, K.; Laurence, A.; Yang, X.P.; Hirahara, K.; O’Shea, J.J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011, 32, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Levings, M.K.; Bacchetta, R.; Schulz, E.; Roncarolo, M.G. The role of IL-10 and TGF-beta in the differentiation and effector function of t regulatory cells. Int. Arch. Allergy Immunol. 2002, 129, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.R.M.; Legrand, N.; Papiernik, M.; Freitas, A.A. Homeostasis of peripheral CD4(+) T cells: IL-2R alpha and il-2 shape a population of regulatory cells that controls CD4(+) T cell numbers. J. Immunol. 2002, 169, 4850–4860. [Google Scholar] [CrossRef]
- Kuwano, Y.; Fujimoto, M.; Watanabe, R.; Ishiura, N.; Nakashima, H.; Ohno, Y.; Yano, S.; Yazawa, N.; Okochi, H.; Tamaki, K. Serum chemokine profiles in patients with alopecia areata. Br. J. Dermatol. 2007, 157, 466–473. [Google Scholar] [CrossRef]
- Teraki, Y.; Imanishi, K.; Shiohara, T. Cytokines in alopecia areata: Contrasting cytokine profiles in localized form and extensive form (alopecia universalis). Acta Dermatovenereol. Venereol. 1996, 76, 421–423. [Google Scholar]
- Tawfic, S.O.; Abdel-halim, M.R.E.; Shaker, O.G. Assessment of interleukin-17 in alopecia areata: A case–control pilot study. J. Egyp. Women’s Dermatol. Soc. 2014, 11, 20–23. [Google Scholar] [CrossRef]
- Tojo, G.; Fujimura, T.; Kawano, M.; Ogasawara, K.; Kambayashi, Y.; Furudate, S.; Mizuashi, M.; Aiba, S. Comparison of interleukin-17-producing cells in different clinical types of alopecia areata. Dermatology 2013, 227, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, A.; Oiso, N.; Nakano, M.; Itoi, S.; Kawada, A.; Katayama, I. Alopecia areata: Infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology 2013, 226, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Lew, B.I.; Cho, H.R.; Haw, S.; Kim, H.J.; Chung, J.H.; Sim, W.Y. Association between IL17a/IL17RA gene polymorphisms and susceptibility to alopecia areata in the korean population. Ann. Dermatol. 2012, 24, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, N.; Akcali, C.; Pehlivan, S.; Kirtak, N.; Inaloz, S. Investigation of interleukin-12, interleukin-17 and interleukin-23 receptor gene polymorphisms in alopecia areata. J. Int. Med. Res. 2015, 43, 526–534. [Google Scholar] [CrossRef] [PubMed]
- El-Morsy, E.H.; Eid, A.A.; Ghoneim, H.; Al-Tameemi, K.A. Serum level of interleukin-17a in patients with alopecia areata and its relationship to age. Int. J. Dermatol. 2016, 55, 869–874. [Google Scholar] [CrossRef]
- Ito, T.; Ito, N.; Saatoff, M. Maintenance of hair follicle immune privilege is linked to prevention of nk cell attack. J. Investig. Dermatol. 2008, 128, 1196–1206. [Google Scholar] [CrossRef]
- Xing, I.Z.; Dai, Z.P.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic t lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Horvath, C.M. The JAK-STAT pathway stimulated by interferon gamma. Sci. STKE 2004, 23, tr8. [Google Scholar]
- Darnell, J.E.; Kerr, I.M.; Stark, G.R. JAK-STAT pathways and transcriptional activation in response to ifns and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef]
- White, I.C.; Wright, K.I.; Felix, N.J.; Ruffner, H.; Ruffner, H.; Reis, L.F.; Pine, R.; Ting, J.P. Regulation of LMP2 and TAP1 genes by IRF-1 explains the paucity of CD8 + t cells in Irf-1(-/-) mice. Immunity 1996, 5, 365–376. [Google Scholar] [CrossRef]
- Lechleitner, S.; Gille, J.; Johnson, D.R.; Petzelbauer, P. Interferon enhances tumor necrosis factor-induced vascular cell adhesion molecule 1 (CD106) expression in human endothelial cells by an interferon-related factor 1-dependent pathway. J. Exp. Med. 1998, 187, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kimura, T.; Kitagawa, M.; Pfeffer, K.; Kawakami, T.; Watanabe, N.; Kündig, T.M.; Amakawa, R.; Kishihara, K.; Wakeham, A.; et al. Targeted disruption of irf-1 or irf-2 results in abnormal type i ifn gene induction and aberrant lymphocyte development. Cell 1993, 75, 83–97. [Google Scholar] [CrossRef]
- Fragale, A.; Gabriele, I.; Stellacci, E.; Borghi, P.; Perrotti, E.; Ilari, R.; Lanciotti, A.; Remoli, A.L.; Venditti, M.; Belardelli, F.; et al. IFN regulatory factor-1 negatively regulates CD4 + CD25 + regulatory t cell differentiation by repressing foxp3 expression. J. Immunol. 2008, 181, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.Y.; Craiglow, B.G.; Dai, F.; King, B.A. Tofacitinib for the treatment of severe alopecia areata and variants: A study of 90 patients. J. Am. Acad. Dermatol. 2017, 76, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Dai, Z.; Xing, I.; Cerise, J.E.; Ramot, Y.; Berkun, Y.; Sanchez, G.A.; Goldbach-Mansky, R.; Christiano, A.M.; Clynes, R.; et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. Ebiomedicine 2015, 2, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Shreberk-Hassidim, R.; Ramot, Y.; Zlotogorski, A. Janus kinase inhibitors in dermatology: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 745–753.e19. [Google Scholar] [CrossRef]
- Yang, C.S.; Maliakal, M.; Meng, X. Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 25–54. [Google Scholar] [CrossRef]
- Cheng, C.W.; Shieh, P.C.; Lin, Y.C.; Chen, Y.J.; Lin, Y.H.; Kuo, D.P.; Liu, J.Y.; Kao, J.; Kao, M.C.; Way, T.D. Indoleamine 2,3-dioxygenase, an immunomodulatory protein, is suppressed by (-)-epigallocatechin-3-gallate via blocking of gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human oral cancer cells. Biochem. Biophys. Res. Commun. 2007, 354, 1004–1009. [Google Scholar]
- Ogawa, K.; Hara, T.; Shimizu, M.; Nagano, J.; Ohno, T.; Hoshi, M.; Ito, H.; Tsurumi, H.; Saito, K.; Seishima, M.; et al. (-)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncol. Lett. 2012, 4, 546–550. [Google Scholar] [CrossRef]
- Pae, M.; Ren, Z.H.; Meydani, M.; Shang, F.; Meydani, S.N.; Wu, D. Epigallocatechin-3-gallate directly suppresses t cell proliferation through impaired il-2 utilization and cell cycle progression. J. Nutr. 2010, 140, 1509–1515. [Google Scholar] [CrossRef]
- Aktas, O.; Prozorovski, T.; Smorodchenko, A.; Savaskan, N.E.; Lauster, R.; Kloetzel, P.M.; Infante-Duarte, C.; Brocke, S.; Zipp, F. Green tea epigallocatechin-3-gallate mediates t cellular nf-kappa b inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004, 173, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Guo, Z.; Ren, Z.; Guo, W.; Meydani, S.N. Green tea EGCG suppresses t cell proliferation through impairment of IL-2/IL-2 receptor signaling. Free Rad. Biol. Med. 2009, 47, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Matsui, M.S.; Elmets, C.A.; Mukhtar, H. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea reduces uvb-induced inflammatory responses and infiltration of leukocytes in human skin. Photochem. Photobiol. 1999, 69, 148–153. [Google Scholar] [CrossRef]
- Chow, H.H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon e in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Scalia, S.; Trotta, V.; Bianchi, A. In vivo human skin penetration of (-)-epigallocatechin-3-gallate from topical formulations. Acta Pharm. 2004, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.; Armstrong, A.W. JAK inhibitors: Treatment efficacy and safety profile in patients with psoriasis. J. Immunol. Res 2014, 2014, 283617. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. The JAK-STAT signaling pathway: Input and output integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef]
- Tefferi, A.; Pardanani, A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin. Proc. 2011, 86, 1188–1191. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L.; et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef]
- Tedeschi, E.; Suzuki, H.; Menegazzi, M. Antiinflammatory action of egcg, the main component of green tea, through stat-1 inhibition. Ann. N. Y. Acad. Sci. 2002, 973, 435–437. [Google Scholar] [CrossRef]
- Watson, J.I.; Ansari, S.; Cameron, H.; Wang, A.; Akhtar, M.; McKay, D.M. Green tea polyphenol (-)-epigallocatechin gallate blocks epithelial barrier dysfunction provoked by IFN-gamma but not by IL-4. Am. J. Physiol. Gast Liver Physiol. 2004, 287, g954. [Google Scholar] [CrossRef] [PubMed]
- Zoller, M.; Mcelwee, K.J.; Vitacolonna, M.; Hoffmann, R. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp. Dermatol. 2004, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Tembhre, M.K.; Sharma, V.K. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br. J. Dermatol. 2013, 169, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ercan, A.R.C.A.; Musabak, U.; Ahmet, A.K.A.R.; Erbil, A.H.; Tatan, H.B. Interferon-gamma in alopecia areata. Eur. J. Dermatol. 2004, 14, 33–36. [Google Scholar]
- Shuai, K.; Stark, G.R.; Kerr, I.M.; Darnell, J.E. A single phosphotyrosine residue of stat91 required for gene activation by interferon-γ. Science 1993, 261, 1744–1746. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Plenge, R. JAK and STAT signalling molecules in immunoregulation and immune-mediated disease. Immunity 2012, 36, 542–550. [Google Scholar] [CrossRef]
- Chang, C.H.; Hammer, J.; Loh, J.E.; Fodor, W.L.; Flavell, R.A. The activation of major histocompatibility complex class I genes by Interferon Regulatory Factor-1 (IRF-1). Immunogenetics 1992, 35, 378–384. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Massa, P.T. Interferon regulatory factor-1 is required for interferon-γ-induced MHC class I genes in astrocytes. J. Neuroimmunol. 2002, 122, 74–84. [Google Scholar] [CrossRef]
- Girdlestone, J.; Isamat, M.; Gewert, D.; Milstein, C. Transcriptional regulation of HLA-A and -B: Differential binding of members of the REL and IRF families of transcription factors. Proc. Natl. Acad. Sci. USA 1993, 90, 11568. [Google Scholar] [CrossRef]
- Lin, A.; Guo, X.; Inman, R.D.; Sivak, J.M. Ocular inflammation in HLA-B27 transgenic mice reveals a potential role for MHC class I in corneal immune privilege. Mol. Vis. 2015, 21, 131. [Google Scholar]
- Harrist, T.J.; Ruiter, D.J.; Mihm, M.C., Jr.; Bhan, A.K. Distribution of major histocompatibility antigens in normal skin. Br. J. Dermatol. 1983, 109, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Winiarska, M.; Olszewska, M.; Rudnicka, L. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin disease. Postep. Dermatol. Alergol. 2016, 33, 247–252. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, F.N.; McDonagh, A.J.G.; Almaghrabi, S.; Bakri, Y.; Messenger, A.G.; Tazi-Ahnini, R. Epigallocatechin-3 Gallate Inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLA-B and Reduces CD8 MKG2D Lymphocytes of Alopecia Areata Patients. Int. J. Environ. Res. Public Health 2018, 15, 2882. https://doi.org/10.3390/ijerph15122882
Hamed FN, McDonagh AJG, Almaghrabi S, Bakri Y, Messenger AG, Tazi-Ahnini R. Epigallocatechin-3 Gallate Inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLA-B and Reduces CD8 MKG2D Lymphocytes of Alopecia Areata Patients. International Journal of Environmental Research and Public Health. 2018; 15(12):2882. https://doi.org/10.3390/ijerph15122882
Chicago/Turabian StyleHamed, Fatma N., Andrew J. G. McDonagh, Sarah Almaghrabi, Youssef Bakri, Andrew G. Messenger, and Rachid Tazi-Ahnini. 2018. "Epigallocatechin-3 Gallate Inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLA-B and Reduces CD8 MKG2D Lymphocytes of Alopecia Areata Patients" International Journal of Environmental Research and Public Health 15, no. 12: 2882. https://doi.org/10.3390/ijerph15122882
APA StyleHamed, F. N., McDonagh, A. J. G., Almaghrabi, S., Bakri, Y., Messenger, A. G., & Tazi-Ahnini, R. (2018). Epigallocatechin-3 Gallate Inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLA-B and Reduces CD8 MKG2D Lymphocytes of Alopecia Areata Patients. International Journal of Environmental Research and Public Health, 15(12), 2882. https://doi.org/10.3390/ijerph15122882





