Disability Adjusted Life Years (DALYs) in Terms of Years of Life Lost (YLL) Due to Premature Adult Mortalities and Postneonatal Infant Mortalities Attributed to PM2.5 and PM10 Exposures in Kuwait
Abstract
1. Introduction
2. Methods
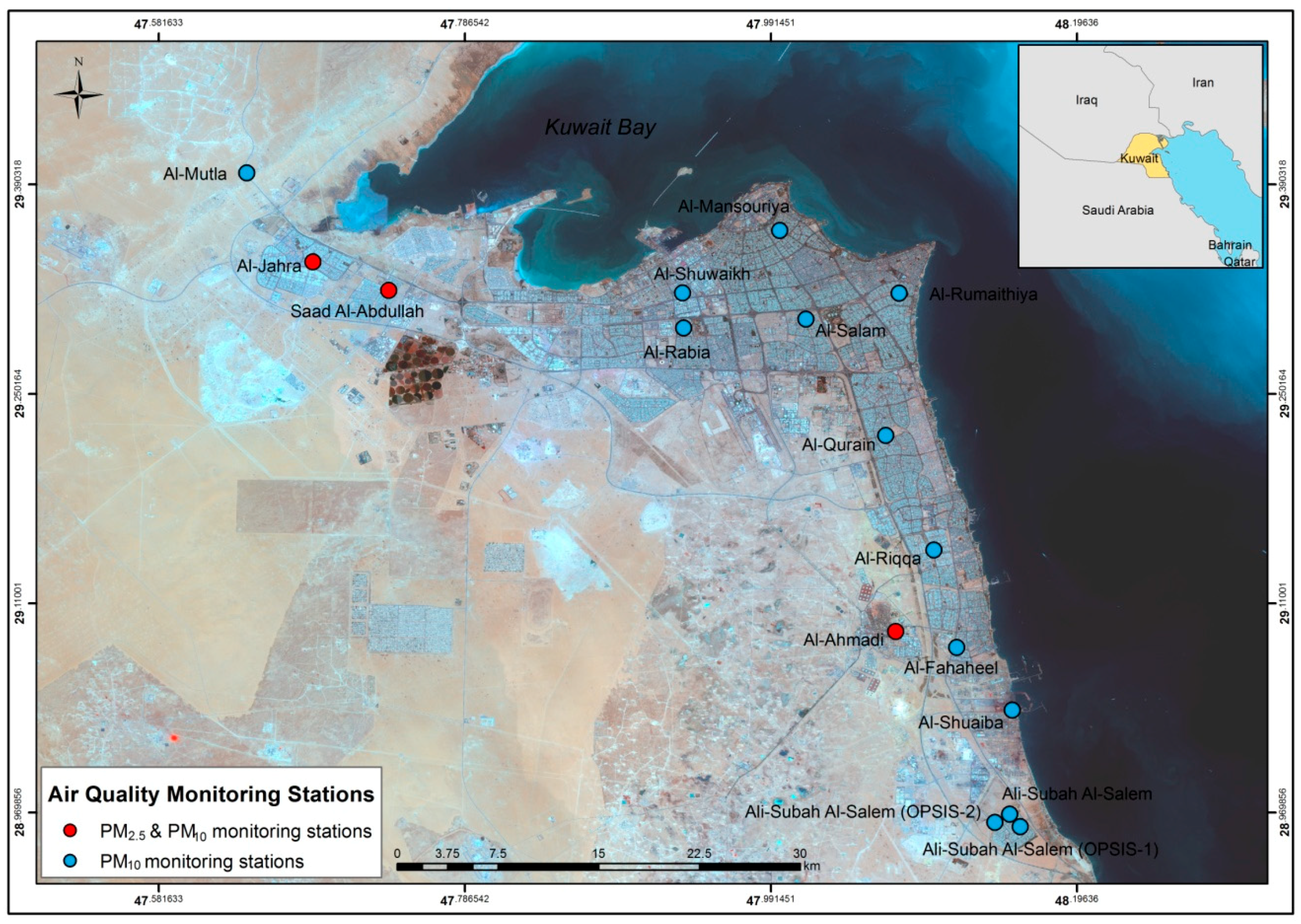
2.1. Air Quality Data
2.2. Life Tables and DALYs
2.3. AirQ+ Model
3. Results
3.1. Baseline Health Data and PM Data
3.2. Premature Adult Mortality
3.3. Postneonatal Infant Mortality
3.4. Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, R.; Kang, S.; Anderson, H.; Mills, I.; Walton, H. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Crouse, D.L.; Peters, P.A.; Hystad, P.; Brook, J.R.; van Donkelaar, A.; Martin, R.V.; Villeneuve, P.J.; Jerrett, M.; Goldberg, M.S.; Pope, C.A., III; et al. Ambient PM2.5, O3, and NO2 exposures and associations with mortality over 16 years of follow-up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Health Perspect. 2015, 123, 1180. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, Y.; Tang, X.; Zhu, J.; Zhu, T. Estimating adult mortality attributable to PM2.5 exposure in China with assimilated PM2.5 concentrations based on a ground monitoring network. Sci. Total Environ. 2016, 568, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization (WHO). Air Quality Guidelines: Global Update 2005; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Son, J.-Y.; Lee, H.J.; Koutrakis, P.; Bell, M.L. Pregnancy and Lifetime Exposure to Fine Particulate Matter and Infant Mortality in Massachusetts, 2001–2007. Am. J. Epidemiol. 2017, 186, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Kashima, S.; Doi, H. Acute exposure to fine and coarse particulate matter and infant mortality in Tokyo, Japan (2002–2013). Sci. Total Environ. 2016, 551, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cambra, K.; Alonso, E.; Medina, S.; Letertre, A.; Cassadou, S.; Pascal, L.; Frank, F.; Dequijano, E.D.; Valero, N.; Knudsen, L.; et al. Health Impact Assessment of PM10 Air Pollution on Postneonatal Mortality in 31 European Cities. Epidemiology 2006, 17, S106. [Google Scholar] [CrossRef]
- Paldy, A.; Bobvos, J.; Lustigova, M.; Moshammer, H.; Niciu, E.M.; Otorepec, P.; Puklova, V.; Szafraniec, K.; Zagargale, T.; Neuberger, M.; et al. Health impact assessment of PM10 on mortality and morbidity in children in central-eastern european cities. Epidemiology 2006, 17, S131. [Google Scholar] [CrossRef]
- Kaiser, R.; Romieu, I.; Medina, S.; Schwartz, J.; Krzyzanowski, M.; Künzli, N. Air pollution attributable postneonatal infant mortality in US metropolitan areas: A risk assessment study. Environ. Health 2004, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six US cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Apte, J.S.; Marshall, J.D.; Cohen, A.J.; Brauer, M. Addressing global mortality from ambient PM2.5. Environ. Sci. Technol. 2015, 49, 8057–8066. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef]
- Krewski, D.; Jerrett, M.; Burnett, R.T.; Ma, R.; Hughes, E.; Shi, Y.; Turner, M.C.; Pope, C.A., III; Thurston, G.; Calle, E.E.; et al. Extended Follow-Up and Spatial Analysis of the American Cancer Society Study Linking Particulate Air Pollution and Mortality; Health Effects Institute: Boston, MA, USA, 2009. [Google Scholar]
- Lepeule, J.; Laden, F.; Dockery, D.; Schwartz, J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ. Health Perspect. 2012, 120, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Hu, J.; Goldberg, D.; Reynolds, P.; Hertz, A.; Bernstein, L.; Kleeman, M.J. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: Results from the California Teachers Study Cohort. Environ. Health Perspect. 2015, 123, 549. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Lipsett, M.; Reynolds, P.; Goldberg, D.; Hertz, A.; Garcia, C.; Henderson, K.D.; Bernstein, L. Long-term exposure to constituents of fine particulate air pollution and mortality: Results from the California Teachers Study. Environ. Health Perspect. 2010, 118, 363. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Murray, C.J.; Ezzati, M.; Flaxman, A.D.; Lim, S.; Lozano, R.; Michaud, C.; Naghavi, M.; Salomon, J.A.; Shibuya, K.; Vos, T.; et al. GBD 2010: Design, definitions, and metrics. Lancet 2012, 380, 2063–2066. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Woodruff, T.J.; Darrow, L.A.; Parker, J.D. Air pollution and postneonatal infant mortality in the United States, 1999–2002. Environ. Health Perspect. 2007, 116, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.J.; Grillo, J.; Schoendorf, K.C. The relationship between selected causes of postneonatal infant mortality and particulate air pollution in the United States. Environ. Health Perspect. 1997, 105, 608. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.-H.; Lee, J.-T.; Kim, H.; Hong, Y.-C.; Lee, B.-E.; Park, H.-S.; Christiani, D.C. Infant susceptibility of mortality to air pollution in Seoul, South Korea. Pediatrics 2003, 111, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Pharoah, P.; Morris, J. Postneonatal mortality. Epidemiol. Rev. 1979, 1, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Geelen, L.M.; Huijbregts, M.A.; den Hollander, H.; Ragas, A.M.; van Jaarsveld, H.A.; de Zwart, D. Confronting environmental pressure, environmental quality and human health impact indicators of priority air emissions. Atmos. Environ. 2009, 43, 1613–1621. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.C.; Chen, R.; Ngo, H.H.; Guo, W. Disability adjusted life year (DALY): A useful tool for quantitative assessment of environmental pollution. Sci. Total Environ. 2015, 511, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Valent, F.; Bertollini, R.; Nemer, L.E.; Barbone, F.; Tamburlini, G. Burden of disease attributable to selected environmental factors and injury among children and adolescents in Europe. Lancet 2004, 363, 2032–2039. [Google Scholar] [CrossRef]
- Murray, C.J.; Acharya, A. Age weights and discounting in health gaps reconsidered. In Summary Measures of Population Health; Book Chapter; CiteSeer: University Park, PA, USA, 2002. [Google Scholar]
- Murray, C.J.; Lopez, A.D. Measuring global health: Motivation and evolution of the Global Burden of Disease Study. Lancet 2017, 390, 1460–1464. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Stubbs, R.W.; Bertozzi-Villa, A.; Morozoff, C.; Callender, C.; Finegold, S.B.; Shirude, S.; Flaxman, A.D.; Laurent, A.; Kern, E.; et al. Variation in life expectancy and mortality by cause among neighbourhoods in King County, WA, USA, 1990–2014: A census tract-level analysis for the Global Burden of Disease Study 2015. Lancet Public Health 2017, 2, e400–e410. [Google Scholar] [CrossRef]
- Chen, A.; Jacobsen, K.H.; Deshmukh, A.A.; Cantor, S.B. The evolution of the disability-adjusted life year (DALY). Socio-Econ. Plan. Sci. 2015, 49, 10–15. [Google Scholar] [CrossRef]
- Prüss-Üstün, A.; Mathers, C.; Corvalán, C.; Woodward, A. Introduction and Methods: Assessing the Environmental Burden of Disease at National and Local Levels; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Ragas, A.M.; Oldenkamp, R.; Preeker, N.; Wernicke, J.; Schlink, U. Cumulative risk assessment of chemical exposures in urban environments. Environ. Int. 2011, 37, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, J.A.; Polinder, S.; Stein, C.E.; Havelaar, A.H. Systematic review of foodborne burden of disease studies: Quality assessment of data and methodology. Int. J. Food Microbiol. 2013, 166, 34–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- WHO. Assessing the Environmental Burden of Disease at National and Local Levels; Fewtrell, L., Kaufmann, R., Prüss-Üstün, A., Eds.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Prüss, A.; Kay, D.; Fewtrell, L.; Bartram, J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ. Health Perspect. 2002, 110, 537. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Shih, T.-S.; Chang, H.-Y.; Yu, H.-S.; Wu, J.-D.; Sheu, S.-C.; Wu, C.-E.; Chou, T.-C. The total body burden of chromium associated with skin disease and smoking among cement workers. Sci. Total Environ. 2008, 391, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.A. Disability-Adjusted Life Years. In Encyclopedia of Health Economics; Culyer, A.J., Ed.; Elsevier: San Diego, CA, USA, 2014; pp. 200–203. [Google Scholar]
- Murray, C.J.; Lopez, A.D.; World Health Organization. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020: Summary; Published by the Harvard School of Public Health on behalf of The World Health Organization and The World Bank; Harvard University Press: Cambridge, MA, USA, 1996; ISBN 0-9655466-0-8. [Google Scholar]
- Fattore, E.; Paiano, V.; Borgini, A.; Tittarelli, A.; Bertoldi, M.; Crosignani, P.; Fanelli, R. Human health risk in relation to air quality in two municipalities in an industrialized area of Northern Italy. Environ. Res. 2011, 111, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Tominz, R.; Mazzoleni, B.; Daris, F. Estimate of potential health benefits of the reduction of air pollution with PM10 in Trieste, Italy. Epidemiol. Prev. 2005, 29, 149–155. [Google Scholar] [PubMed]
- Skotak, K.; Swiatczak, J. Potential human health effects of PM10 exposure in Poland. Prz. Lek. 2008, 65, 18–25. [Google Scholar] [PubMed]
- Moustris, K.P.; Ntourou, K.; Nastos, P.T. Estimation of particulate matter impact on human health within the urban environment of Athens City, Greece. Urban Sci. 2017, 1, 6. [Google Scholar] [CrossRef]
- Orru, H.; Teinemaa, E.; Lai, T.; Tamm, T.; Kaasik, M.; Kimmel, V.; Kangur, K.; Merisalu, E.; Forsberg, B. Health impact assessment of particulate pollution in Tallinn using fine spatial resolution and modeling techniques. Environ. Health 2009, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Wheida, A.; Nasser, A.; El Nazer, M.; Borbon, A.; El Ata, G.A.A.; Wahab, M.A.; Alfaro, S.C. Tackling the mortality from long-term exposure to outdoor air pollution in megacities: Lessons from the Greater Cairo case study. Environ. Res. 2018, 160, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Faridi, S.; Shamsipour, M.; Krzyzanowski, M.; Künzli, N.; Amini, H.; Azimi, F.; Malkawi, M.; Momeniha, F.; Gholampour, A.; Hassanvand, M.S.; et al. Long-term trends and health impact of PM2.5 and O3 in Tehran, Iran, 2006–2015. Environ. Int. 2018, 114, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Ghozikali, M.G.; Heibati, B.; Naddafi, K.; Kloog, I.; Conti, G.O.; Polosa, R.; Ferrante, M. Evaluation of chronic obstructive pulmonary disease (COPD) attributed to atmospheric O3, NO2, and SO2 using Air Q Model (2011–2012 year). Environ. Res. 2016, 144, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, G.; Daryanoosh, S.; Godini, H.; Hopke, P.; Sicard, P.; De Marco, A.; Rad, H.; Harbizadeh, A.; Jahedi, F.; Mohammadi, M.; et al. Health risk assessment of exposure to the Middle-Eastern Dust storms in the Iranian megacity of Kermanshah. Public Health 2017, 148, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hadei, M.; Hashemi-Nazari, S.S.; Eslami, A.; Khosravi, A.; Yarahmadi, M.; Naghdali, Z.; Shahsavani, A. Distribution and number of ischemic heart disease (IHD) and stroke deaths due to chronic exposure to PM2.5 in 10 cities of iran (2013–2015); an airq+ modelling. J. Air Pollut. Health 2018, 2, 129–136. [Google Scholar]
- Hopke, P.K.; Nazari, S.S.H.; Hadei, M.; Yarahmadi, M.; Kermani, M.; Yarahmadi, E.; Shahsavani, A. Spatial and Temporal Trends of Short-Term Health Impacts of PM2.5 in Iranian Cities; a Modelling Approach (2013–2016). Aerosol Air Qual. Res. 2018, 18, 497–504. [Google Scholar] [CrossRef]
- Khaniabadi, Y.O.; Daryanoosh, M.; Sicard, P.; Takdastan, A.; Hopke, P.K.; Esmaeili, S.; de Marco, A.; Rashidi, R. Chronic obstructive pulmonary diseases related to outdoor PM10, O3, SO2, and NO2 in a heavily polluted megacity of Iran. Environ. Sci. Pollut. Res. 2018, 25, 17726–17734. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.; Aval, H.E.; Ehrampoush, M.H.; Mohammadi, A.; Toolabi, A.; Nikonahad, A.; Derakhshan, Z.; Abdollahnejad, A. Human health impact assessment of exposure to particulate matter: An AirQ software modeling. Environ. Sci. Pollut. Res. 2017, 24, 16513–16519. [Google Scholar] [CrossRef] [PubMed]
- Habeebullah, T. Health impacts of PM10 using AirQ2. 2.3 model in Makkah. J. Basic Appl. Sci. 2013, 9, 259–268. [Google Scholar]
- Jeong, S.J. The impact of air pollution on human health in Suwon City. Asian J. Atmos. Environ. 2013, 7, 227–233. [Google Scholar] [CrossRef]
- Pascal, M.; Corso, M.; Ung, A.; Declercq, C.; Medina, S. Improving Knowledge and Communication for Decision Making on Air Pollution and Health in Europe–Aphekom Project in Guidelines for Assessing the Health Impacts of Strategies to Reduce Air Pollution in Europe; French Institute for Public Health Surveillance: Saint-Maurice, France, 2011. [Google Scholar]
- Ostro, B.; World Health Organization (WHO). Outdoor Air Pollution: Assessing the Environmental Burden of Disease at National and Local Levels; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Review of the National Ambient Air Quality Standards for Particulate Matter: Policy Assessment of Scientific and Technical Information (OAQPS Staff Paper); USEPA: Research Triangle Park, NC, USA, 1996.
- Ostro, B.D.; Hurley, S.; Lipsett, M.J. Air pollution and daily mortality in the Coachella Valley, California: A study of PM10 dominated by coarse particles. Environ. Res. 1999, 81, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Homedes, N. The Disability-Adjusted Life Year (DALY) Definition, Measurement and Potential Use; World Bank: Washington, DC, USA, 1996. [Google Scholar]
- Conti, G.O.; Heibati, B.; Kloog, I.; Fiore, M.; Ferrante, M. A review of AirQ Models and their applications for forecasting the air pollution health outcomes. Environ. Sci. Pollut. Res. 2017, 24, 6426–6445. [Google Scholar] [CrossRef] [PubMed]
- Héroux, M.-E.; Anderson, H.R.; Atkinson, R.; Brunekreef, B.; Cohen, A.; Forastiere, F.; Hurley, F.; Katsouyanni, K.; Krewski, D.; Krzyzanowski, M.; et al. Quantifying the health impacts of ambient air pollutants: Recommendations of a WHO/Europe project. Int. J. Public Health 2015, 60, 619–627. [Google Scholar] [CrossRef] [PubMed]
- WHO. Review of Evidence on Health Aspects of Air Pollution: REVIHAAP Project: Final Technical Report; WHO Regional Office for Europe: København, Denmark, 2013. [Google Scholar]
- Sudhir, A.; Kara, H. Disability-adjusted life years: A critical review. J. Health Econ. 1997, 16, 685–702. [Google Scholar]
- Tsuchiya, A. Age weighting and time discounting: Technical imperative versus social choice. In Summary Measures of Population Health: Concepts, Ethics, Measurement and Applications; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- van Zelm, R.; Huijbregts, M.A.; den Hollander, H.A.; Van Jaarsveld, H.A.; Sauter, F.J.; Struijs, J.; van Wijnen, H.J.; van de Meent, D.; et al. European characterization factors for human health damage of PM10 and ozone in life cycle impact assessment. Atmos. Environ. 2008, 42, 441–453. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Peters, G.M.; Ashbolt, N.J.; Shiels, S.; Khan, S.J. Assessing burden of disease as disability adjusted life years in life cycle assessment. Sci. Total Environ. 2015, 530–531, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Boldo, E.; Medina, S.; Le Tertre, A.; Hurley, F.; Mücke, H.-G.; Ballester, F.; Aguilera, I. Apheis: Health impact assessment of long-term exposure to PM2.5 in 23 European cities. Eur. J. Epidemiol. 2006, 21, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Nel, A. Air pollution-related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Al-Hemoud, A.; Al-Dabbous, A.N.; Gasana, J.; Al-Shatti, A. Burden of Disease Associated with Long- and Short-Term Exposures to High Ambient Concentrations of PM2.5 using AirQ+. Environ. Res. 2018. under review. [Google Scholar]
- Künzli, N.; Medina, S.; Kaiser, R.; Quenel, P.; Horak, F., Jr.; Studnicka, M. Assessment of deaths attributable to air pollution: Should we use risk estimates based on time series or on cohort studies? Am. J. Epidemiol. 2001, 153, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.W.; Pope, C.A., III; Dockery, D.W.; Wang, Y.; Ezzati, M.; Dominici, F. The effect of air pollution control on life expectancy in the United States: An analysis of 545 US counties for the period 2000 to 2007. Epidemiology 2013, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.T.; Pope, C.A., III; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014, 122, 397. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.F.; Almutairi, S.K.; Al-Hemoud, A. Dust storms backward Trajectories’ and source identification over Kuwait. Atmos. Res. 2018, 212, 158–171. [Google Scholar] [CrossRef]
- Al-Hemoud, A.; Al-Sudairawi, M.; Neelamanai, S.; Naseeb, A.; Behbehani, W. Socioeconomic effect of dust storms in Kuwait. Arab. J. Geosci. 2017, 10, 18. [Google Scholar] [CrossRef]
- Al-Dabbous, A.N.; Kumar, P. Number and size distribution of airborne nanoparticles during summertime in Kuwait: First observations from the Middle East. Environ. Sci. Technol. 2014, 48, 13634–13643. [Google Scholar] [CrossRef] [PubMed]
- Al-Hemoud, A.; Al-Dousari, A.; Al-Shatti, A.; Al-Khayat, A.; Behbehani, W.; Malak, M. Health Impact Assessment Associated with Exposure to PM10 and Dust Storms in Kuwait. Atmosphere 2018, 9, 6. [Google Scholar] [CrossRef]
- Popoola, L.; Adebanjo, S.; Adeoye, B. Assessment of atmospheric particulate matter and heavy metals: A critical review. Int. J. Environ. Sci. Technol. 2018, 1–14. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.; Stockman, L.; Hicks, L.; Garrison, L.; Zhou, F.; Beach, M. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol. Infect. 2012, 140, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
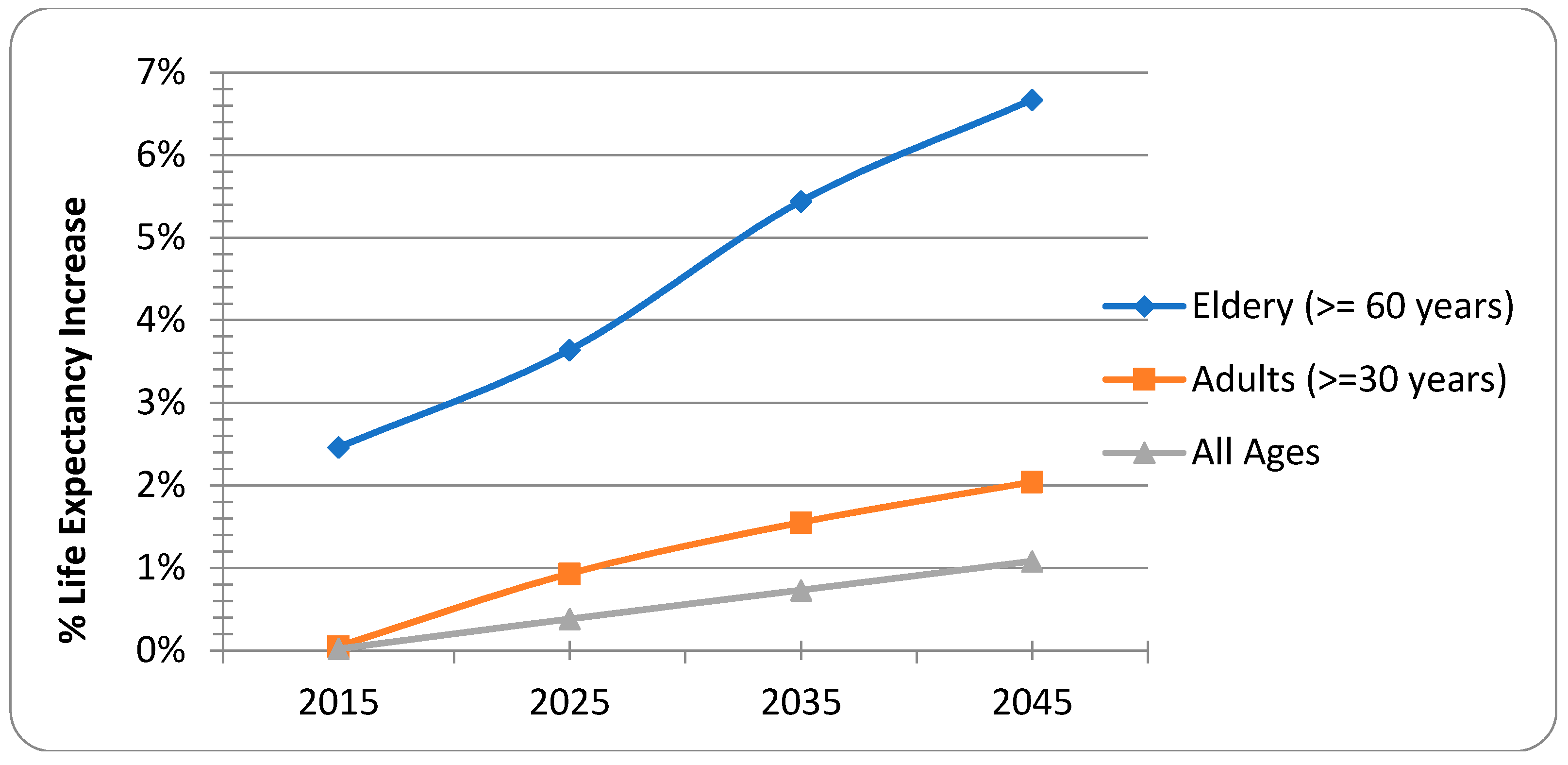
| Country * | PM2.5 | PM10 | AP | YLL | Reference |
|---|---|---|---|---|---|
| Egypt | √ | √ | Wheida et al., 2018 | ||
| Estonia | √ | √ | √ | √ | Orru et al., 2009 ** |
| 23 European Cities | √ | √ | √ | Boldo et al., 2006 | |
| Greece | √ | √ | Moustris et al., 2017 | ||
| Iran | √ | √ | √ | √ | Faridi et al., 2018 **; Ghozikali et al., 2016; Goudarzi et al., 2017; Hadei et al., 2018; Hopke et al., 2018; Khaniabadi et al., 2018; Miri et al., 2017 |
| Italy | √ | √ | √ | √ | Fattore et al., 2011 **; Tominz et al., 2005 |
| Poland | √ | √ | Skotak and Swiatczak, 2008 | ||
| Saudi Arabia | √ | √ | Habeebullah, 2013 | ||
| South Korea | √ | √ | Jeong, 2013 |
| Health Endpoint | Exposure | Cut-off Value (μg/m3) | Annual Mean (μg/m3) | RR * | β Coefficient * |
|---|---|---|---|---|---|
| Premature adult mortality, all causes | PM2.5 | 35 | 87.9 | 1.062 (1.04,1.083) | 0.2454 (0.1600, 0.3252) |
| Postneonatal infant mortality, all causes | PM10 | 70 | 167.5 | 1.04 (1.02, 1.07) | 0.2976 (0.1502, 0.5134) |
| Mortality | 2014 | 2015 | 2016 |
|---|---|---|---|
| Adult | 2561 | 2735 | 2685 |
| Postneonatal | 73 | 76 | 86 |
| Measure | Age | Central | Lower | Upper |
|---|---|---|---|---|
| YLL–2015 | all ages | 252.18 | 170.69 | 322.92 |
| YLL–2015 | age 0–64 | 89.53 | 60.60 | 114.64 |
| YLL over 10 Years–2025 | all ages | 27,474.61 | 18,483.02 | 35,370.58 |
| YLL over 10 Years–2025 | age 0–64 | 8487.28 | 5741.46 | 10,873.33 |
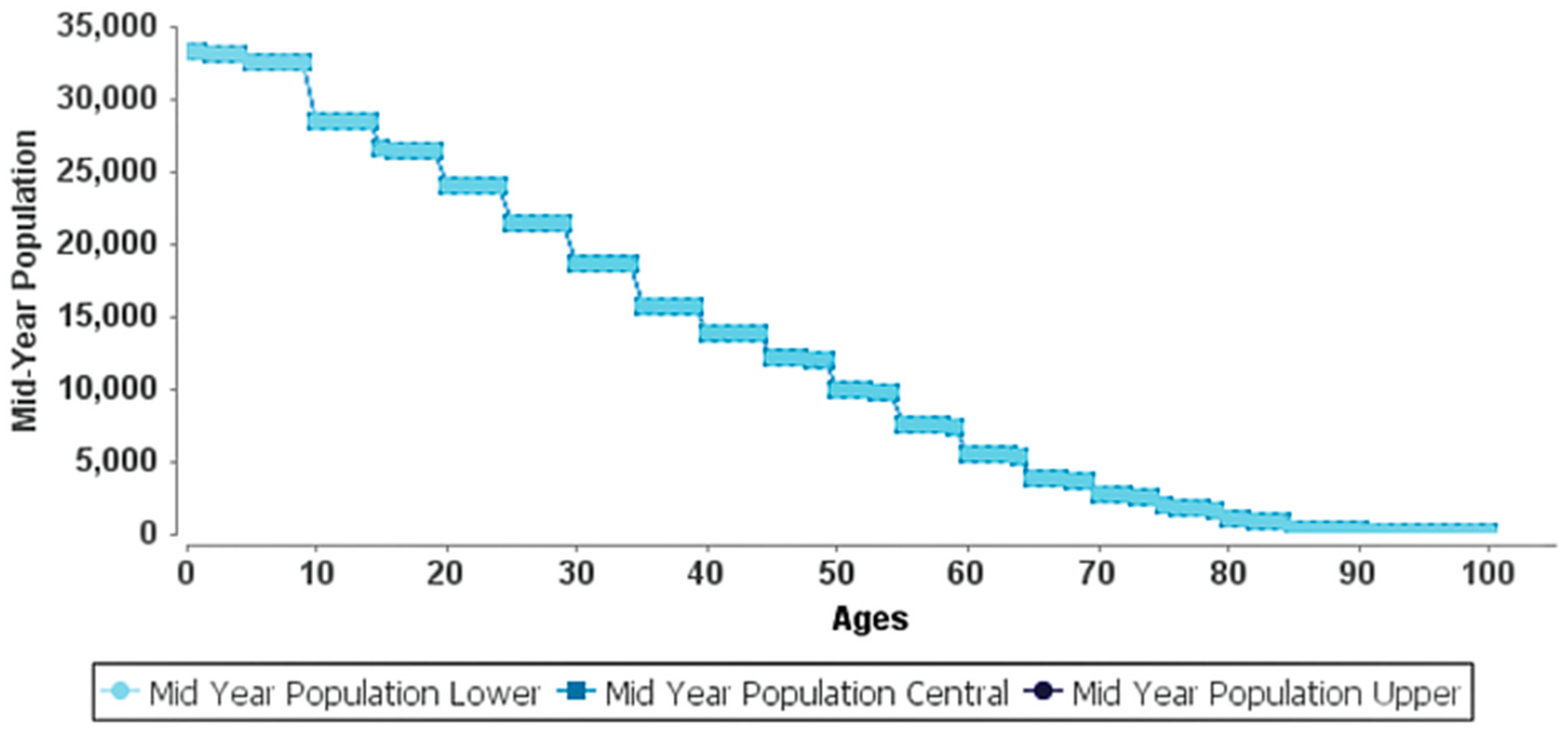
| Age | ELR (years) | Delta ELR * | Entry Population | Years of Life | Hazard Rate | Survival Probability |
|---|---|---|---|---|---|---|
| 0 | 79.81 | 2.65 (1.35, 4.51) | 33,269 | 33,242 | 0.16% | 99.84% |
| 1 | 78.94 | 2.66 (1.35, 4.52) | 33,215 | 33,189 | 0.16% | 99.84% |
| 30 | 51.12 | 2.34 (1.53, 3.08) | 18,576 | 18,571 | 0.06% | 99.94% |
| 35 | 46.29 | 2.31 (1.51, 3.05) | 15,735 | 15,730 | 0.07% | 99.93% |
| 40 | 41.47 | 2.28 (1.49, 3.01) | 13,885 | 13,878 | 0.11% | 99.89% |
| 45 | 36.73 | 2.24 (1.47, 2.96) | 12,123 | 12,113 | 0.16% | 99.84% |
| 50 | 32.08 | 2.19 (1.43, 2.89) | 9910 | 9898 | 0.25% | 99.75% |
| 55 | 27.54 | 2.12 (1.39, 2.81) | 7564 | 7550 | 0.38% | 99.62% |
| 60 | 23.14 | 2.04 (1.33, 2.70) | 5561 | 5542 | 0.66% | 99.34% |
| 65 | 19.02 | 1.93 (1.26, 2.56) | 3895 | 3871 | 1.23% | 98.77% |
| 70 | 15.36 | 1.78 (1.16, 2.36) | 2812 | 2782 | 2.16% | 97.84% |
| 75 | 12.25 | 1.58 (1.03, 2.10) | 1939 | 1907 | 3.29% | 96.71% |
| 80 | 9.54 | 1.37 (0.89, 1.81) | 1042 | 1014 | 5.28% | 94.72% |
| 85 | 7.46 | 1.10 (0.72, 1.45) | 381 | 364 | 8.47% | 91.53% |
| Central | Lower | Upper | |
|---|---|---|---|
| AP * | 22.68% | 12.18% | 35.84% |
| Excess Cases ** | 17 | 9 | 27 |
| Cases per 100,000 *** | 53.77 | 28.87 | 84.95 |
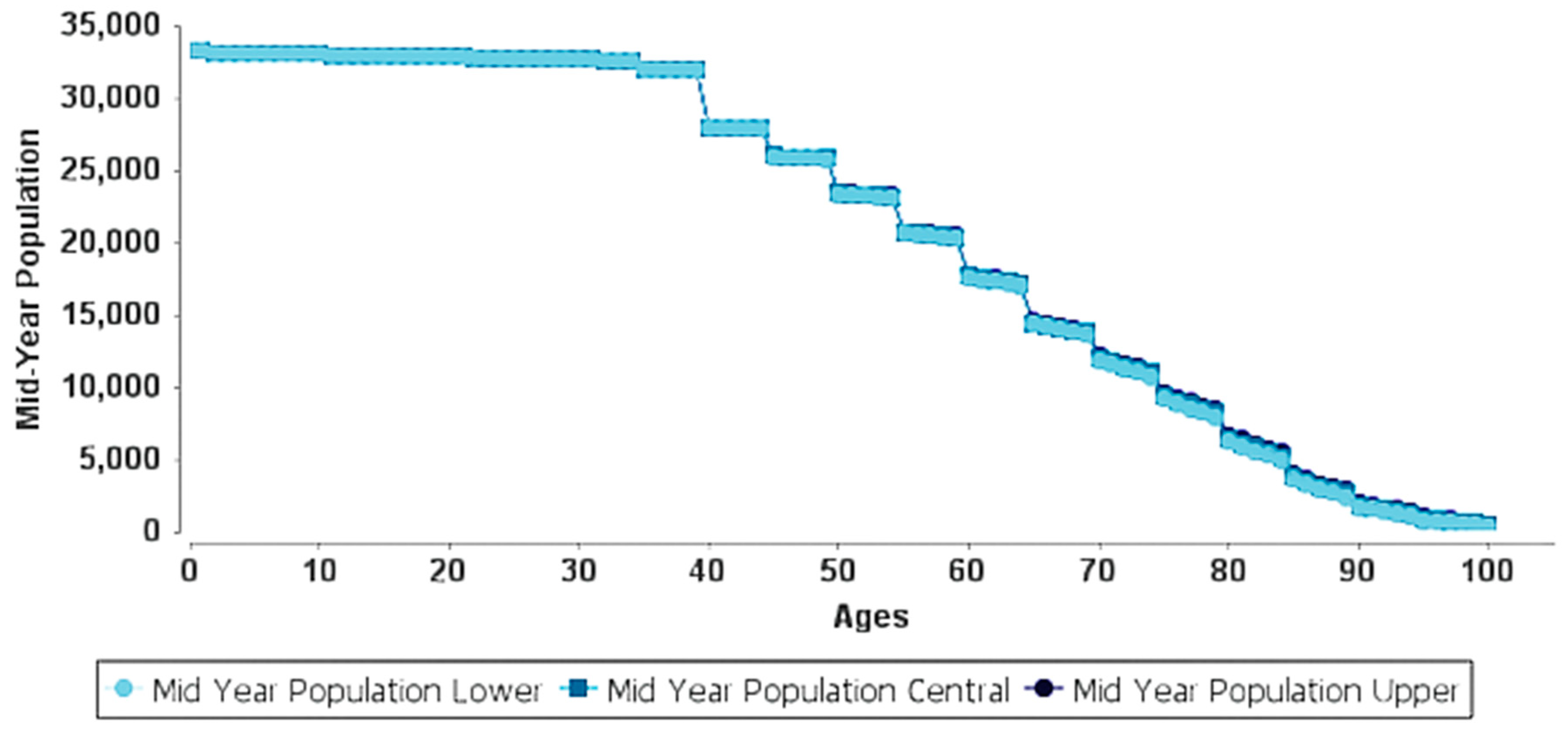
| Age | Years of Life at the Current PM2.5 | Years of Life at the Cut-Off PM2.5 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2015 | 2025 | 2035 | 2045 | 2015 | 2025 | 2035 | 2045 | |
| 0–1 | 66,431 | 66,431 | 66,431 | 66,431 | 66,431 | 66,431 | 66,431 | 66,431 |
| 2–9 | 261,498 | 264,168 | 264,168 | 264,168 | 261,498 | 264,168 | 264,168 | 264,168 |
| 10–19 | 274,269 | 326,535 | 329,197 | 329,197 | 274,269 | 326,535 | 329,197 | 329,197 |
| 20–29 | 226,802 | 271,655 | 324,606 | 327,251 | 226,802 | 271,655 | 324,606 | 327,251 |
| 30–39 | 171,239 | 225,351 | 270,909 | 322,526 | 171,251 | 225,508 | 271,099 | 322,757 |
| 40–49 | 129,500 | 169,332 | 222,815 | 267,848 | 129,521 | 169,714 | 223,473 | 268,644 |
| 50–59 | 86,543 | 126,119 | 164,919 | 216,988 | 86,576 | 126,807 | 166,172 | 218,779 |
| 60–69 | 45,971 | 80,746 | 117,527 | 153,736 | 46,022 | 81,899 | 119,836 | 157,075 |
| 70–79 | 21,891 | 37,249 | 65,321 | 94,749 | 21,962 | 38,888 | 69,098 | 100,819 |
| ≥80 | 7251 | 14,884 | 26,376 | 45,951 | 7314 | 16,929 | 31,683 | 56,197 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hemoud, A.; Gasana, J.; Al-Dabbous, A.N.; Al-Shatti, A.; Al-Khayat, A. Disability Adjusted Life Years (DALYs) in Terms of Years of Life Lost (YLL) Due to Premature Adult Mortalities and Postneonatal Infant Mortalities Attributed to PM2.5 and PM10 Exposures in Kuwait. Int. J. Environ. Res. Public Health 2018, 15, 2609. https://doi.org/10.3390/ijerph15112609
Al-Hemoud A, Gasana J, Al-Dabbous AN, Al-Shatti A, Al-Khayat A. Disability Adjusted Life Years (DALYs) in Terms of Years of Life Lost (YLL) Due to Premature Adult Mortalities and Postneonatal Infant Mortalities Attributed to PM2.5 and PM10 Exposures in Kuwait. International Journal of Environmental Research and Public Health. 2018; 15(11):2609. https://doi.org/10.3390/ijerph15112609
Chicago/Turabian StyleAl-Hemoud, Ali, Janvier Gasana, Abdullah N. Al-Dabbous, Ahmad Al-Shatti, and Ahmad Al-Khayat. 2018. "Disability Adjusted Life Years (DALYs) in Terms of Years of Life Lost (YLL) Due to Premature Adult Mortalities and Postneonatal Infant Mortalities Attributed to PM2.5 and PM10 Exposures in Kuwait" International Journal of Environmental Research and Public Health 15, no. 11: 2609. https://doi.org/10.3390/ijerph15112609
APA StyleAl-Hemoud, A., Gasana, J., Al-Dabbous, A. N., Al-Shatti, A., & Al-Khayat, A. (2018). Disability Adjusted Life Years (DALYs) in Terms of Years of Life Lost (YLL) Due to Premature Adult Mortalities and Postneonatal Infant Mortalities Attributed to PM2.5 and PM10 Exposures in Kuwait. International Journal of Environmental Research and Public Health, 15(11), 2609. https://doi.org/10.3390/ijerph15112609






