Histopathologic Analysis of Lung Cancer Incidence Associated with Radon Exposure among Ontario Uranium Miners
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Estimates of Radon Progeny Exposure
2.3. Morphology and Histology
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The International Agency for Research on Cancer. Ionizing Radiation, Part 2, Some Internally Deposited Radionuclides; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (IARC): Lyon, France, 2001; Volume 78, ISBN 978-92-832-1578-3. [Google Scholar]
- National Research Council (US) Committee on the Biological Effects of Ionizing Radiations. Health Risks of Radon and Other Internally Deposited Alpha-Emitters: Beir IV; National Academies Press (US): Washington, DC, USA, 1988; ISBN 0-309-03789-1. [Google Scholar]
- National Research Council Staff. Health Effects of Exposure to Radon: BEIR; Joseph Henry Press; National Academies Press: Washington, DC, USA, 1999; Volume VI, ISBN 978-0-309-05645-8. [Google Scholar]
- National Research Council, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006; Volume VII, ISBN 978-0-309-09156-5. [Google Scholar]
- Schubauer-Berigan, M.K.; Daniels, R.D.; Pinkerton, L.E. Radon exposure and mortality among White and American Indian uranium miners: An update of the Colorado Plateau cohort. Am. J. Epidemiol. 2009, 169, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Boice, J.D., Jr.; Cohen, S.S.; Mumma, M.T.; Chadda, B.; Blot, W.J. A cohort study of uranium millers and miners of Grants, New Mexico, 1979–2005. J. Radiol. Prot. 2008, 28, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Rage, E.; Vacquier, B.; Blanchardon, E.; Allodji, R.S.; Marsh, J.W.; Caër-Lorho, S.; Acker, A.; Laurier, D. Risk of lung cancer mortality in relation to lung doses among French uranium miners: Follow-up 1956–1999. Radiat. Res. 2012, 177, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Rage, E.; Caër-Lorho, S.; Drubay, D.; Ancelet, S.; Laroche, P.; Laurier, D. Mortality analyses in the updated French cohort of uranium miners (1946–2007). Int. Arch. Occup. Environ. Health 2014, 88, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Vacquier, B.; Rogel, A.; Leuraud, K.; Caer, S.; Acker, A.; Laurier, D. Radon-associated lung cancer risk among French uranium miners: Modifying factors of the exposure-risk relationship. Radiat. Environ. Biophys. 2009, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, L. Lung cancer mortality among Czech uranium miners-60 years since exposure. J. Radiol. Prot. 2012, 32, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Kulich, M.; Řeřicha, V.; Řeřicha, R.; Shore, D.L.; Sandler, D.P. Incidence of non-lung solid cancers in Czech uranium miners: A case–cohort study. Environ. Res. 2011, 111, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Tomásek, L.; Darby, S.C. Recent results from the study of West Bohemian uranium miners exposed to radon and its progeny. Environ. Health Perspect. 1995, 103 (Suppl. 2), 55–57. [Google Scholar] [PubMed]
- Kreuzer, M.; Walsh, L.; Schnelzer, M.; Tschense, A.; Grosche, B. Radon and risk of extrapulmonary cancers: Results of the German uranium miners’ cohort study, 1960–2003. Br. J. Cancer 2008, 99, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Fenske, N.; Schnelzer, M.; Walsh, L. Lung cancer risk at low radon exposure rates in German uranium miners. Br. J. Cancer 2015, 113, 1367–1369. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Tschense, A.; Schnelzer, M.; Dufey, F.; Grosche, B.; Kreuzer, M. The influence of radon exposures on lung cancer mortality in German uranium miners, 1946–2003. Radiat. Res. 2010, 173, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Bergdahl, I.A.; Akerblom, G.; Eriksson, K.; Andersson, K.; Kågström, L.; Järvholm, B.; Damber, L. Lung cancer risk and radon exposure in a cohort of iron ore miners in Malmberget, Sweden. Occup. Environ. Med. 2010, 67, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.J.; Morrison, H.I.; Lane, R. Radon and lung cancer risk: An extension of the mortality follow-up of the Newfoundland fluorspar cohort. Health Phys. 2007, 92, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Navaranjan, G.; Berriault, C.; Do, M.; Villeneuve, P.J.; Demers, P.A. Cancer incidence and mortality from exposure to radon progeny among Ontario uranium miners. Occup. Environ. Med. 2016, 73, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, J.; Coyle, D.; Whyte, J.; Krewksi, D. Global Estimate of Lung Cancer Mortality Attributable to Residential Radon. Environ. Health Perspect. 2018, 126, 057009. [Google Scholar] [CrossRef] [PubMed]
- Meza, R.; Meernik, C.; Jeon, J.; Cote, M.L. Lung cancer incidence trends by gender, race and histology in the United States, 1973–2010. PLoS ONE 2015, 10, e0121323. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Müller, K.M.; Brachner, A.; Gerken, M.; Grosche, B.; Wiethege, T.; Wichmann, H.E. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000, 89, 2613–2621. [Google Scholar] [CrossRef]
- Kusiak, R.A.; Ritchie, A.C.; Muller, J.; Springer, J. Mortality from lung cancer in Ontario uranium miners. Br. J. Ind. Med. 1993, 50, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Tomásek, L.; Placek, V. Radon exposure and lung cancer risk: Czech cohort study. Radiat. Res. 1999, 152, 59–63. [Google Scholar] [CrossRef]
- Kreyberg, L. Lung cancer in workers in a nickel refinery. Br. J. Ind. Med. 1978, 35, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ilar, A.; Plato, N.; Lewné, M.; Pershagen, G.; Gustavsson, P. Occupational exposure to diesel motor exhaust and risk of lung cancer by histological subtype: A population-based case-control study in Swedish men. Eur. J. Epidemiol. 2017, 32, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Navaranjan, G.; Berriault, C.; Do, M.; Villeneuve, P.; Demers, P. Ontario Uranium Miners Cohort Study Report; Cancer Care Ontario: Toronto, ON, Canada, 2015; p. 123. [Google Scholar]
- Muller, J.; Kusiak, R.A.; Ritchie, A.C. Factors Modifying Lung Cancer Risk in Ontario Uranium Miners, 1955–1981; Ontario Ministry of Labour: Toronto, ON, Canada; Ontario Workers’ Compensation Board: Toronto, ON, Canada; Atomic Energy Control Board of Canada: Ottawa, ON, Canada, 1989; p. 37. [Google Scholar]
- Do, M.T. Ionizing Radiation Exposure and Risk of Gastrointestinal Cancer: A Study of the Ontario Uranium Miners. Ph.D. Thesis, The University of Toronto, Toronto, ON, Canada, 2009. [Google Scholar]
- Ashmore, J.P.; Krewski, D.; Zielinski, J.M.; Jiang, H.; Semenciw, R.; Band, P.R. First analysis of mortality and occupational radiation exposure based on the National Dose Registry of Canada. Am. J. Epidemiol. 1998, 148, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Wheeler, W.; Gentleman, J.; Suranyi, G.; Kusiak, R. Study of Mortality of Ontario Miners 1955–1977, Part I; Ontario, Ministry of Labour: Toronto, ON, Canada; Ontario Workers’ Compensation Board: Toronto, ON, Canada; Atomic Energy Control Board of Canada: Ottawa, ON, Canada, 1983; p. 84. [Google Scholar]
- Statistics Canada Canadian Cancer Registry (CCR). Available online: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207 (accessed on 19 July 2018).
- Lane, R.S.D.; Frost, S.E.; Howe, G.R.; Zablotska, L.B. Mortality (1950–1999) and cancer incidence (1969–1999) in the cohort of Eldorado uranium workers. Radiat. Res. 2010, 174, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. Induction and latent periods. Am. J. Epidemiol. 1981, 114, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Archer, V.E. Lung cancer risks of underground miners: Cohort and case-control studies. Yale J. Biol. Med. 1988, 61, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Thomas, C.L.; Snider, A.M.; Picchi, M.A.; Chen, W.; Willis, D.G.; Carr, T.G.; Krzeminski, J.; Desai, D.; Shantu, A.; et al. Radon Exposure, IL-6 Promoter Variants, and Lung Squamous Cell Carcinoma in Former Uranium Miners. Environ. Health Perspect. 2016, 124, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Barros-Dios, J.M.; Ruano-Ravina, A.; Pérez-Ríos, M.; Castro-Bernárdez, M.; Abal-Arca, J.; Tojo-Castro, M. Residential radon exposure, histologic types, and lung cancer risk. A case-control study in Galicia, Spain. Cancer Epidemiol. Biomark. Prev. 2012, 21, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, Á.; Torres-Durán, M.; Barros-Dios, J.M.; Ruano-Ravina, A. Residential radon and small cell lung cancer. A systematic review. Cancer Lett. 2018, 426, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Taeger, D.; Fritsch, A.; Wiethege, T.; Johnen, G.; Eisenmenger, A.; Wesch, H.; Ko, Y.; Stier, S.; Michael Muller, K.; Bruning, T.; et al. Role of exposure to radon and silicosis on the cell type of lung carcinoma in German uranium miners. Cancer 2006, 106, 881–889. [Google Scholar] [CrossRef] [PubMed]
| Lung Cancer Histology | ICD-O-3 Morphology Codes |
|---|---|
| Squamous cells (SqCC) | 8050–8053, 8060, 8070–8078, 8083–8084 |
| Adenocarcinoma (AdC) | 8140, 8211, 8230–8231, 8250–8260, 8323, 8480–8481, 8490, 8550–8551, 8570–8574, 8576 |
| Large cells (LCC) | 8010–8012, 8014, 8015, 8020, 8021, 8022, 8030, 8031, 8035, 8310, 8046 |
| Small cells (SmCC) | 8041–8045 |
| Other or Unspecified | Other specified carcinoma (8246), sarcoma (8800–8811, 8830, 8840–8921, 8990–8991, 9040–9044, 9120–9133, 9150, 9540–9581), unspecified (8000–8005) |
| Characteristics | Cases n = 1274 | Non-Cases n = 27,272 | Test for Difference p-Value |
|---|---|---|---|
| Age at entry into study (Years) | |||
| Median | 32 | 26 | <0.0001 |
| Mean ± SD | 32.85 ± 8.88 | 28.57 ± 8.53 | |
| Range | 17–65 | 16–65 | |
| Total Duration of Employment (Years) | |||
| Median | 3.0 | 2.5 | <0.0001 |
| Mean ± SD | 5.04 ± 5.10 | 4.01 ± 4.05 | |
| Range | 0.5–31.0 | 0.5–32.0 | |
| Cumulative radon exposure (WLM) | |||
| Median | 18.89 | 6.03 | <0.0001 |
| Mean ± SD | 45.86 ± 70.33 | 23.06 ± 45.06 | |
| Range | 0–875.13 | 0–800.00 | |
| Birth year, n (%) | |||
| <1900 | 0 (0%) | 26 (0.10%) | <0.0001 |
| 1900–1919 | 198 (15.54%) | 2104 (7.71%) | |
| 1920–1939 | 950 (74.57%) | 12,081 (44.30%) | |
| 1940–1959 | 125 (9.81%) | 11,314 (41.49%) | |
| ≥1960 | 1 (0.08%) | 1747 (6.41%) | |
| Year first employed, n (%) | |||
| <1960 | 956 (75.04%) | 11,780 (43.19%) | <0.0001 |
| 1960–1969 | 107 (8.40%) | 2426 (8.90%) | |
| 1970–1979 | 157 (12.32%) | 8496 (31.15%) | |
| 1980–1989 | 52 (4.08%) | 4475 (16.41%) | |
| ≥1990 | 2 (0.16%) | 95 (0.35%) |
| Characteristics | Squamous Cells (n = 391) | Adenocarcinoma (n = 249) | Large Cells (n = 225) | Small Cells (n = 181) | Other/Unspecified (n = 210) |
|---|---|---|---|---|---|
| Age at entry into study (years) | |||||
| Median | 32 | 29 | 33 | 30 | 34 |
| Mean ± SD | 32.9 ± 8.4 | 30.8 ± 8.6 | 33.7 ± 9.3 | 31.8 ± 8.5 | 35.2 ± 9.3 |
| Range | 17–61 | 17–62 | 17–63 | 19–61 | 17–65 |
| Total Duration of Employment (years) | |||||
| Median | 3.25 | 3 | 3 | 4 | 3 |
| Mean ± SD | 5.3 ± 5.2 | 4.2 ± 4.2 | 4.9 ± 4.7 | 6.0 ± 6.2 | 5.1 ± 5.2 |
| Range | 0.5–30 | 0.5–26.5 | 0.5–31 | 0.5–29 | 0.5–25.5 |
| Cumulative radon exposure (WLM) | |||||
| Median | 22.6 | 14.9 | 16.3 | 23.1 | 18.6 |
| Mean ± SD | 51.4 ± 75.3 | 37.0 ± 61.2 | 41.9 ± 57.2 | 55.7 ± 77.4 | 43.3 ± 78.0 |
| Range | 0–759.2 | 0–579.0 | 0–267.3 | 0–355.9 | 0–875.1 |
| Birth year, n (%) | |||||
| <1900 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 1900–1919 | 60 (15.35%) | 22 (8.84%) | 41 (18.22%) | 22 (12.15%) | 48 (22.86%) |
| 1920–1939 | 305 (78.01%) | 192 (77.11%) | 155 (68.89%) | 138 (76.24%) | 147 (70.00%) |
| 1940–1959 | 26 (6.65%) | 34 (13.65%) | 29 (12.89%) | 21 (11.60%) | 15 (7.14%) |
| ≥1960 | 0 (0%) | 1 (0.40%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Year first employed, n (%) | |||||
| <1960 | 306 (78.26%) | 180 (72.29%) | 156 (69.33%) | 136 (75.14%) | 160 (76.19%) |
| 1960–1969 | 32 (8.18%) | 20 (8.03%) | 20 (8.89%) | 16 (8.84%) | 19 (9.05%) |
| 1970–1979 | 39 (9.97%) | 35 (14.06%) | 39 (17.33%) | 23 (12.71%) | 21 (10.00%) |
| 1980–1989 | 14 (3.58%) | 12 (4.82%) | 10 (4.44%) | 6 (3.31%) | 10 (4.76%) |
| ≥1990 | 0 (0%) | 2 (0.80%) | 0 (0%) | 0 (0%) | 0 (0%) |
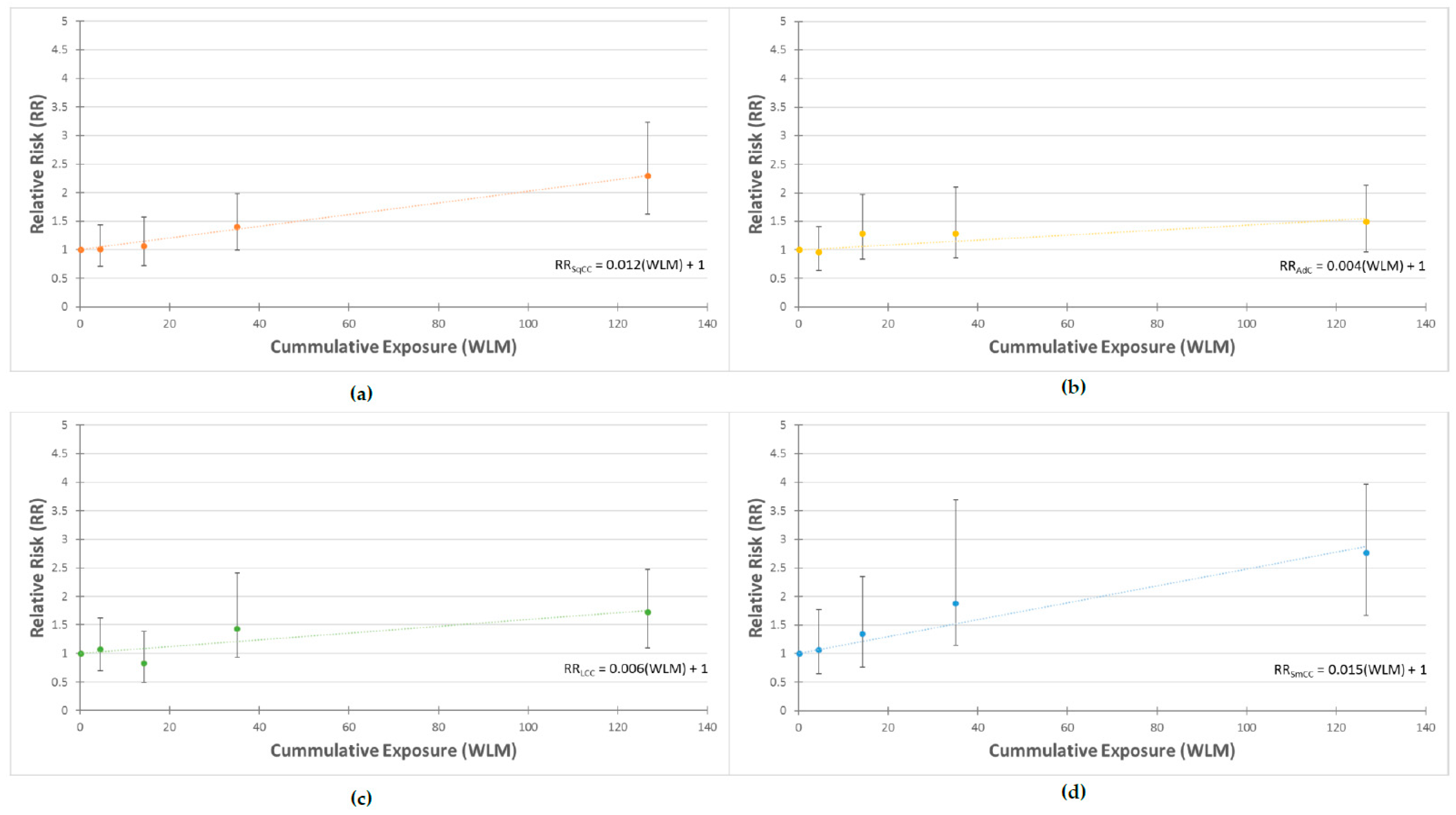
| Cumulative Exposure to Radon Progeny (WLM) | No Lag | 5 Year Lag | 10 Year Lag | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (n) | Relative Risk * (95% CI) | Test for Linear Trend p-Value | Cases (n) | Relative Risk * (95% CI) | Test for Linear Trend p-Value | Cases (n) | Relative Risk * (95% CI) | Test for Linear Trend p-Value | |
| All Lung Cancers | |||||||||
| <1 | 188 | Reference | <0.0001 | 188 | Reference | <0.0001 | 197 | Reference | <0.0001 |
| 1–10 | 290 | 0.91 (0.75, 1.09) | 290 | 1.00 (0.83, 1.21) | 286 | 1.05 (0.87, 1.26) | |||
| 10–20 | 185 | 1.03 (0.84, 1.27) | 186 | 1.13 (0.92, 1.39) | 183 | 1.17 (0.95, 1.44) | |||
| 20–60 | 313 | 1.30 (1.08, 1.57) | 312 | 1.40 (1.16, 1.68) | 313 | 1.46 (1.21, 1.76) | |||
| >60 | 298 | 1.84 (1.52, 2.22) | 298 | 1.98 (1.64, 2.40) | 295 | 2.03 (1.68, 2.45) | |||
| Squamous cells | |||||||||
| <1 | 49 | Reference | <0.0001 | 49 | Reference | <0.0001 | 54 | Reference | <0.0001 |
| 1–10 | 87 | 0.99 (0.69, 1.40) | 87 | 1.03 (0.72, 1.47) | 83 | 1.01 (0.71, 1.43) | |||
| 10–20 | 52 | 1.01 (0.68, 1.50) | 52 | 1.06 (0.71, 1.57) | 52 | 1.06 (0.72, 1.57) | |||
| 20–60 | 97 | 1.37 (0.96, 1.94) | 97 | 1.42 (1.00, 2.02) | 96 | 1.40 (0.99, 1.98) | |||
| >60 | 106 | 2.20 (1.55, 3.12) | 106 | 2.29 (1.62, 3.25) | 106 | 2.29 (1.63, 3.23) | |||
| Adenocarcinoma | |||||||||
| <1 | 44 | Reference | 0.2168 | 44 | Reference | 0.2471 | 45 | Reference | 0.1736 |
| 1–10 | 61 | 0.88 (0.59, 1.30) | 61 | 0.99 (0.67, 1.46) | 61 | 0.95 (0.64, 1.41) | |||
| 10–20 | 44 | 1.18 (0.77, 1.81) | 44 | 1.26 (0.82, 1.95) | 43 | 1.28 (0.84, 1.97) | |||
| 20–60 | 56 | 1.18 (0.78, 1.77) | 56 | 1.28 (0.85, 1.92) | 56 | 1.28 (0.85, 1.92) | |||
| >60 | 44 | 1.37 (0.89, 2.13) | 44 | 1.49 (0.96, 2.31) | 44 | 1.49 (0.96, 2.31) | |||
| Large cells | |||||||||
| <1 | 36 | Reference | 0.0088 | 36 | Reference | 0.0087 | 39 | Reference | 0.0148 |
| 1–10 | 56 | 0.92 (0.60, 1.40) | 56 | 1.03 (0.67, 1.57) | 55 | 1.07 (0.7, 1.62) | |||
| 10–20 | 27 | 0.79 (0.48, 1.31) | 27 | 0.88 (0.53, 1.46) | 26 | 0.83 (0.5, 1.39) | |||
| 20–60 | 55 | 1.20 (0.78, 1.86) | 55 | 1.32 (0.85, 2.04) | 56 | 1.43 (0.93, 2.19) | |||
| >60 | 51 | 1.68 (1.08, 2.62) | 51 | 1.84 (1.18, 2.88) | 49 | 1.72 (1.09, 2.69) | |||
| Small cells | |||||||||
| <1 | 28 | Reference | <0.0001 | 28 | Reference | <0.0001 | 28 | Reference | <0.0001 |
| 1–10 | 34 | 0.77 (0.46, 1.27) | 34 | 0.87 (0.52, 1.44) | 35 | 1.07 (0.64, 1.77) | |||
| 10–20 | 26 | 1.10 (0.64, 1.90) | 27 | 1.28 (0.74, 2.20) | 26 | 1.34 (0.77, 2.35) | |||
| 20–60 | 47 | 1.52 (0.94, 2.47) | 46 | 1.64 (1.00, 2.69) | 46 | 1.88 (1.15, 3.09) | |||
| >60 | 46 | 2.28 (1.39, 3.73) | 46 | 2.50 (1.52, 4.12) | 46 | 2.76 (1.67, 4.57) | |||
| Other/ Unspecified | |||||||||
| <1 | 29 | Reference | 0.0339 | 29 | Reference | 0.0323 | 29 | Reference | 0.0248 |
| 1–10 | 45 | 0.85 (0.53, 1.36) | 45 | 0.94 (0.58, 1.50) | 45 | 1.05 (0.65, 1.69) | |||
| 10–20 | 36 | 1.16 (0.71, 1.91) | 36 | 1.30 (0.79, 2.14) | 36 | 1.51 (0.92, 2.49) | |||
| 20–60 | 51 | 1.19 (0.74, 1.89) | 51 | 1.25 (0.78, 2.01) | 52 | 1.34 (0.83, 2.17) | |||
| >60 | 49 | 1.65 (1.03, 2.65) | 49 | 1.78 (1.10, 2.87) | 48 | 1.93 (1.19, 3.13) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramkissoon, A.; Navaranjan, G.; Berriault, C.; Villeneuve, P.J.; Demers, P.A.; Do, M.T. Histopathologic Analysis of Lung Cancer Incidence Associated with Radon Exposure among Ontario Uranium Miners. Int. J. Environ. Res. Public Health 2018, 15, 2413. https://doi.org/10.3390/ijerph15112413
Ramkissoon A, Navaranjan G, Berriault C, Villeneuve PJ, Demers PA, Do MT. Histopathologic Analysis of Lung Cancer Incidence Associated with Radon Exposure among Ontario Uranium Miners. International Journal of Environmental Research and Public Health. 2018; 15(11):2413. https://doi.org/10.3390/ijerph15112413
Chicago/Turabian StyleRamkissoon, Avinash, Garthika Navaranjan, Colin Berriault, Paul J. Villeneuve, Paul A. Demers, and Minh T. Do. 2018. "Histopathologic Analysis of Lung Cancer Incidence Associated with Radon Exposure among Ontario Uranium Miners" International Journal of Environmental Research and Public Health 15, no. 11: 2413. https://doi.org/10.3390/ijerph15112413
APA StyleRamkissoon, A., Navaranjan, G., Berriault, C., Villeneuve, P. J., Demers, P. A., & Do, M. T. (2018). Histopathologic Analysis of Lung Cancer Incidence Associated with Radon Exposure among Ontario Uranium Miners. International Journal of Environmental Research and Public Health, 15(11), 2413. https://doi.org/10.3390/ijerph15112413




