Effects of Weir Construction on Phytoplankton Assemblages and Water Quality in a Large River System
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Physical and Chemical Variables and Phytoplankton Density
2.3. Data Analysis
3. Results
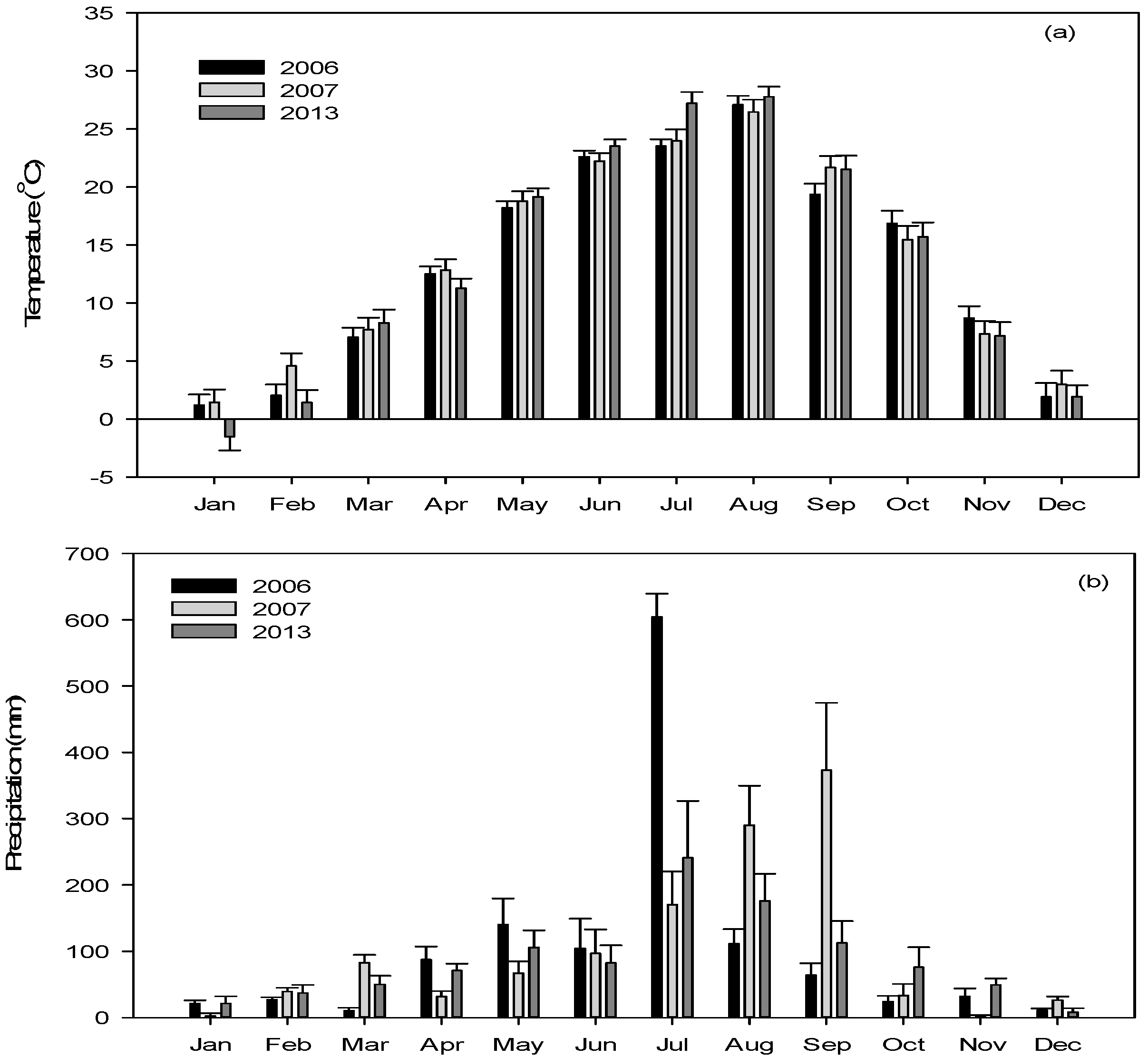
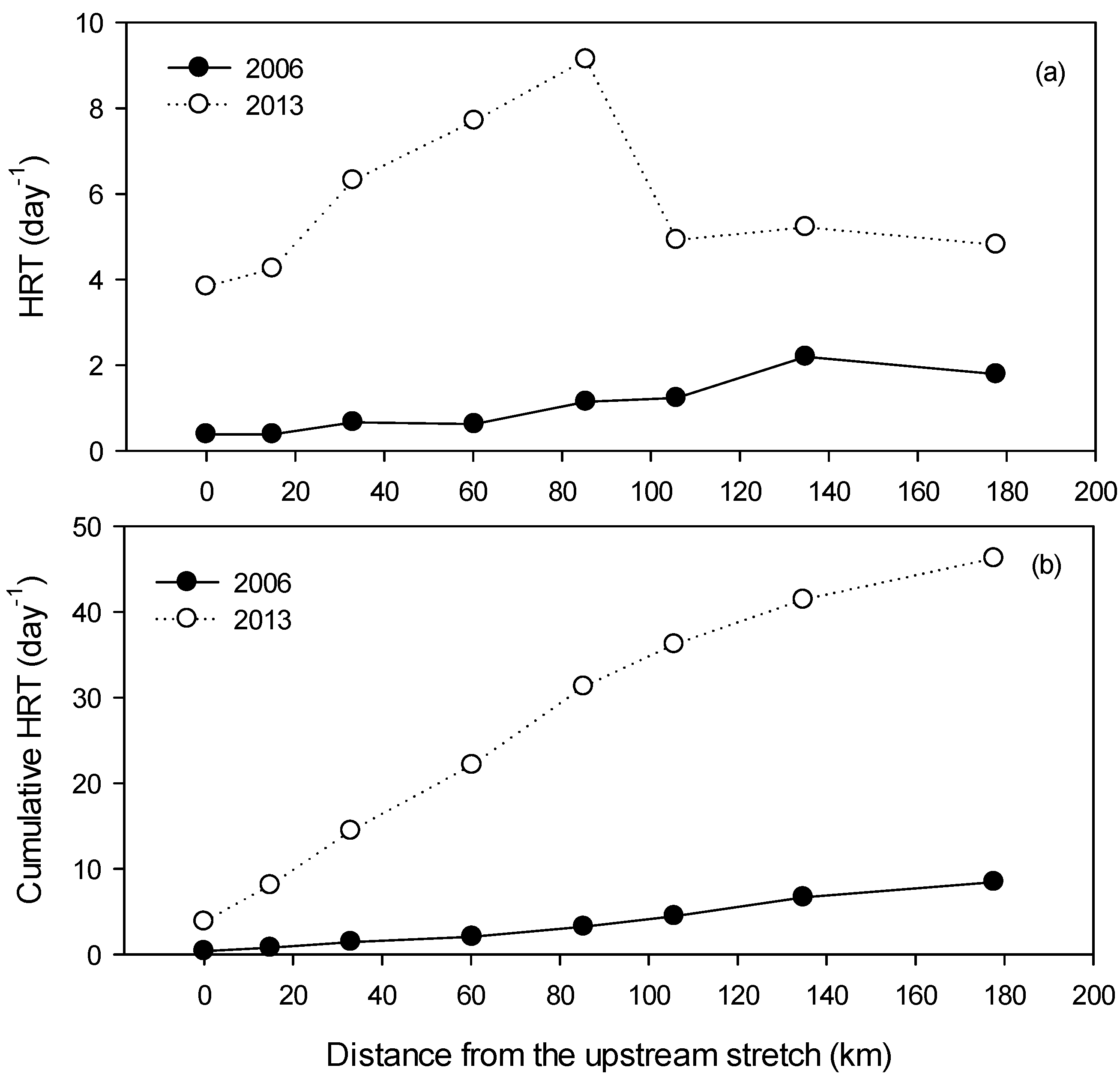
3.1. Atmospheric, Hydraulic, and Hydrological Conditions in the Weir Section
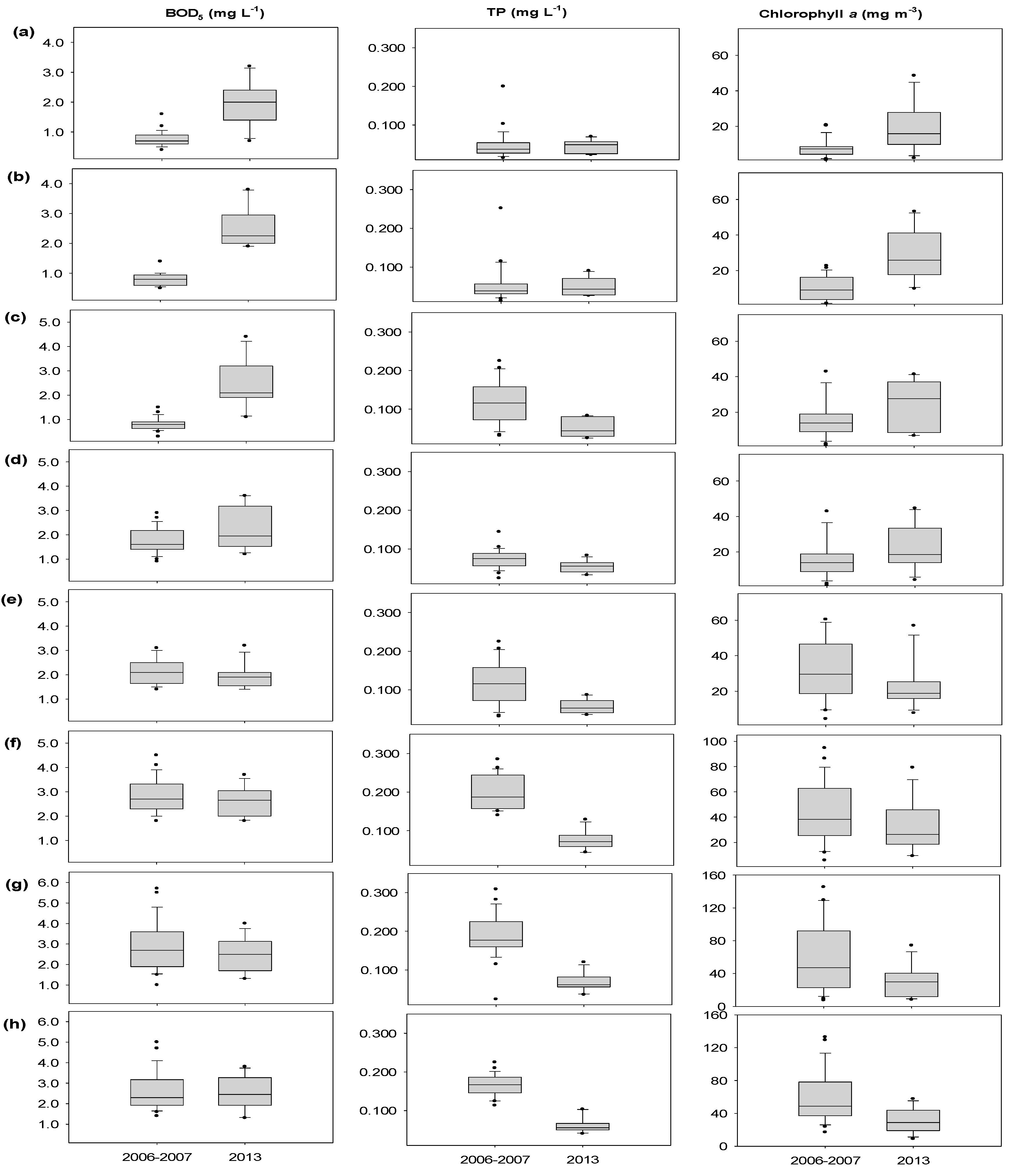
3.2. Variations in Physical and Chemical Parameters
3.3. Variations in Phytoplankton Density
3.4. Correlation between Phytoplankton Species and Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bain, M.B.; Fimm, J.T.; Booke, H.E. Streamflow regulation and fish community structure. Ecology 1988, 69, 382–392. [Google Scholar] [CrossRef]
- Humborg, C.; Ittekkot, V.; Cociasu, A.; Boudungen, B. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature 1997, 386, 385–388. [Google Scholar] [CrossRef]
- Kelly, V.J. Influence of reservoir on solute transport: A regional-scale approach. Hydrol. Process. 2001, 15, 1227–1249. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Guo, J.; Fang, F.; Cao, X.; Long, M. Responses of phytoplankton diversity to physical disturbance under manual operation in a large reservoir, China. Hydrobiologia 2012, 684, 45–56. [Google Scholar] [CrossRef]
- Poff, N.L.; Ward, J.V. Implications of streamflow variability and predictability for lotic community structure: A regional analysis of streamflow patterns. Can. J. Fish. Aquat. Sci. 1989, 46, 1805–1818. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; McCully, P.; Pringle, C.M. Global-scale environmental effects of hydrological alterations: Introduction. Bioscience 2000, 50, 746–751. [Google Scholar] [CrossRef]
- Horne, D.J.; Cohen, A.; Martens, K. Biology, taxonomy and identification techniques. In The Ostracoda: Applications in Quaternary Research, 1st ed.; Holmes, J.A., Chivas, A., Eds.; American Geophysical Union: Washington, DC, USA, 2002; pp. 6–36. [Google Scholar]
- Lindeman, R. The trophic dynamics aspect of ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [PubMed]
- Bowling, L.; Egan, S.; Holliday, J.; Honeyman, G. Did spatial and temporal variations in water quality influence cyanobacterial abundance, community composition and cell size in the Murray River, Australia during a drought-affected low-flow summer? Hydrobiologia 2016, 765, 356–377. [Google Scholar] [CrossRef]
- Istvánovics, V.; Honti, M.; Vörös, L.; Kozma, Z. Phytoplankton dynamics in relation to connectivity flow dynamics and resource availability—The case of a large, lowland river, the Hungarian Tisza. Hydrobiologia 2010, 637, 121–141. [Google Scholar] [CrossRef]
- Livingston, R.J. Eutrophication Processes in Coastal Systems: Origin and Succession of Plankton Blooms and Effect on Secondary Production in Gulf Coast Estuaries Center for Aquatic Research and Resources Management; CRC Press: Tallahassee, FL, USA, 2001. [Google Scholar]
- Perona, E.; Bonilla, I.; Mateo, P. Epilithic cyanobacterial communities and water quality: An alternative tool for monitoring eutrophication in the Alberche River (Spain). J. Appl. Phycol. 1998, 10, 183–191. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Descy, J.P. The production, biomass and structure of phytoplankton in large rivers. Large Rivers 1996, 18, 161–187. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Q.H.; Tan, L.; Kong, L.H. Longitudinal differences of phytoplankton community during a period of small water level fluctuations in a subtropical reservoir bay (Xingxi Bay, Three Gorges reservoir, China). Int. Rev. Hydrobiol. 2011, 96, 381–396. [Google Scholar] [CrossRef]
- Choi, A.R.; Oh, H.M.; Lee, J.A. Ecological study on the toxic Microcystis in the lower Nakdong River. Algae 2002, 17, 171–185. [Google Scholar] [CrossRef]
- Ha, K.; Kim, H.W.; Joo, G.J. The phytoplankton succession in the lower part of hypertrophic Nakdong River (Mulgum), South Korea. Hydrobiologia 1998, 369, 217–227. [Google Scholar] [CrossRef]
- Ha, K.; Jang, M.H.; Joo, G.J. Winter Stephanodiscus bloom development in the Nakdong River regulated by estuary dam and tributaries. Hydrobiologia 2003, 506, 221–227. [Google Scholar] [CrossRef]
- Li, Z.; Fang, F.; Chen, J.; Zhang, C.; Tian, G. Spring algal bloom and nutrients characteristics in Xiaojiang River backwater area, Three Gorges reservoir, 2007. J. Lake Sci. 2009, 21, 36–44. [Google Scholar]
- Meng, C.; Zhao, B. Study on the trend of eutrophication after impounding in Three Gorges reservoir. J. Agro-Environ. Sci. 2007, 26, 863–867. [Google Scholar]
- Hwang, D.J.; Lee, H.J.; Yoon, J.S.; Hur, S.N.; Lim, T.H.; Kwon, Y.H.; Shin, C.K.; Kim, H.W. Study on Ecological Characteristic of Algal Species Forming Bloom during Spring-Winter Season in the Nakdong River; NIER 2007-56-912; Nakdong River Environment Research Center: Goryeong, Korea; Sunchon National University: Seokhyeon-dong, Korea, 2007; pp. 1–144. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WDF): Washington, DC, USA, 2005.
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Freshwater Flora of Central Europe Vol. 19/1 Cyanoprokaryota, 1st Part: Chroococcales; Gustav Fischer Verlag: Jena, Germany, 1999. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Freshwater Flora of Central Europe, Volume 19/2 Cyanoprokaryota, Part 2: Oscillatoriales; Elsevier GmbH: München, Germany, 2005. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Cheon, S.U.; Lee, H.J.; Yu, J.J.; Park, H.K.; Lim, T.H.; Lee, J.J.; Lee, I.J.; Shin, S.H.; Yoon, J.S.; Lee, K.L.; et al. Changes of Water Environment and Phytoplankton Community Structures in the Nakdong River; NIER-RP 2013-245; Nakdong River Environment Research Center: Goryeong, Korea; Sunchon National University: Seokhyeon-dong, Korea, 2013; pp. 1–35. [Google Scholar]
- Ahn, J.M.; Lee, S.; Kang, T. Evaluation of dams and weirs operation for water resource management of the Geum River. Sci. Total Environ. 2014, 478, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.S.; Kim, J.S. The four major rivers restoration projects: Impact on river flows. J. Civ. Eng. 2011, 15, 217–224. [Google Scholar] [CrossRef]
- Seo, D.I.; Kim, M.; Ahn, J.H. Prediction of chlorophyll-a changes due to weir constructions in the Nakdong river using EFDC-WASP modeling. Environ. Eng. Res. 2012, 17, 95–102. [Google Scholar] [CrossRef]
- Wang, X.L.; Lu, L.Y.; He, G.Z.; Han, J.J.; Wang, T.Y. Exploration of relationship between phytoplankton biomass and related environmental variables using multivariate statistic analysis in a eutrophic shallow lake: A 5-year study. J. Environ. Sci. 2007, 19, 920–927. [Google Scholar] [CrossRef]
- Wehr, J.D.; Descy, J.P. Use of phytoplankton in large river management. J. Phycol. 1998, 34, 741–749. [Google Scholar] [CrossRef]
- Grücker, B.; Brauns, M.; Pusch, T. Effects of wastewater treatment plant discharge on ecosystem structure and function of lowland streams. J. N. Am. Benthol. Soc. 2006, 25, 313–329. [Google Scholar] [CrossRef]
- Harris, G.P. Phytoplankton Ecology: Structure, Function and Fluctuation; Chapman and Hall: London, UK, 1986. [Google Scholar]
- Kilham, P.A. Hypothesis concerning silica and the freshwater planktonic diatoms. Limnol. Oceanogr. 1971, 16, 10–18. [Google Scholar] [CrossRef]
- Krivtsov, V.; Bellinger, E.; Sigee, D. Ecological study of Stephanodiscus during a spring diatom bloom: Dynamics of intracellular elemental concentration and correlations in relation to water chemistry, and implications for overall chemical cycling in a temperate lake. Acta Oecol. 2003, 24, 265–274. [Google Scholar] [CrossRef]
- van Donk, E.; Kilham, S.S. Temperature effects on silicon- and phosphorus-limited growth and competitive interactions among three diatoms. J. Phycol. 1990, 26, 40–50. [Google Scholar] [CrossRef]
- Salmaso, N.; Braioni, M.G. Factors controlling the seasonal development and distribution of the phytoplankton community in the lowland course of a large river in Northern Italy (River Adige). Aquat. Ecol. 2008, 42, 533–545. [Google Scholar] [CrossRef]
- Kölhler, J. Origin and succession of phytoplankton in a river-lake system (Spree, Germany). Hydrobiologia 1994, 289, 78–83. [Google Scholar]
- Hilton, J.; O’Hare, M.; Bowes, M.J.; Jones, J.I. How green is my river? A new paradigm of eutrophication in rivers. Sci. Total Environ. 2006, 365, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.C.; Chu, Z.C.; Yi, W.L.; Hu, X.Z. Influence of phosphorus on Microcystis growth and the changes of other environmental factors. J. Environ. Sci. (China) 2005, 17, 931–941. [Google Scholar]
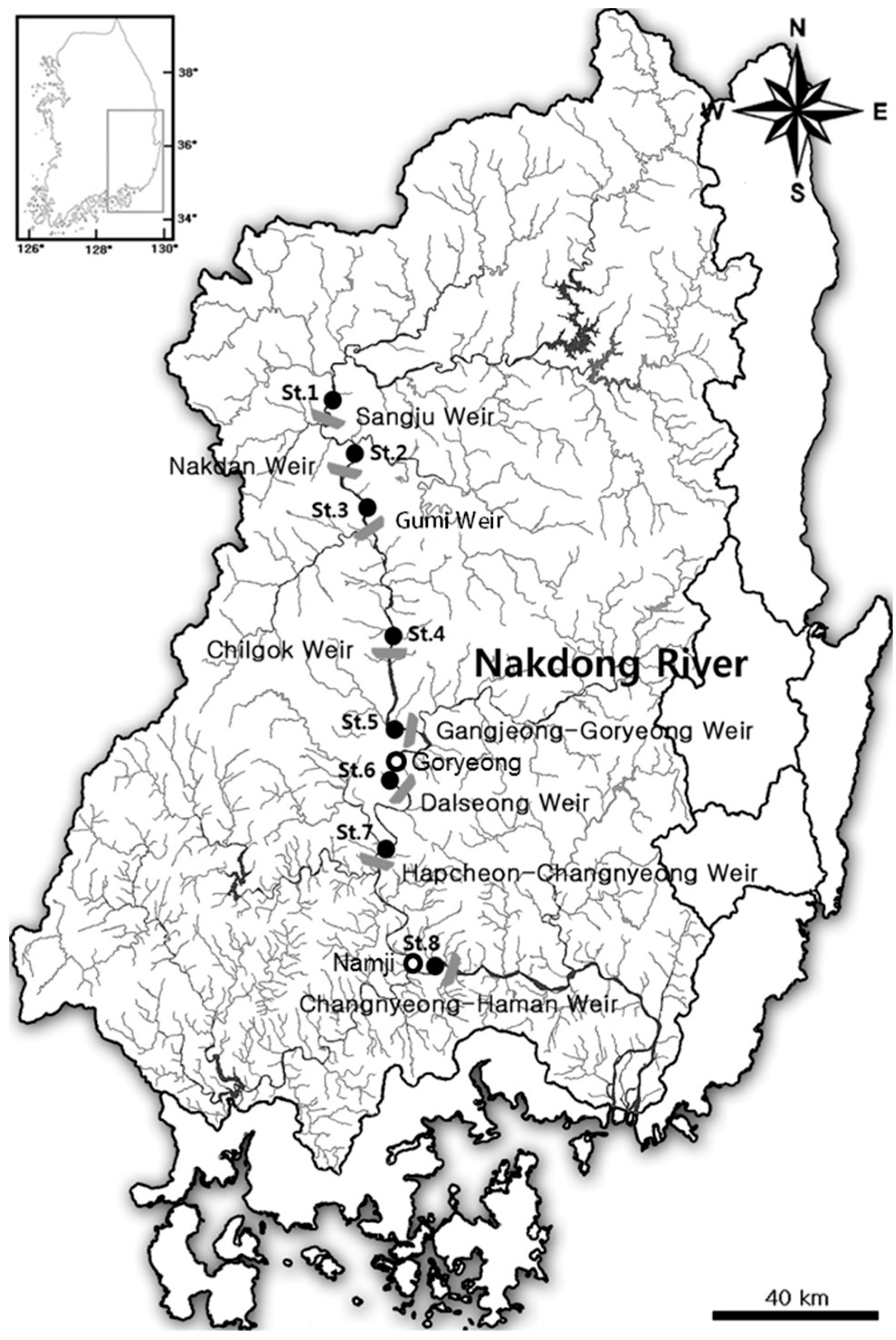



| Site | Elevation (EL m) | Distance (km) | Volume (106 m3) | Catchment Area (km2) | Average Depth (m) | Geographic Coordinates (Latitude/Longitude) |
|---|---|---|---|---|---|---|
| Sangju (St. 1) | 47.0 | 28.2 | 27 | 7407 | 11.0 | 36°25′51.1″ N/128°15′5.19″ E |
| Nakdan (St. 2) | 40.0 | 15.7 | 35 | 9221 | 11.5 | 36°21′28.16″ N/128°18′27.96″ E |
| Gumi (St. 3) | 32.5 | 18.2 | 53 | 9557 | 12.0 | 36°14′2.11″ N/128°20′52.48″ E |
| Chilgok (St. 4) | 25.5 | 27.5 | 75 | 11,040 | 11.5 | 36°0′45.51″ N/128°24′3.58″ E |
| Gangjeong-Goryeong (St. 5) | 19.5 | 26.2 | 92 | 11,667 | 10.5 | 35°50′27.73″ N/128°27′31.1″ E |
| Dalseong (St. 6) | 14.0 | 19.2 | 59 | 14,248 | 10.5 | 35°43′55.18″ N/128°25′10.9″ E |
| Hapcheon-Changnyeong (St. 7) | 10.5 | 29.8 | 70 | 15,074 | 9.0 | 35°35′16.13″ N/128°21′29.39″ E |
| Changnyeong-Haman (St. 8) | 5.0 | 42.5 | 101 | 20,697 | 13.2 | 35°22′39.42″ N/128°33′15.96″ E |
| Parameter | Year(s) | Sangju (St. 1) | Nakdan (St. 2) | Gumi (St. 3) | Chilgok (St. 4) | Gangjeong-Goryeong (St. 5) | Dalseong (St. 6) | Hapcheon-Changnyeong (St. 7) | Changnyeong-Haman (St. 8) |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 2006–2007 2013 | 15.5 ± 8.4 17.4 ± 9.8 | 15.2 ± 8.7 19.1 ± 8.7 * | 15.5 ± 8.9 18.5 ± 10.0 * | 15.4 ± 8.0 17.1 ± 110.5 | 16.2 ± 8.9 16.4 ± 10.0 | 16.3 ± 8.3 17.1 ± 9.8 | 16.5 ± 8.7 17.5 ± 9.9 | 16.4 ± 8.5 17.9 ± 10.3 |
| pH | 2006–2007 2013 | 7.9 ± 0.2 8.4 ± 0.4 * | 7.8 ± 0.2 8.6 ± 0.2 *** | 7.9 ± 0.2 8.6 ± 0.4 *** | 7.8 ± 0.3 8.4 ± 0.4 *** | 8.2 ± 0.4 8.2 ± 0.3 | 8.0 ± 0.4 8.2 ± 0.3 * | 7.7 ± 0.7 8.3 ± 0.3 ** | 7.6 ± 0.6 8.4 ± 0.3 ** |
| DO (mg·L−1) | 2006–2007 2013 | 9.2 ± 2.1 11.5 ± 1.3 * | 8.9 ± 2.1 11.1 ± 1.7 *** | 9.1 ± 2.1 11.2 ± 1.9 *** | 9.1 ± 2.1 11.3 ± 2.3 *** | 11.4 ± 2.1 11.2 ± 2.6 | 10.7 ± 2.2 11.5 ± 2.6 * | 11.0 ± 3.1 11.5 ± 2.6 | 10.9 ± 2.7 11.4 ± 2.2 |
| Conductivity (µS·cm−1) | 2006–2007 2013 | 125 ± 25 189 ± 27 *** | 128 ± 22 206 ± 23 *** | 129 ± 25 195 ± 24 *** | 202 ± 50 244 ± 42 * | 250 ± 48 244 ± 40 | 383 ± 107 349 ± 82 | 305 ± 58 324 ± 71 | 255 ± 37 268 ± 50 |
| BOD5 (mg·L−1) | 2006–2007 2013 | 0.8 ± 0.2 1.9 ± 0.7 *** | 0.8 ± 0.2 2.5 ± 0.7 *** | 0.8 ± 0.2 2.4 ± 1.0 *** | 1.8 ± 0.4 2.3 ± 0.9 | 2.2 ± 0.5 1.9 ± 0.5 | 2.8 ± 0.7 2.6 ± 0.6 | 2.9 ± 1.0 2.5 ± 0.8 | 2.6 ± 0.9 2.6 ± 0.8 |
| CODMn (mg·L−1) | 2006–2007 2013 | 3.1 ± 0.9 4.3 ± 0.8 ** | 3.2 ± 1.0 5.3 ± 1.0 *** | 3.3 ± 0.9 5.3 ± 1.2 *** | 4.3 ± 0.9 5.5 ± 1.2 ** | 4.9 ± 0.7 5.4 ± 0.9 | 6.2 ± 0.9 6.6 ± 1.0 | 6.3 ± 1.0 6.4 ± 1.2 | 5.9 ± 1.0 6.2 ± 1.2 |
| Chlorophyll a (mg·m−3) | 2006–2007 2013 | 7.7 ± 3.7 18.6 ± 12.7 * | 9.9 ± 5.6 29.3 ± 14.1 * | 11.5 ± 7.1 24.6 ± 13.0 ** | 17.2 ± 13.4 23.2 ± 12.8 | 33.6 ± 18.4 22.9 ± 13.2 | 44.2 ± 23.1 32.7 ± 19.8 | 57.0 ± 36.0 29.6 ± 19.3 * | 58.7 ± 29.4 31.2 ± 14.7 * |
| TN (mg·L−1) | 2006–2007 2013 | 2.457 ± 0.288 2.506 ± 0.331 | 2.509 ± 0.336 2.470 ± 0.315 | 2.478 ± 0.344 2.465 ± 0.391 | 3.162 ± 0.610 2.869 ± 0.494 * | 2.830 ± 0.477 2.718 ± 0.542 | 4.045 ± 0.926 3.699 ± 0.829 ** | 3.669 ± 0.863 3.343 ± 0.718 * | 3.249 ± 0.667 2.944 ± 0.641 ** |
| NH3+-N (mg·L−1) | 2006–2007 2013 | 0.069 ± 0.050 0.049 ± 0.050 | 0.061 ± 0.036 0.040 ± 0.019 ** | 0.052 ± 0.027 0.053 ± 0.040 | 0.290 ± 0.268 0.099 ± 0.066 ** | 0.115 ± 0.099 0.083 ± 0.044 | 0.194 ± 0.147 0.108 ± 0.052 * | 0.088 ± 0.071 0.090 ± 0.051 | 0.064 ± 0.062 0.072 ± 0.041 |
| NO3−-N (mg·L−1) | 2006–2007 2013 | 2.172 ± 0.360 2.011 ± 0.343 | 2.272 ± 0.336 1.839 ± 0.420 | 2.263 ± 0.344 1.841 ± 0.411 ** | 2.551 ± 0.426 2.101 ± 0.480 *** | 2.319 ± 0.038 1.960 ± 0.502 *** | 3.199 ± 0.813 2.722 ± 0.775 *** | 2.388 ± 0.604 2.456 ± 0.704 | 2.014 ± 0.439 2.095 ± 0.062 |
| TP (mg·L−1) | 2006–2007 2013 | 0.047 ± 0.026 0.044 ± 0.017 | 0.053 ± 0.038 0.050 ± 0.023 | 0.054 ± 0.038 0.051 ± 0.025 | 0.116 ± 0.047 0.057 ± 0.018 ** | 0.074 ± 0.017 0.054 ± 0.015 * | 0.200 ± 0.037 0.075 ± 0.025 *** | 0.189 ± 0.037 0.069 ± 0.024 *** | 0.165 ± 0.021 0.063 ± 0.020 *** |
| PO43−-P (mg·L−1) | 2006–2007 2013 | 0.018 ± 0.010 0.009 ± 0.009 | 0.019 ± 0.012 0.006 ± 0.007 ** | 0.016 ± 0.013 0.008 ± 0.008 * | 0.070 ± 0.037 0.012 ± 0.009 *** | 0.016 ± 0.010 0.010 ± 0.006 | 0.121 ± 0.041 0.013 ± 0.011 *** | 0.097 ± 0.023 0.013 ± 0.011 *** | 0.071 ± 0.011 0.008 ± 0.008 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Park, H.-K.; Cheon, S.-U. Effects of Weir Construction on Phytoplankton Assemblages and Water Quality in a Large River System. Int. J. Environ. Res. Public Health 2018, 15, 2348. https://doi.org/10.3390/ijerph15112348
Lee H-J, Park H-K, Cheon S-U. Effects of Weir Construction on Phytoplankton Assemblages and Water Quality in a Large River System. International Journal of Environmental Research and Public Health. 2018; 15(11):2348. https://doi.org/10.3390/ijerph15112348
Chicago/Turabian StyleLee, Hae-Jin, Hae-Kyung Park, and Se-Uk Cheon. 2018. "Effects of Weir Construction on Phytoplankton Assemblages and Water Quality in a Large River System" International Journal of Environmental Research and Public Health 15, no. 11: 2348. https://doi.org/10.3390/ijerph15112348
APA StyleLee, H.-J., Park, H.-K., & Cheon, S.-U. (2018). Effects of Weir Construction on Phytoplankton Assemblages and Water Quality in a Large River System. International Journal of Environmental Research and Public Health, 15(11), 2348. https://doi.org/10.3390/ijerph15112348





