Lung Function Assessment as an Early Biomonitor of Mercury-Induced Health Disorders in Artisanal and Small-Scale Gold Mining Areas in Indonesia
Abstract
1. Introduction
1.1. Background
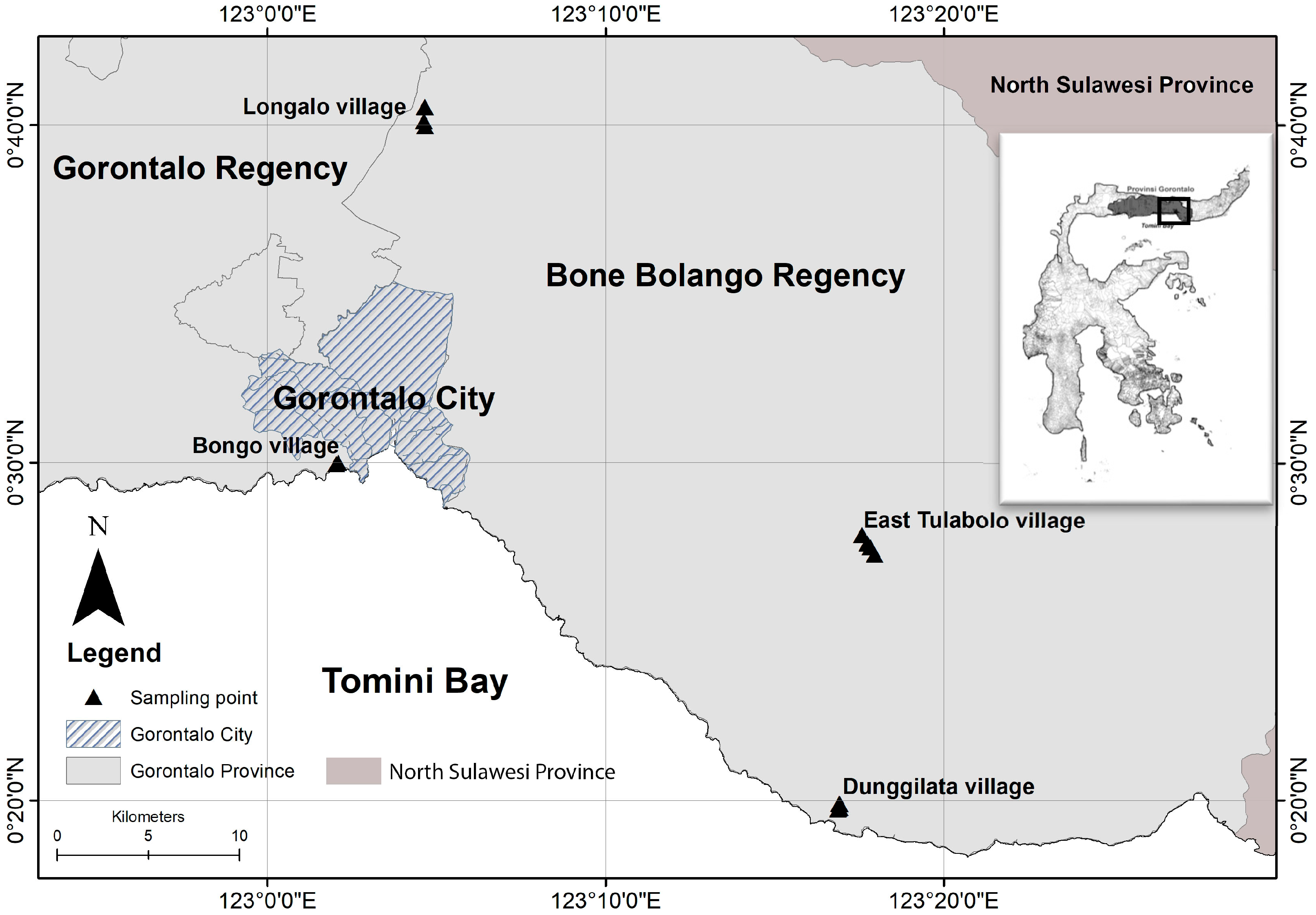
1.2. Study Area and Selected Population
1.2.1. Study Area
1.2.2. Population and Samples
2. Materials and Methods
2.1. Characteristics of Respondents
2.2. General Health Assessment
2.3. Spirometry Test
2.4. Nose and Scalp Hair Samples
- Pre-washing stageHair samples must be selected with a hair length greater than 2.5 cm and not excessively lean. We must pick out the part that is closer to the hair root.
- Washing stageThe hair samples were put into a beaker glass filled with Milli-Q water (18.2 MΩ·cm), and were then washed in an ultrasonic cleaner bath (AS ONE corporation, Osaka, Japan) for 5 min. Afterwards, the samples were dried on sterile paper towels at room temperature. After drying, the samples were washed by stirring in acetone (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 5 min and washed again with Milli-Q water, then dried on sterile paper towels at room temperature.
- Post-washing stageThe eight strands of dried hair samples were attached in parallel to the midway position of the sample holder. Sample labels were written on the side part of the holder.
2.5. Analytical Method
3. Results
3.1. Characteristics of Respondents
3.2. Analysis of Lung Function
3.3. The Relationship between Mercury Levels in Head and Nose Hair with Spirometry Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veiga, M.M.; Baker, R.F. Protocols for Environment and Health Assessment of Mercury Released by Artisanal and Small-Scale Miners; GEF/UNDP/UNIDO: Vienna, Austria, 2005; ISBN 9211064295. [Google Scholar]
- Yorifuji, T.; Tsuda, T.; Inoue, S.; Takao, S.; Harada, M. Long-term exposure to methylmercury and psychiatric symptoms in residents of Minamata, Japan. Environ. Int. 2011, 37, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.; Ahern, M. Worker and community health impacts related to mining operations internationally. A rapid review of the literature. Lond. Min. Miner. 2001, 32, 129–163. [Google Scholar]
- Bundschuh, J.; Maity, J.P.; Mushtaq, S.; Vithanage, M.; Seneweera, S.; Schneider, J.; Bhattacharya, P.; Khan, N.I.; Hamawand, I.; Guilherme, L.R.G.; et al. Medical geology in the framework of the sustainable development goals. Sci. Total Environ. 2017, 581–582, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Steckling, N.; Tobollik, M.; Plass, D.; Hornberg, C.; Ericson, B.; Fuller, R.; Bose-O’Reilly, S. Global Burden of Disease of Mercury Used in Artisanal Small-Scale Gold Mining. Ann. Glob. Heal. 2017, 83, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Ozuah, P.O. Mercury poisoning. Curr. Probl. Pediatr. 2000, 30, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Arifin, Y.; Sakakibara, M.; Sera, K. Impacts of Artisanal and Small-Scale Gold Mining (ASGM) on Environment and Human Health of Gorontalo Utara Regency, Gorontalo Province, Indonesia. Geosciences 2015. [Google Scholar] [CrossRef]
- Arifin, Y.I.; Sakakibara, M.; Sera, K. Arsenic, Lead, and Mercury Concentrations of Scalp Hairs in ASGM Miners and Inhabitants of Gorontalo Utara regency, Gorontalo province, Indonesia. NMCC Ann. Rep. 2014, 21, 133–138. [Google Scholar]
- Manovita, S.; Indriati, Y.; Novarina, V. Mapping of health disorders related to mercury on community around the Bone River, Gorontalo Province. Int. J. Sci. Basic Appl. Res. 2017, 36, 83–93. [Google Scholar]
- United Nations Environment Programme (UNEP). Global Mercury Assessment 2013. Available online: http:// H:/ARTICLES/Global%20mercury%20assessment%20_%20UN%20Environment.html (accessed on 20 March 2018).
- Telmer, K.H.; Veiga, M.M. World emissions of mercury from artisanal and small scale gold mining. In Mercury Fate and Transport in the Global Atmosphere: Emissions, Measurements and Models; Springer: Berlin, Germany, 2009; pp. 131–172. ISBN 9780387939575. [Google Scholar]
- Keane, S.E. Artisanal and Small Scale Gold Mining Area. UNEP Global Mercury Partnership. Available online: https://wedocs.unep.org/bitstream/handle/20.500.1182/11572/ASGM_final_3.pdf?sequence=1&isAllowed=y (accessed on 21 November 2017).
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Nagafuchi, O.; Kawakami, T.; Inoue, T.; Yokota, K.; Serikawa, Y.; Cyio, B.; Elvince, R. Human health risk assessment of mercury vapor around artisanal small-scale gold mining area, Palu city, Central Sulawesi, Indonesia. Ecotoxicol. Environ. Saf. 2016, 124. [Google Scholar] [CrossRef] [PubMed]
- Basri; Sakakibara, M.; Sera, K. Current Mercury Exposure from Artisanal and Small-Scale Gold Mining in Bombana, Southeast Sulawesi, Indonesia—Future Significant Health Risks. Toxics 2017, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Alhamshari, Y.S.; Salahuddin, M. European Journal of Case Reports in Internal Medicine. Available online: http://eprints.bice.rm.cnr.it/16427/1/557-3526-1-PB.pdf (accessed on 21 November 2017).
- Edwards, W.G. Geology and health. Jbsd 1955, 12, 131–135. [Google Scholar]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, N.A.; Le Blond, J.S. Assessing correlations between geological hazards and health outcomes: Addressing complexity in medical geology. Environ. Int. 2015, 84, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Bose-O’Reilly, S.; Schierl, R.; Nowak, D.; Siebert, U.; William, J.F.; Owi, F.T.; Ir, Y.I. A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia. Environ. Res. 2016, 149, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hnizdo, E.; Murray, J. Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South African gold miners. Occup. Environ. Med. 1998, 55, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.B.; Bulatao, R.A. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life; National Academies Press: Washington, DC, USA, 2004; ISBN 0309532000. [Google Scholar]
- Olson, D.A. Medscape_Mercury Toxicity: Background, Etiology, Epidemiology. Available online: https://emedicine.medscape.com/article/1175560-overview (accessed on 20 March 2018).
- Harada, M. Minamata disease:methylomercury poisoning in Japan causes by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, M.; Sera, K.; Kurniawan, I.A. Mercury Contamination of Cattle in Artisanal and Small-Scale Gold Mining in Bombana, Southeast Sulawesi, Indonesia. Geosciences 2017, 7, 133. [Google Scholar] [CrossRef]
- Gao, Z.; Ying, X.; Yan, J.; Wang, J.; Cai, S.; Yan, C. Acute mercury vapor poisoning in a 3-month-old infant: A case report. Clin. Chim. Acta 2017, 465, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hachiya, N.; Murata, K.Y.; Nakanishi, I.; Kondo, T.; Yasutake, A.; Miyamoto, K.; Ser, P.H.; Omi, S.; Furusawa, H.; Watanabe, C.; Usuki, F.; Sakamoto, M. Methylmercury exposure and neurological outcomes in Taiji residents accustomed to consuming whale meat. Environ. Int. 2014, 68, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and cardiovascular risk: A systematic review and meta-analysis of observational studies. Br. Med. J. 2018, 14–16. [Google Scholar] [CrossRef]
- Selinus, O.; Alloway, B.J.; Centeno, J.A.; Finkelman, R.B.; Fuge, R.; Lindh, U.; Smedley, P. Essentials Of Medical Geology; Elsevier Academic Press: London, UK, 2005; ISBN 0126363412. [Google Scholar]
- Eisler, R. Health risk of gold miners: A synoptic review. Env. Geochem. Heal. 2003, 25, 325–345. [Google Scholar] [CrossRef]
- Perhimpunan Dokter Paru Indonesia. Penyakit Paru Obstruktif Kronik. Available online: https://www.klikpdpi.com/konsensus/konsensus-ppok/ppok.pdf (accessed on 14 October 2018).
- WinspiroPro. User Manual. Available online: https://system.na2.netsuite.com/core/media/media.nl?id=6831&c=728341&h=f86713d690bb97d25a50&_xt=.pdf (accessed on 29 March 2018).
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Angerer, J.; Ewers, U.; Kolossa-Gehring, M. The German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2007, 210, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.B.; Schierl, R.; Nowak, D.; Siebert, U.; Frederick, J.; Teorgi, F.; Ismawati, Y. A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia. Environ. Res. 2016, 1–8. [Google Scholar]

 nose hair (PIXE);
nose hair (PIXE); scalp hair (PIXE). Lung function by spirometry test:
scalp hair (PIXE). Lung function by spirometry test:  severe disorder;
severe disorder;  middle disorder;
middle disorder;  normal
normal  mild to no smoker
mild to no smoker  moderate smoker. PIXE: particle-induced X-ray emissions.
moderate smoker. PIXE: particle-induced X-ray emissions.
 nose hair (PIXE);
nose hair (PIXE); scalp hair (PIXE). Lung function by spirometry test:
scalp hair (PIXE). Lung function by spirometry test:  severe disorder;
severe disorder;  middle disorder;
middle disorder;  normal
normal  mild to no smoker
mild to no smoker  moderate smoker. PIXE: particle-induced X-ray emissions.
moderate smoker. PIXE: particle-induced X-ray emissions.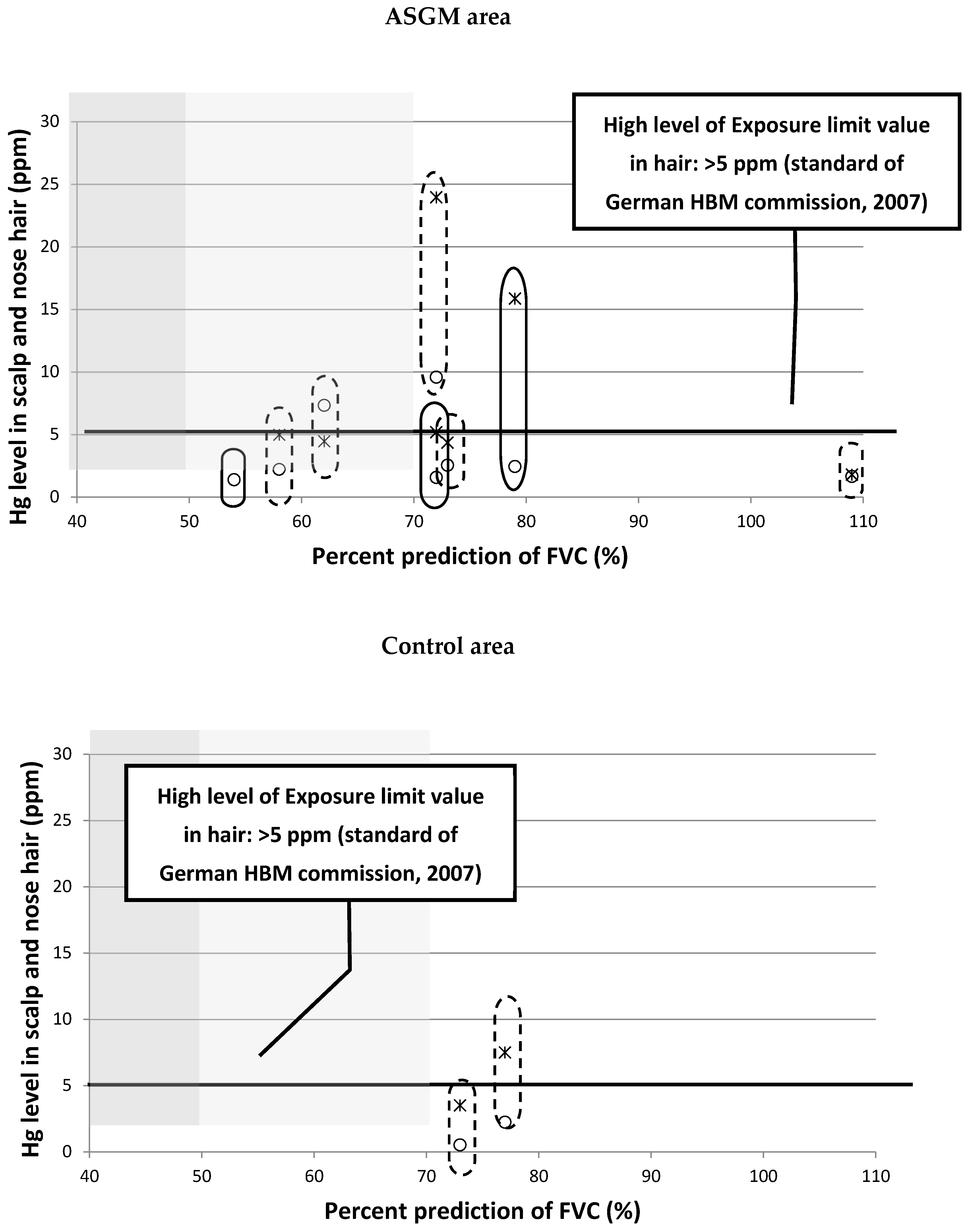
| Characteristics | ASGM Area | Control Area | Mean | ||
|---|---|---|---|---|---|
| East Tulabolo | Dunggilata | Longalo | Bongo | ||
| n (respondents) | 27 | 31 | 35 | 40 | 133 |
| Age (years old) | |||||
| • Modus | 21 | 43 | 46 | 32 | 35,5 |
| • Mean (SD) | 37.1 (2.91) | 41.7 (3.08) | 45.8 (2.70) | 38.2 (1.74) | 40.7 (2.61) |
| • Median | 35 | 43 | 46 | 36 | 40 |
| Sex (%) | |||||
| • Male | 51.9 | 58 | 43 | 63 | 54 |
| • Female | 48.1 | 42 | 57 | 37 | 46 |
| Body Mass Index (kg/m2) | |||||
| • Mean (SD) | 22.6 (0.75) | 21.9 (1.08) | 22.7 (0.77) | 25.3 (0.74) | 23.1 (0.80) |
| • Modus | 23.2 | 21.6 | 19.8 | 19.7 | 21.1 |
| Work as a miner (%) | |||||
| • Yes | 44 | 32 | 0 | 0 | 19 |
| • No | 56 | 68 | 100 | 100 | 81 |
| Respiratory Assessment | ASGM Area | Control Area | ||
|---|---|---|---|---|
| East Tulabolo | Dunggilata | Longalo | Bongo | |
| n (respondents) | 27 | 31 | 35 | 40 |
| Smoking Status (n) | ||||
| • Yes | 13 | 16 | 25 | 26 |
| • No | 14 | 15 | 10 | 14 |
| Smoking Grade (%) | ||||
| • Mild | 25.9 | 19.4 | 17.1 | 25.0 |
| • Moderate | 18.5 | 9.7 | 11.4 | 10.0 |
| • Heavy | 3.7 | 19.4 | 0.0 | 0.0 |
| FVC (Liter) | ||||
| • Mean (SD) | 2.32 (0.14) | 2.13 (0.16) | 2.29 (0.14) | 2.67 (0.13) |
| • Modus | 2.57 | 1.54 | 1.58 | 2.15 |
| % prediction of FVC | ||||
| • Mean (SD) | 67.0 (18.0) | 61.8 (19.9) | 74.6 (19.4) | 72.0 (15.1) |
| • Modus | 58 | 70 | 73 | 72 |
| FEV1 (Liter) | ||||
| • Mean (SD) | 1.86 (0.14) | 1.69 (0.15) | 1.82 (0.12) | 2.19 (0.13) |
| • Modus | 2.07 | 0.89 | 0.68 | 1.79 |
| % prediction of FEV1 | ||||
| • Mean (SD) | 61.8 (20.2) | 56.4 (22.6) | 68.0 (17.3) | 67.1 (17.6) |
| • Modus | 66 | 31 | 64 | 78 |
| Spirometry Interpretation (%) | ||||
| • Normal | 29.6 | 41.9 | 57.1 | 62.5 |
| • Middle | 44.4 | 25.8 | 28.6 | 27.5 |
| • Severe | 25.9 | 32.3 | 14.3 | 10.0 |
| Classification | n | Spirometry Test Results (%) | p | ||
|---|---|---|---|---|---|
| Normal | Middle | Severe | |||
| Based on Area | 0.03 0 | ||||
| ― ASGM area | 58 | 36.2 | 34.5 | 29.3 | |
| ― Control area | 75 | 60.0 | 28.0 | 12.0 | |
| Smoking Grade | |||||
| ― Mild | 29 | 55.2 | 27.6 | 17.2 | 0.04 0 |
| ― Moderate | 16 | 37.5 | 37.5 | 25.0 | |
| ― Heavy | 7 | 14.3 | 14.3 | 71.4 | |
| Occupation in ASGM area | 0.63 0 | ||||
| ― Miner | 22 | 36.4 | 27.3 | 36.4 | |
| ― Non-miner | 36 | 36.1 | 38.9 | 25.0 | |
| Duration of work (as a miner) | 0.69 * r = −0.07 | ||||
| ― Acute (<5 years) | 8 | 25.0 | 33.3 | 50.0 | |
| ― Chronic (≥5 years) | 14 | 75.0 | 66.7 | 50.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pateda, S.M.; Sakakibara, M.; Sera, K. Lung Function Assessment as an Early Biomonitor of Mercury-Induced Health Disorders in Artisanal and Small-Scale Gold Mining Areas in Indonesia. Int. J. Environ. Res. Public Health 2018, 15, 2480. https://doi.org/10.3390/ijerph15112480
Pateda SM, Sakakibara M, Sera K. Lung Function Assessment as an Early Biomonitor of Mercury-Induced Health Disorders in Artisanal and Small-Scale Gold Mining Areas in Indonesia. International Journal of Environmental Research and Public Health. 2018; 15(11):2480. https://doi.org/10.3390/ijerph15112480
Chicago/Turabian StylePateda, Sri Manovita, Masayuki Sakakibara, and Koichiro Sera. 2018. "Lung Function Assessment as an Early Biomonitor of Mercury-Induced Health Disorders in Artisanal and Small-Scale Gold Mining Areas in Indonesia" International Journal of Environmental Research and Public Health 15, no. 11: 2480. https://doi.org/10.3390/ijerph15112480
APA StylePateda, S. M., Sakakibara, M., & Sera, K. (2018). Lung Function Assessment as an Early Biomonitor of Mercury-Induced Health Disorders in Artisanal and Small-Scale Gold Mining Areas in Indonesia. International Journal of Environmental Research and Public Health, 15(11), 2480. https://doi.org/10.3390/ijerph15112480






