Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains
Abstract
1. Introduction
2. Polyphenols
2.1. Structure and Division of Polyphenols
2.2. Structure-Antibacterial Activity Relationship
3. Antistaphylococcal Phenolic Compounds
3.1. Flavonols
3.2. Flavanols
3.3. Non-Flavonoids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aldulaimi, O.A. General overview of phenolics from plant to laboratory, good antibacterials or not. Pharmacogn. Rev. 2017, 11, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Mularz, T.; Idzik, D. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative staphylococcus strains in vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Investig. 2003, 111, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The combination of catechin and epicatechin gallate from Fructus Crataegi potentiates beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef] [PubMed]
- Miklasińska, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Zdebik, A.; Orlewska, K.; Wąsik, T.J. Antibacterial activity of protocatechuic acid ethyl ester on Staphylococcus aureus clinical strains alone and in combination with antistaphylococcal drugs. Molecules 2015, 20, 13536–13549. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W.; Stapleton, P.D.; Paul, L.J. New ways to treat bacterial infections. Drug Discov. 2002, 7, 1086–1091. [Google Scholar] [CrossRef]
- Kyaw, B.M.; Arora, S.; Lim, C.S. Bactericidal antibiotic-phytochemical combinations against methicillin resistant Staphylococcus aureus. Braz. J. Microbiol. 2012, 43, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kępa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wąsik, T.J. Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.; Ip, M.; Lau, C.B.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Litaudon, M.; Reiner, N.E.; Gong, H.; See, R.H.; et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J. Ethnopharmacol. 2011, 137, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Cuschnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Jiang, Y.; Xia, L.; Xiang, H.; Feng, H.; Pu, S.; Huang, N.; Yu, L.; Deng, X. Subinhibitory concentrations of licochalcone A decrease alpha-toxin production in both methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Appl. Microbiol. 2010, 50, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Anderson, J.C.; Hara, Y.; Hamilton-Miller, J.M.; Taylor, P.W. Modulation of beta-lactam resistance in Staphylococcus aureus by catechins and gallates. Int. J. Antimicrob. Agents 2004, 23, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Cuschnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Miklasińska, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Dziedzic, A.; Wąsik, T.J. Catechin hydrate augments the antibacterial action of selected antibiotics against Staphylococcus aureus clinical strains. Molecules 2016, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Zacchino, S.A.; Butassi, E.; Liberto, M.D.; Raimondi, M.; Postigo, A.; Sortino, M. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 15, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Chem. Int. Ed. Engl. 2011, 17, 586–621. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Inst. Super Sanita 2007, 43, 348–361. [Google Scholar]
- Pereira, D.M.; Valentao, P.; Pereira, J.A.; Andrade, P.B. Phenolisc: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Liu, X.L.; Xu, Y.J.; Go, M.L. Functionalized chalcones with basic functionalities have antibacterial activity against drug sensitive Staphylococcus aureus. Eur. J. Med. Chem. 2008, 43, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Avila, H.P.; Smânia, F.A.; Monache, F.D.; Smânia Júnior, A. Structure-activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Boesen, T.; Larsen, M.; Schonning, K.; Kromann, H. Antibacterial chalcones-bioisosteric replacement of the 4’-hydroxy group. Bioorg. Med. Chem. 2004, 12, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, Z.; Kedzia, B.; Schroeder, G. Synthesis, physicochemical properties and antimicrobial evaluation of new (E)-chalcones. Eur. J. Med. Chem. 2008, 43, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 44, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Babu, T.H.; Srinivas, P.V.; Sastry, B.S.; Kishore, K.H.; Murty, U.S.N. Synthesis and in vitro study of novel 7-O-acyl derivatives of oroxylin A as antibacterial agents. Bioorg. Med. Chem. Lett. 2005, 15, 3953–3956. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Alcaraz, L.E.; Blanco, S.E.; Puig, O.N.; Tomas, F.; Ferretti, F.H. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol. 2000, 205, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, K.; Chudik, S.; Klouˇcek, P.; Marek, R.; Cvaˇcka, J.; Urbanova, M. Antibacterial C-geranylflavonoids from Paulownia tomentosa fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, N.; Liu, M.H.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T. Anti-methicillin resistant Staphylococcus aureus (MRSA) compounds isolated from Laurus nobilis. Biol. Pharm. Bull. 2008, 31, 1794–1797. [Google Scholar] [CrossRef] [PubMed]
- Mughal, E.U.; Ayaz, M.; Hussain, Z.; Hasan, A.; Sadiq, A.; Riaz, M. Synthesis and antibacterial activity of substituted flavones, 4-thioflavones and 4-iminoflavones. Bioorg. Med. Chem. 2006, 14, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Babu, T.H.; Srinivas, P.V.; Kishore, K.H.; Murthy, U.S.N.; Rao, J.M. Synthesis and biological evaluation of novel C (7) modified chrysin analogues as antibacterial agents. Bioorg. Med. Chem. Lett. 2006, 16, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The β-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Hamilton, V.E.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the antibacterial activity of 3-O-octanoyl-(−)-epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Hamilton-Miller, J.M.; Hara, Y.; Nagaoka, Y.; Kumagai, A.; Uesato, S.; Taylor, P.W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, J.G.; Lee, T.H.; Oh, K.B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.D.; Chin, Y.P.; Hou, W.C.; Lee, M.H. The effects of antibiotics combined with natural polyphenols against clinical methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Bernard, F.X.; Sable, S.; Cameron, B.; Provost, J.; Desnottes, J.F.; Crouzet, J.; Blanche, F. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrob. Agents. Chemother. 1997, 41, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic effect of kaempferol glycosides purified from Laurus nobilis and fluoroquinolones on methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Novy, P.; Rondevaldova, J.; Kourimska, L.; Kokoska, L. Synergistic interactions of epigallocatechin gallate and oxytetracycline against various drug resistant Staphylococcus aureus strains in vitro. Phytomedicine 2013, 20, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Schiller, N.L.; Oh, K.H. Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus. Curr. Microbiol. 2008, 57, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Zhao, W.H.; Yoda, Y.; Asano, N.; Hara, Y.; Shimamura, T. Additive, indifferent and antagonistic effects in combinations of epigallocatechin gallate with 12 non β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2002, 50, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Sudano Roccaro, A.; Blanco, A.R.; Giuliano, F.; Rusciano, D.; Enea, V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob. Agents Chemother. 2004, 48, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Headley, C.; Stapleton, P.D.; Taylor, P.W. Synthesis and antibacterial activity of hydrolytically stable (−)-epicatechin gallate analogues for the modulation of β-lactam resistance in Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2005, 15, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Cho, S.J. Synthesis and antimicrobial activities of 3-O-alkyl analogues of (+)-catechin: Improvement of stability and proposed action mechanism. Eur. J. Med. Chem. 2010, 45, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. Biomed Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cheng, K.; Zhang, Z.M.; Fang, R.Q.; Zhu, H.L. Synthesis, structure and structure-activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.N.; Oliveira-Tintino, C.D.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.; Cruz, R.P.; Menezes, I.R.; et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: Gallic acid, caffeic acid and pyrogallol. Microb. Pathog. 2016, 99, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Wang, W.; Bai, F.; Zhu, J.; Li, J.; Li, X. Antimicrobial effect and membrane-active mechanism of tea polyphenols against Serratia marcescens. World J. Microbiol. Biotechnol. 2014, 30, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Yoda, Y.; Hu, Z.Q.; Zhao, W.H.; Shimamura, T. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Hara, Y.; Taylor, P.W. Potentiation of catechin gallate-mediated sensitization of Staphylococcus aureus to oxacillin by nongalloylated catechins. Antimirob. Agents. Chemoter. 2006, 50, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N.; Vera-Samper, E.; Villalaín, J.; Mateo, C.R.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Steric effects on interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 2001, 65, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Kumazawa, S.; Nakayama, T. Effects of external factors on the interaction of tea catechins with lipid bilayers. Biosci. Biotechnol. Biochem. 2002, 66, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Xiao, P.; Fujita, K. Anti-MRSA activity of alkyl gallates. Bioorg. Med. Chem. Lett. 2002, 12, 113–116. [Google Scholar] [CrossRef]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The polyphenol (−)-epicatechin gallate disrupts the secretion of virulence-related proteins by Staphylococcus aureus. Lett. Appl. Microbiol. 2008, 46, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Zloh, M.; Taylor, P.W. Disruption of D-alanyl esterification of Staphylococcus aureus cell wall teichoic acid by the b-lactam resistance modifier (2)-epicatechin gallate. J. Antimicrob. Chemother. 2009, 63, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Yam, T.S.; Hamilton-Miller, J.M.; Shah, S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2’ synthesis, and beta-lactamase production in Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Taylor, P.W. Methicillin resistance in Staphylococcus aureus: Mechanisms and modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Lemaire, S.; Pinho, M.G.; Mobashery, S.; Hinds, J.; Taylor, P.W. Insertion of epicatechin gallate into the cytoplasmic membrane of methicillin-resistant Staphylococcus aureus disrupts penicillin-binding protein (PBP) 2a-mediated beta-lactam resistance by delocalizing PBP2. J. Biol. Chem. 2010, 285, 24055–24065. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Moser, E.; Kaatz, G.W. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta Med. 2004, 70, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.R.; Sudano-Roccaro, A.; Spoto, G.C.; Nostro, A.; Rusciano, D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob. Agents 2005, 49, 4339–4343. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.; Shah, S. Activity of the tea component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 46, 852–853. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Zhao, W.H.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergismwith ampicillin/sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.W.; Sharili, A.S.; Phee, L.M.; Wareham, D.W. In vitro activity of epigallocatechin gallate and quercetin alone and in combination versus clinical isolates of methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2015, 78, 2145–2148. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; Sato, Y.; Murakami, K.; Takaishi, Y.; Arakaki, N.; Higuti, T. Alkyl gallates intensifiers of β-lactam susceptibilities to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Nakano, T.; Parvez, M.A.; Furukawa, Y.; Tomoishi, A.; Niimi, S.; Arakaki, N.; Higuti, T. Triple combinations of lower and lower alkyl gallates and oxacillin improve antibiotic synergy against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 2218–2220. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of Northeastern Portugal wild plant extracts and respective phenolic compounds. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.J.R.; Alberto, M.R.; Nadra, M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Nquefack, B.; Budde, B.; Jakobsen, M. Five essential oils from aromatic plants of Cameroon: Their antibacterial activity and ability to permeabilize the cytoplasmic membrane of Listeria innocua examined by flow cytometry. Lett. Appl. Microbiol. 2004, 39, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Bouix, M.; Abedrabba, M.; Leveau, J.Y.; Hamdi, M. Mechanism of action of Melaleuca armillaris (Sol. Ex Gaertu) Sm. essential oil on six lab strains as assessed by multiparametric flow cytometry and automated microtiter-based assay. Food Chem. 2008, 111, 707–718. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković M., J.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
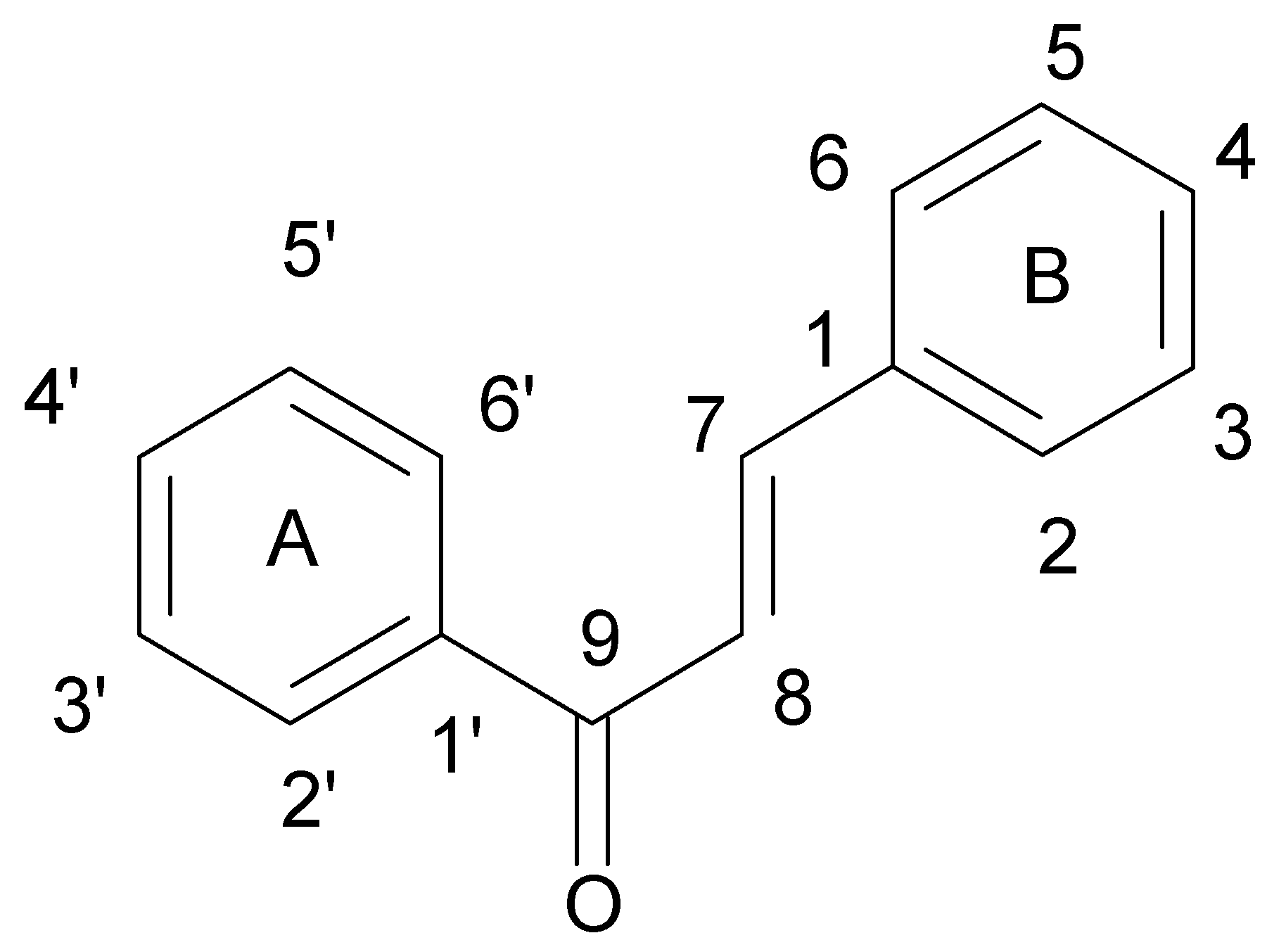
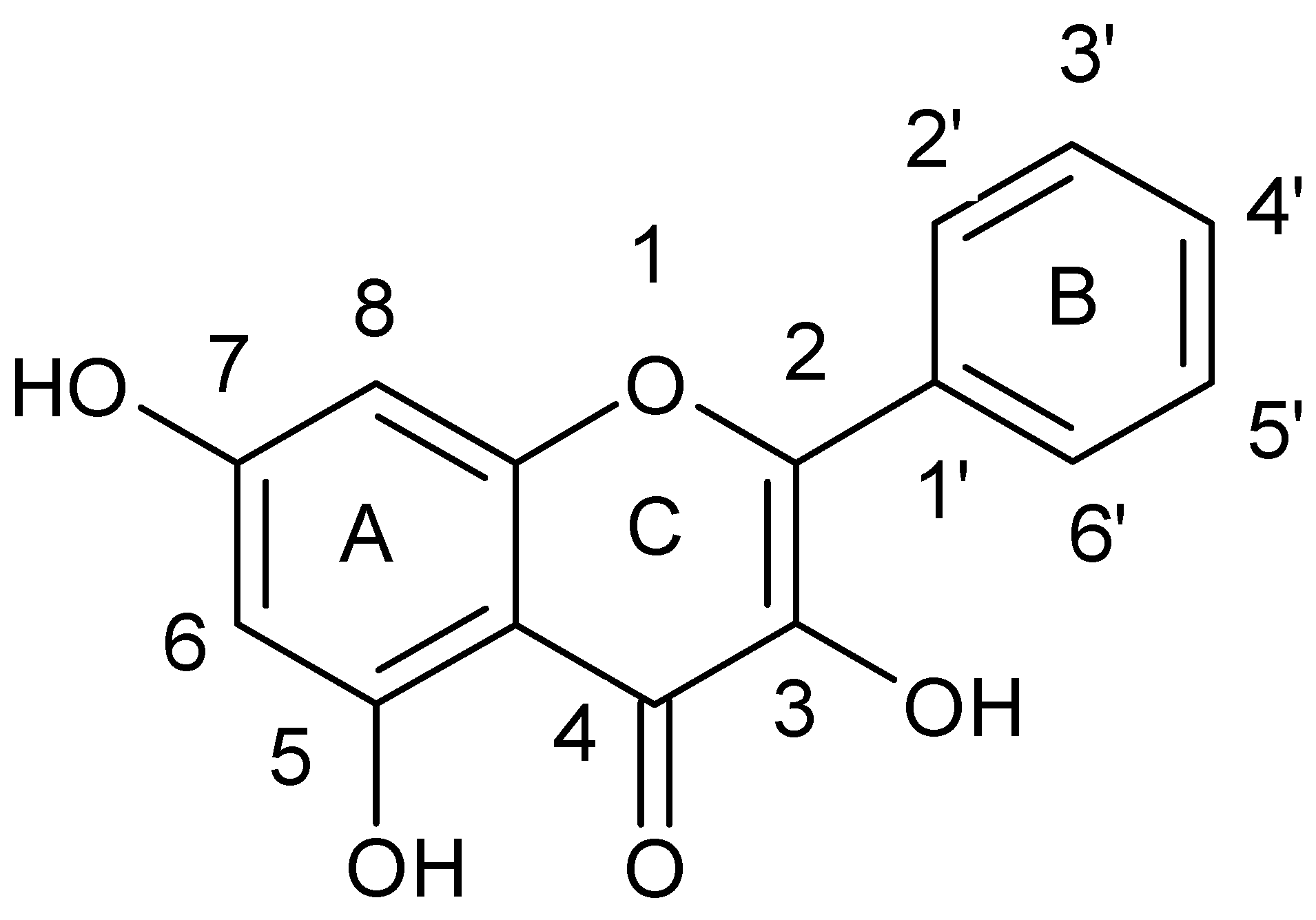
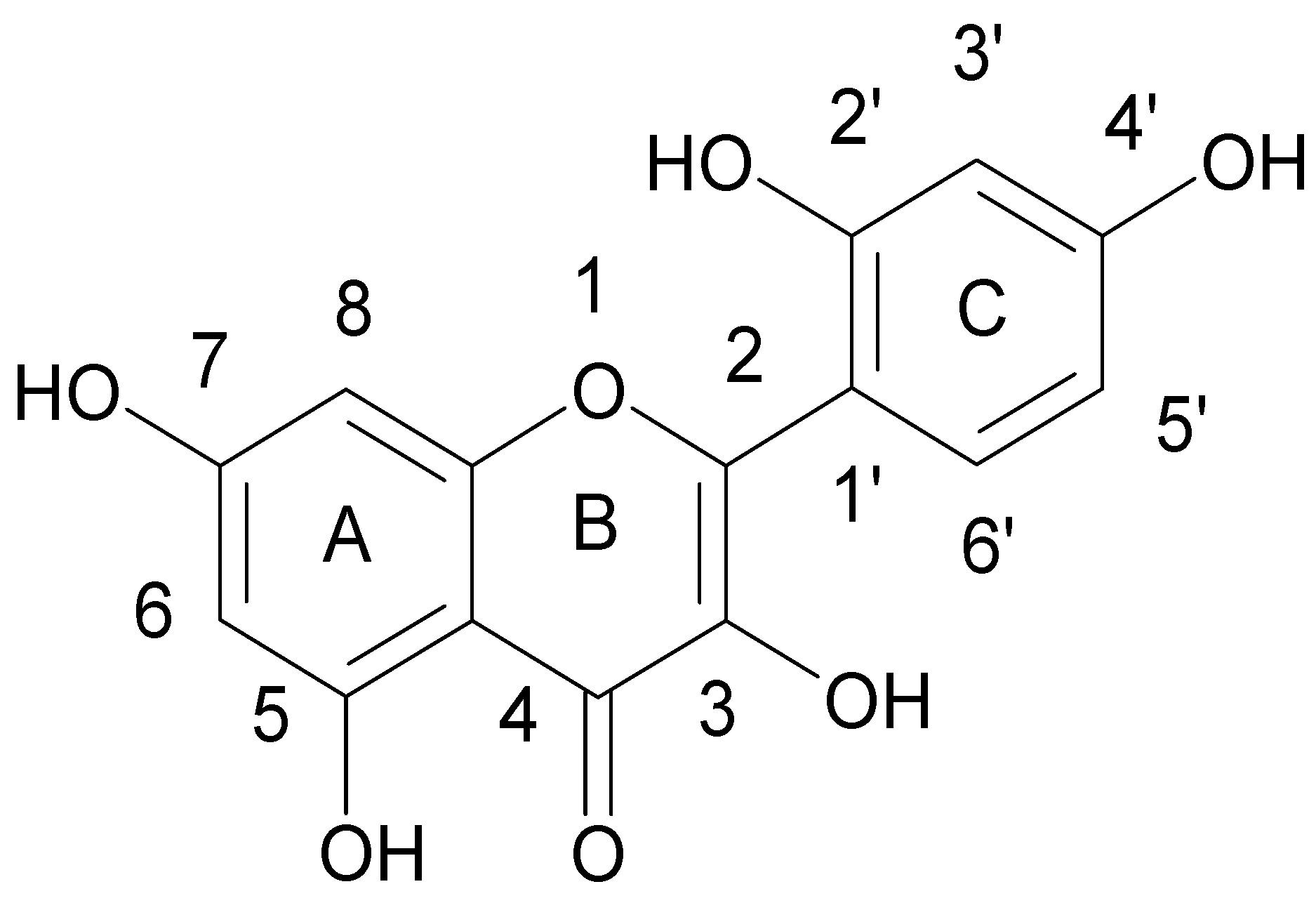
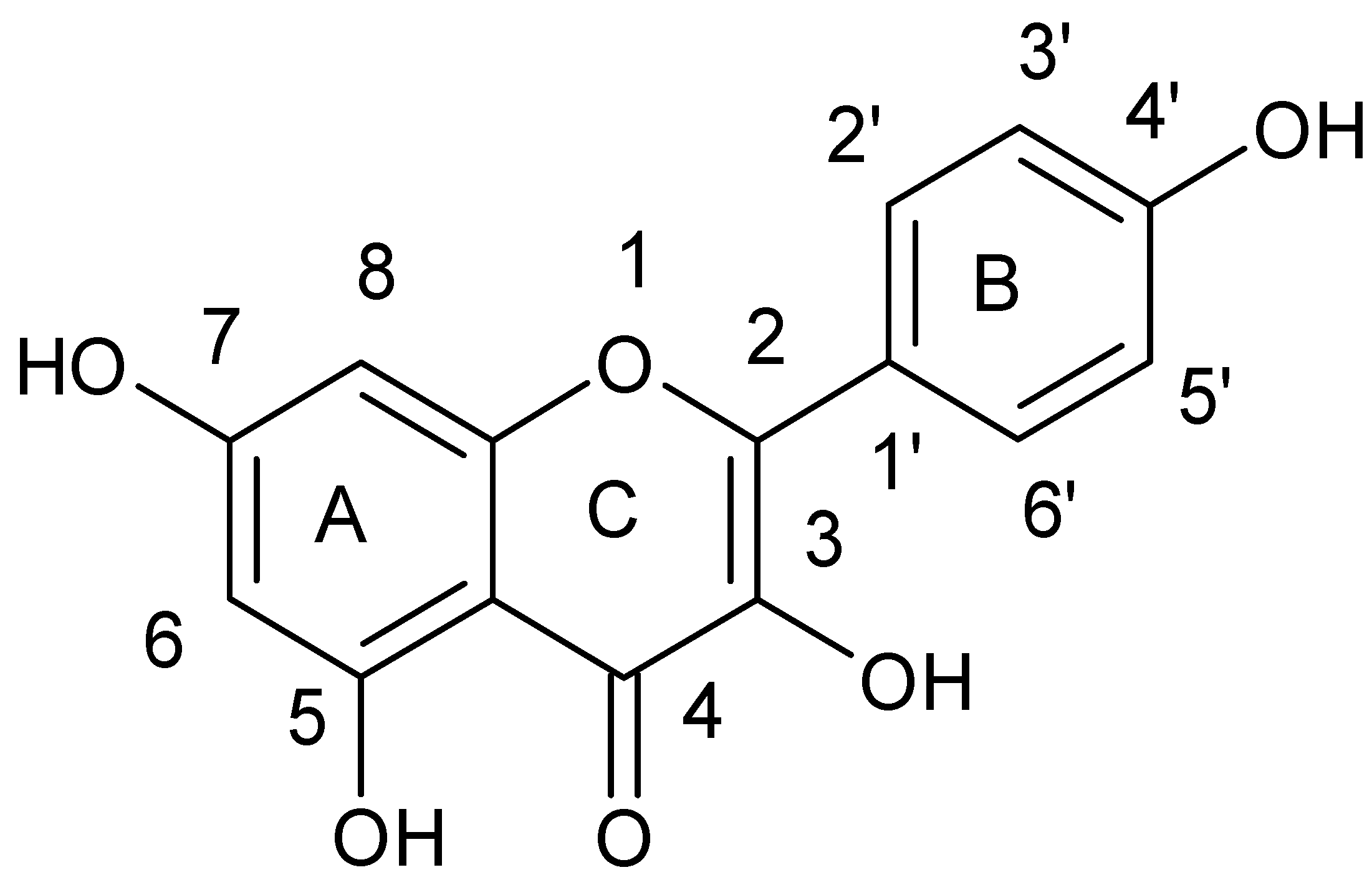
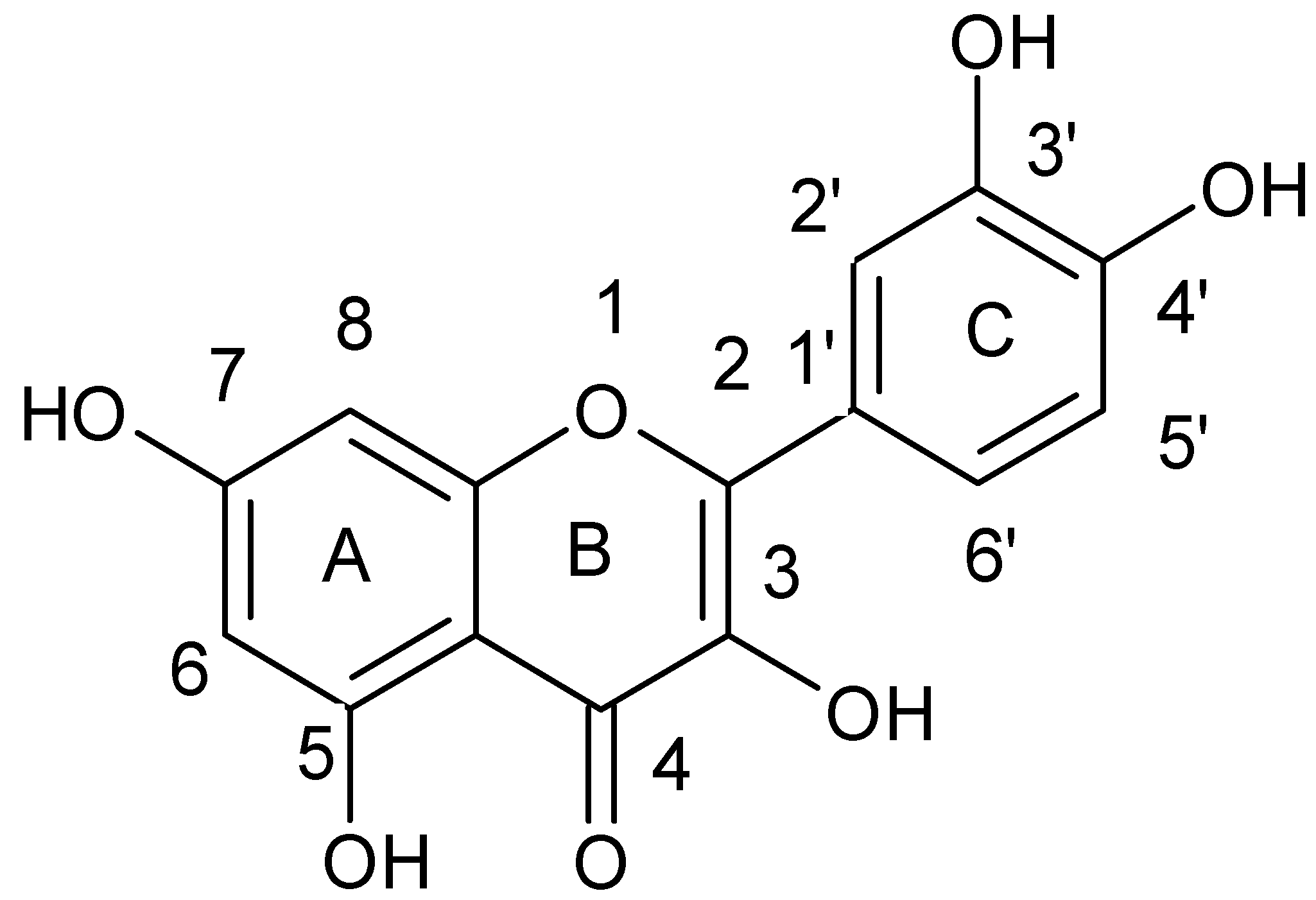
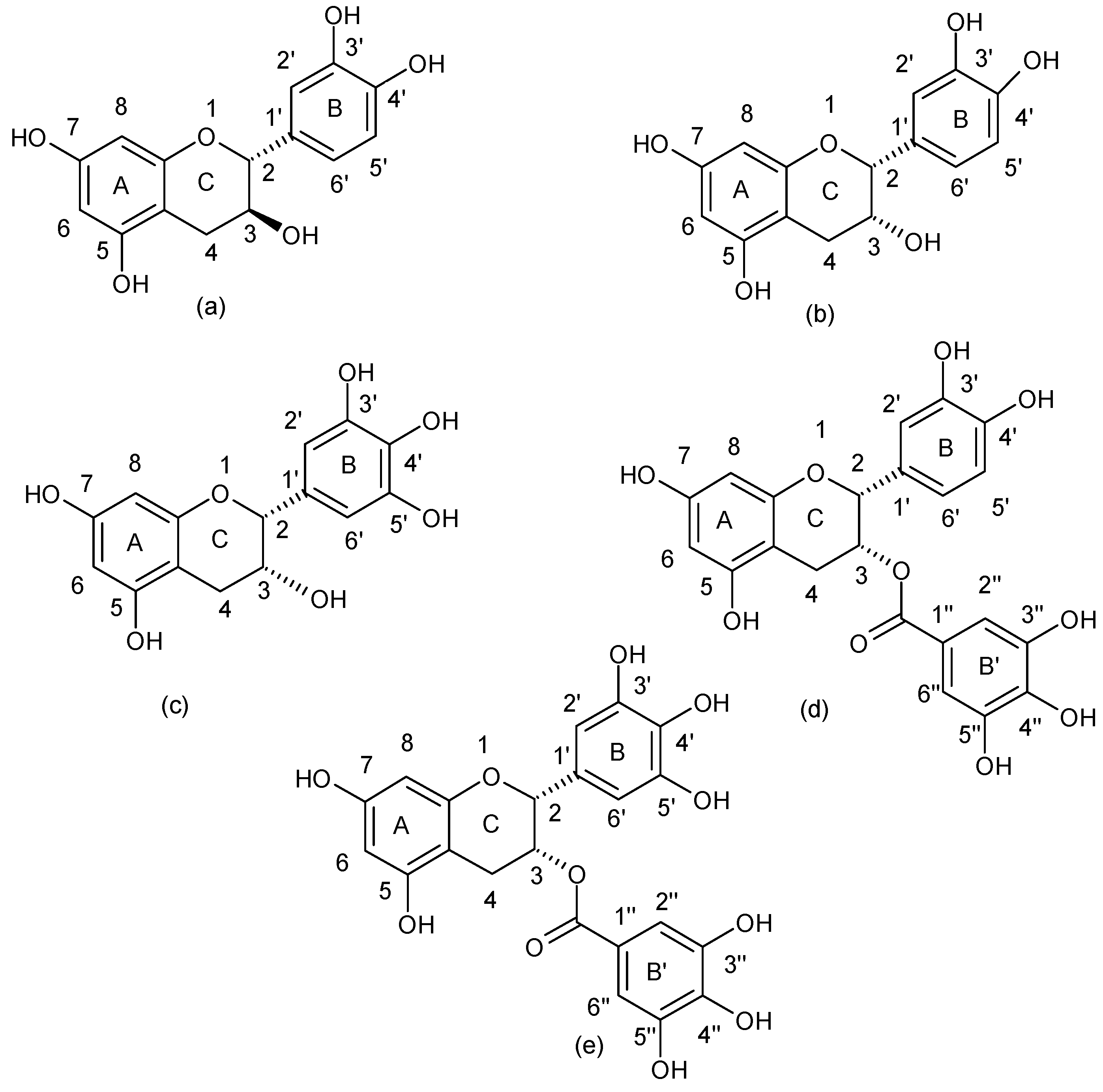
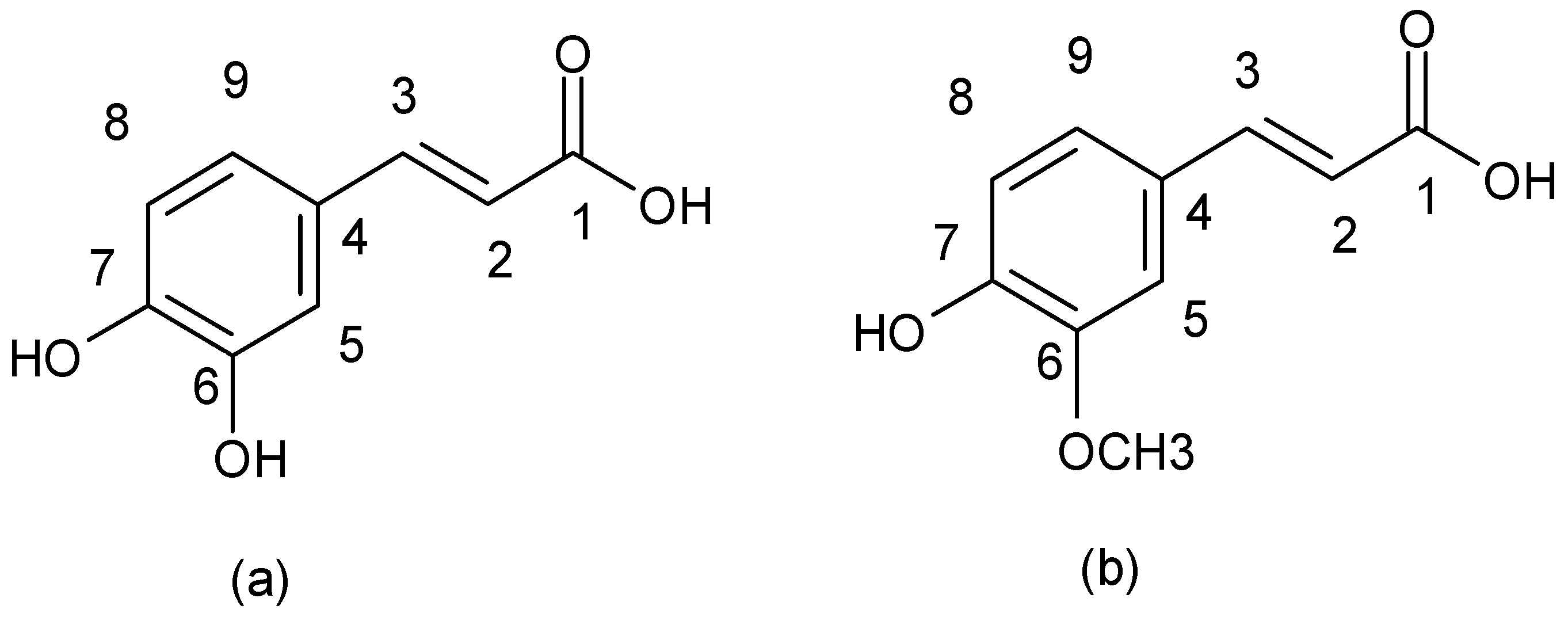
| Flavonoids | |
| Flavonols | 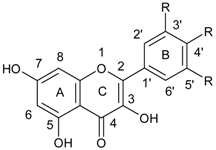 |
| Flavones | 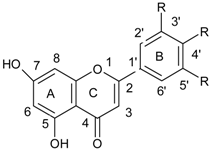 |
| Flavanols | 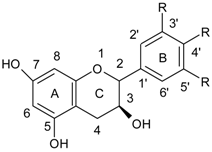 |
| Flavanones | 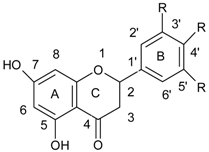 |
| Anthocyanidins | 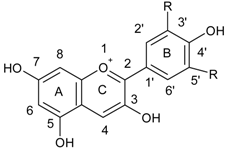 |
| Isoflavonoids | 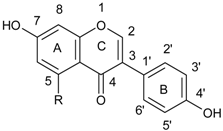 |
| Phenolic acids | |
| Benzoic acid derivatives | 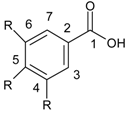 |
| Cinnamic acid derivatives | 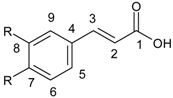 |
| Phenolic Compound | Proposed Mechanism of Action | Examined Strains | Synergism with Antibiotics | References |
|---|---|---|---|---|
| Flavolons | ||||
| Galangin | a | S. aureus NCTC 6571 | Penicillin G | [38,41] |
| Morin | b | S. aureus clinical strains | - | [42] |
| Quercetin | c | MRSA clinical strains | Rifampicin Ciprofloxacin | [43,44,45] |
| Kaempferol | c | MRSA clinical strains | Rifampicin Ciprofloxacin Fluoroquinolone | [43,44,45,46] |
| Flavanols and Derivatives | ||||
| (−)-Epigallocatechin gallate | b,d | MRSA and MSSA clinical and standard strains | Oxacillin Ampicillin/Sulbactam Penicillin Imipenem Panipenem Meropenem Tetracyclin Oxytetracycline | [47,48,49,50,51,52,53] |
| (+)-catechin acyl derivatives | a | MRSA clinical strains | - | [40] |
| Epicatechin gallate | a,e | MRSA clinical strains | β-lactams Ampicillin Ampicillin/Sulbactam Cefazolin Cefepime Imipenem/Cilastatin | [4,37,54] |
| 3-O-decyl-(+)-catechin | a | MRSA and MSSA clinical strains | - | [55] |
| (+)-catechin | e | MRSA clinical strains | Ampicillin Ampicillin/Sulbactam Cefazolin Cefepime Imipenem/Cilastatin | [4] |
| Catechin hydrate | nk | MRSA and MSSA clinical and standard strains | Clindamycin Erythromycin | [18] |
| Phenolic Acids and Derivatives | ||||
| Ferulic acid | a | S. aureus ATCC 6538 | - | [56] |
| Coumaric acid | a | S. aureus ATCC 6538 | - | [56] |
| Chlorogenic acid | a | S. aureus ATCC 6538 | - | [56] |
| Protocatechuic acid ethyl ester | nk | MRSA and MSSA clinical and standard strains | Clindamycin | [5] |
| Caffeic acid | a | MRSA and MSSA clinical and standard strains | Clindamycin Erythromycin Cefoxitin | [16,57,58,59] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. https://doi.org/10.3390/ijerph15102321
Miklasińska-Majdanik M, Kępa M, Wojtyczka RD, Idzik D, Wąsik TJ. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. International Journal of Environmental Research and Public Health. 2018; 15(10):2321. https://doi.org/10.3390/ijerph15102321
Chicago/Turabian StyleMiklasińska-Majdanik, Maria, Małgorzata Kępa, Robert D. Wojtyczka, Danuta Idzik, and Tomasz J. Wąsik. 2018. "Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains" International Journal of Environmental Research and Public Health 15, no. 10: 2321. https://doi.org/10.3390/ijerph15102321
APA StyleMiklasińska-Majdanik, M., Kępa, M., Wojtyczka, R. D., Idzik, D., & Wąsik, T. J. (2018). Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. International Journal of Environmental Research and Public Health, 15(10), 2321. https://doi.org/10.3390/ijerph15102321







