Mitigation of Particulate Matter-Induced Inflammation and Vasoactivity in Human Vascular Endothelial Cells by Omega-3 Polyunsaturated Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cells
2.2. Fatty Acid Oil Saponification
2.3. Stock Preparation
2.4. Cell Culture
2.5. Experimental Design
2.6. Enzyme-Linked Immunosorbent Assays (ELISA)
2.7. Statistical Analysis
3. Results
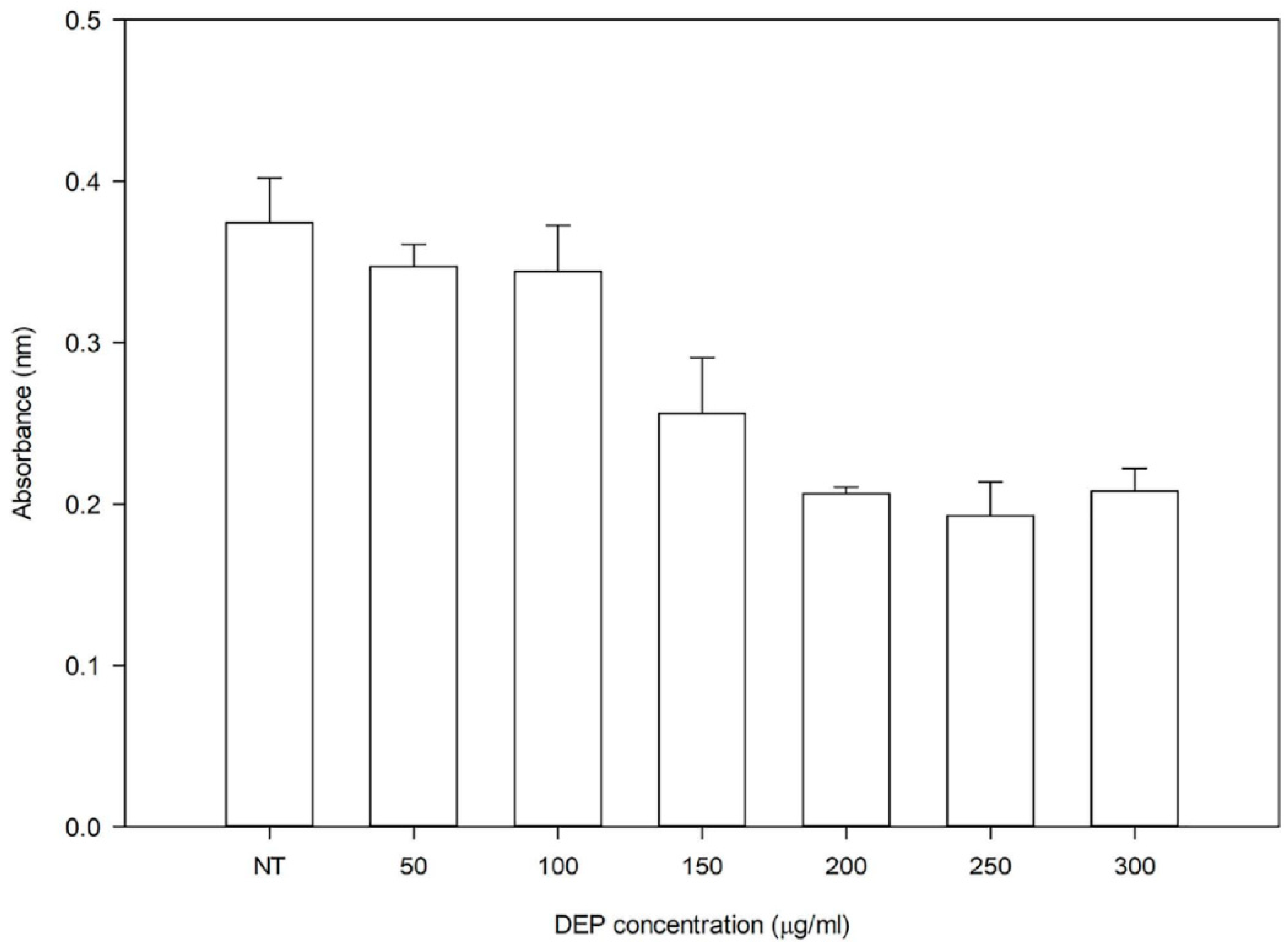
3.1. Effect of DEP
3.2. Effect of ω-3-PUFAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.S.B.; Rollins, A. Environmental exposures and cardiovascular disease: A challenge for health and development in low-and middle-income countries. Cardiol. Clin. 2017, 35, 71–86. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, G.; Murray, F. Air Pollution and Health in Rapidly Developing Countries; Earthscan: London, UK, 2003; pp. 1–15. [Google Scholar]
- The World Bank. PM2.5 Air Pollution, Mean Annual Exposure (Micrograms per Cubic Meter). Available online: https://data.worldbank.org/indicator/EN.ATM.PM25.MC.M3 (accessed on 6 June 2018).
- Krzyzanowski, M.; Cohen, A. Update of WHO air quality guidelines. Air Qual. Atmos. Health 2008, 1, 7–13. [Google Scholar] [CrossRef]
- Bo, L.; Jiang, S.; Xie, Y.; Kan, H.; Song, W.; Zhao, J. Effect of vitamin E and omega-3 fatty acids on protecting ambient PM2.5-induced inflammatory response and oxidative stress in vascular endothelial cells. PLoS ONE 2016, 11, e0152216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Hao, L.; Liu, Y.-H.; Chen, C.-Y.; Pai, V.J.; Kang, J.X. Protection against fine particle-induced pulmonary and systemic inflammation by omega-3 polyunsaturated fatty acids. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Garcia-Esteban, R.; Sunyer, J.; Rios, C.; Alcaraz-Zubeldia, M.; Velasco, S.R.; Holguin, F. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM2.5. Environ. Health Perspect. 2008, 116, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Rappold, A.G.; Diaz-Sanchez, D.; Steck, S.E.; Berntsen, J.; Cascio, W.E.; Devlin, R.B.; Samet, J.M. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution–induced cardiac effects and lipid changes in healthy middle-aged adults. Environ. Health Perspect. 2012, 120, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Marasciulo, F.L.; Montagnani, M.; Potenza, M.A. Endothelin-1: The yin and yang on vascular function. Curr. Med. Chem. 2006, 13, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Miyazawa, T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000, 148, 173–179. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.-N.; Hwang, I.-Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef] [PubMed]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Yang, Z.; Wang, X.; Shi, H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS ONE 2012, 7, e45990. [Google Scholar] [CrossRef] [PubMed]
- Kocbach, A.; Herseth, J.I.; Låg, M.; Refsnes, M.; Schwarze, P.E. Particles from wood smoke and traffic induce differential pro-inflammatory response patterns in co-cultures. Toxicol. Appl. Pharmacol. 2008, 232, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Kocbach, A.; Namork, E.; Schwarze, P.E. Pro-inflammatory potential of wood smoke and traffic-derived particles in a monocytic cell line. Toxicology 2008, 247, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Salnikow, K.; Li, X.; Lippmann, M. Effect of nickel and iron co-exposure on human lung cells. Toxicol. Appl. Pharmacol. 2004, 196, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Téllez-Rojo, M.M.; Lazo, M.; Manzano-Patiño, A.; Cortez-Lugo, M.; Julien, P.; Bélanger, M.C.; Hernandez-Avila, M.; Holguin, F. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am. J. Respir. Crit. Care Med. 2005, 172, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J. Nutr. 2012, 142, 614S–625S. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Moller, P.; Jensen, K.A.; Sharma, A.K.; Wallin, H.; Bossi, R.; Autrup, H.; Molhave, L.; Ravant, J.-L.; Briede, J.J.; et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chem. Res. Toxicol. 2011, 24, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, G.; Lin, Z.; Wang, Y.; He, H.; Liu, T.; Kamp, D.W. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol. 2014, 31, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tian, D.; He, J.; Wang, W.; Zhang, L.; Cui, L.; Jia, L.; Zhang, L.; Li, L.; Shu, Y.; et al. Repeated PM2.5 exposure inhibits BEAS-2B cell p53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli-Rocha, F.; Fernandes, S.; Einicker-Lamas, M.; Zin, W.A. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol. Toxicol. 2010, 26, 481–498. [Google Scholar] [CrossRef] [PubMed]

| Comparisons | 24-Hours | 48-Hours | ||
|---|---|---|---|---|
| t-Value | p-Value | t-Value | p-Value | |
| ET-1 | ||||
| DHA vs. NT | 2.20 | 0.24 | 3.33 | 0.30 |
| EPA vs. NT | 1.75 | 0.53 | 1.58 | 0.71 |
| OSU45 vs. NT | −0.32 | 1.00 | 1.62 | 0.66 |
| DHA vs. EPA | 0.45 | 1.00 | 1.75 | 0.53 |
| DHA vs. OSU45 | 2.52 | 0.13 | 1.71 | 0.56 |
| EPA vs. OSU45 | 2.07 | 0.30 | −0.04 | 1.00 |
| IL-6 | ||||
| DHA vs. NT | −1.98 | 0.35 | −4.33 | <0.01 |
| EPA vs. NT | 0.05 | 1.00 | 2.08 | 0.30 |
| OSU45 vs. NT | −1.82 | 0.47 | −1.96 | 0.37 |
| DHA vs. EPA | −2.03 | 0.33 | −6.41 | <0.01 |
| DHA vs. OSU45 | −0.16 | 1.00 | −2.37 | 0.18 |
| EPA vs. OSU45 | 1.87 | 0.43 | 4.04 | <0.01 |
| IL-8 | ||||
| DHA vs. NT | 0.53 | 1.00 | 1.46 | 0.83 |
| EPA vs. NT | 3.19 | 0.04 | 2.08 | <0.01 |
| OSU45 vs. NT | −0.55 | 1.00 | 0.60 | 1.00 |
| DHA vs. EPA | −2.66 | 0.10 | −2.61 | 0.11 |
| DHA vs. OSU45 | 1.08 | 1.00 | −0.86 | 1.00 |
| EPA vs. OSU45 | 3.74 | 0.01 | −3.47 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriram, J.; Adetona, O.; Orchard, T.; Wu, C.-M.; Odei, J. Mitigation of Particulate Matter-Induced Inflammation and Vasoactivity in Human Vascular Endothelial Cells by Omega-3 Polyunsaturated Fatty Acids. Int. J. Environ. Res. Public Health 2018, 15, 2293. https://doi.org/10.3390/ijerph15102293
Sriram J, Adetona O, Orchard T, Wu C-M, Odei J. Mitigation of Particulate Matter-Induced Inflammation and Vasoactivity in Human Vascular Endothelial Cells by Omega-3 Polyunsaturated Fatty Acids. International Journal of Environmental Research and Public Health. 2018; 15(10):2293. https://doi.org/10.3390/ijerph15102293
Chicago/Turabian StyleSriram, Jaya, Olorunfemi Adetona, Tonya Orchard, Chieh-Ming Wu, and James Odei. 2018. "Mitigation of Particulate Matter-Induced Inflammation and Vasoactivity in Human Vascular Endothelial Cells by Omega-3 Polyunsaturated Fatty Acids" International Journal of Environmental Research and Public Health 15, no. 10: 2293. https://doi.org/10.3390/ijerph15102293
APA StyleSriram, J., Adetona, O., Orchard, T., Wu, C.-M., & Odei, J. (2018). Mitigation of Particulate Matter-Induced Inflammation and Vasoactivity in Human Vascular Endothelial Cells by Omega-3 Polyunsaturated Fatty Acids. International Journal of Environmental Research and Public Health, 15(10), 2293. https://doi.org/10.3390/ijerph15102293