Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion of Subjects
2.2. Environmental Monitoring Data
2.3. Data Management and Analysis
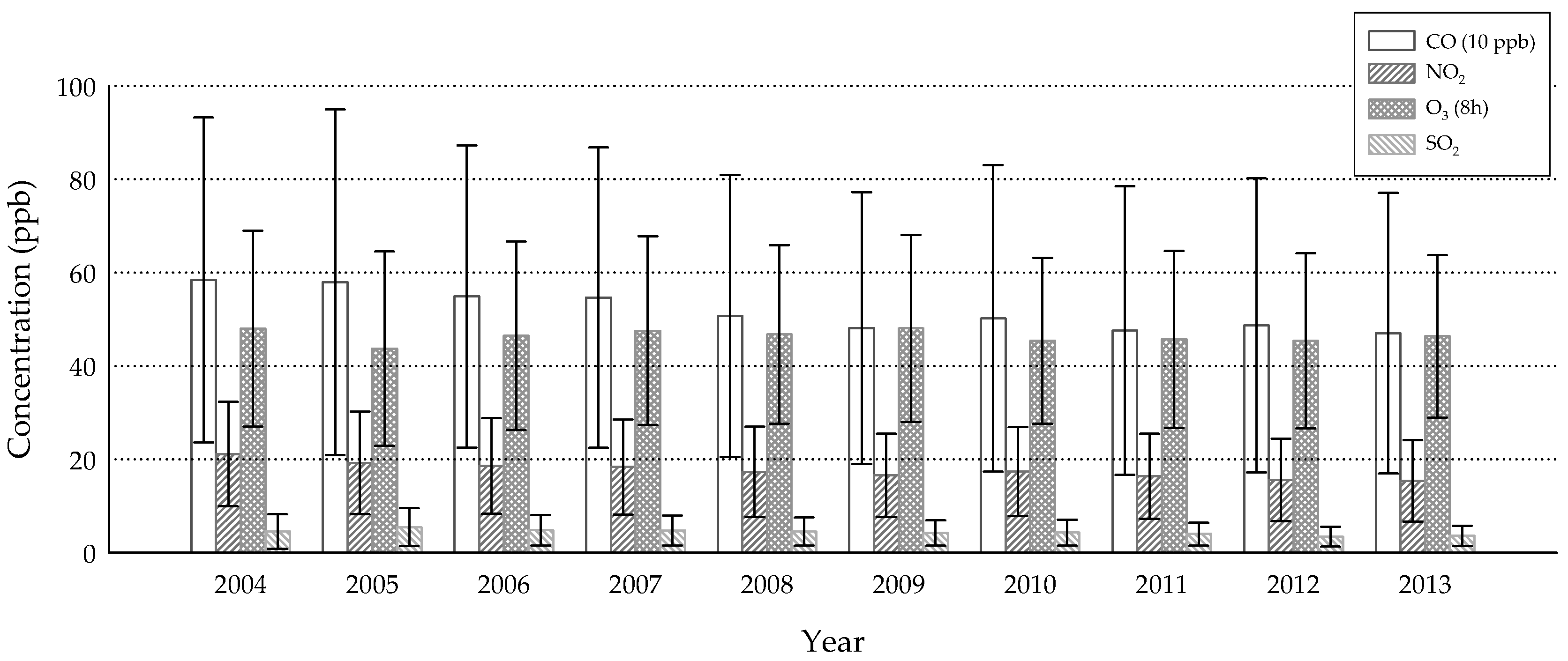
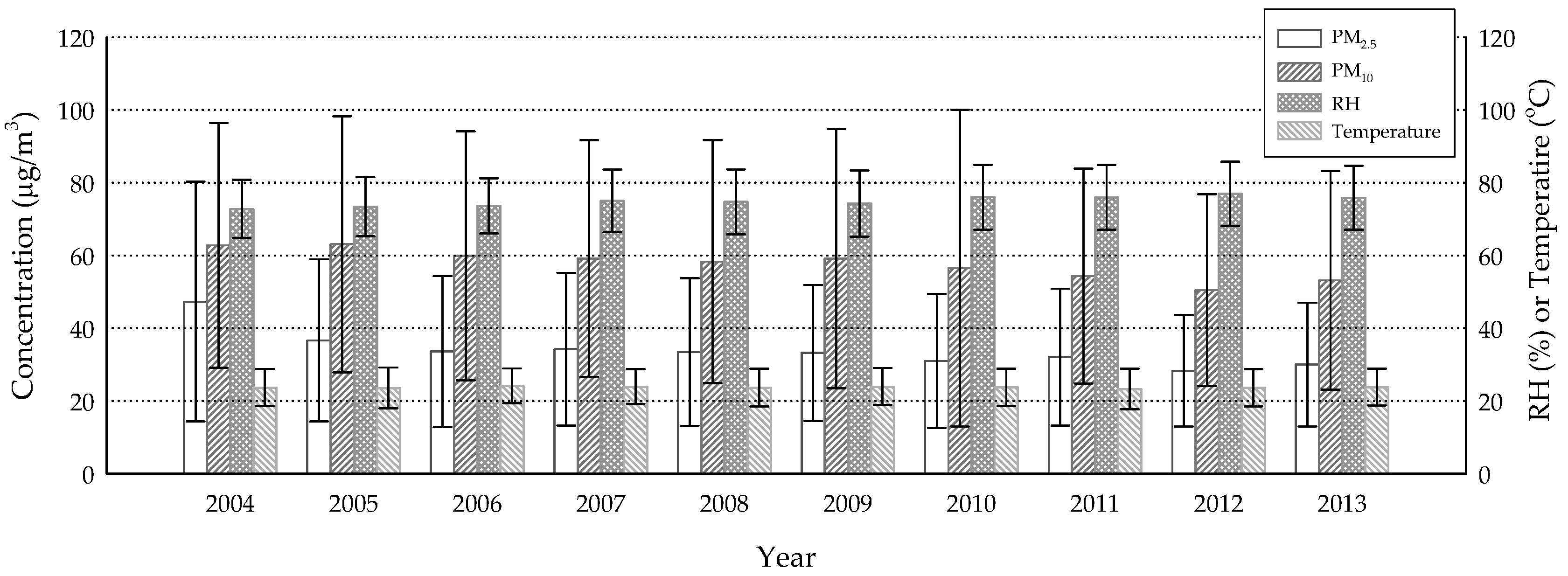
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Lee, J.; Saw, S.M.; Gazzard, G.; Koh, D.; Widjaja, D.; Tan, D.T. Prevalence and risk factors associated with dry eye symptoms: A population based study in Indonesia. Br. J. Ophthalmol. 2002, 86, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology 2011, 118, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Farrand, K.F.; Fridman, M.; Stillman, I.O.; Schaumberg, D.A. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Xu, L.; Wu, Y.Y.; Jonas, J.B. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye 2009, 23, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Tsai, S.-Y.; Cheng, C.-Y.; Liu, J.-H.; Chou, P.; Hsu, W.-M. Prevalence of dry eye among an elderly Chinese population in Taiwan. Ophthalmology 2003, 110, 1096–1101. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Dana, R.; Buring, J.E.; Sullivan, D.A. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Lekhanont, K.; Rojanaporn, D.; Chuck, R.S.; Vongthongsri, A. Prevalence of dry eye in Bangkok, Thailand. Cornea 2006, 25, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Sullivan, D.A.; Buring, J.E.; Dana, M.R. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003, 136, 318–326. [Google Scholar] [CrossRef]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.H.; Klein, B.E.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.M.; Lee, S.H.; Rim, T.H.; Park, R.J.; Yang, H.S.; Kim, T.I.; Yoon, K.C.; Seo, K.Y. Prevalence of and risk factors associated with dry eye: The Korea National Health and Nutrition Examination Survey 2010–2011. Am. J. Ophthalmol. 2014, 158, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Man, R.E.K.; Veerappan, A.R.; Tan, S.P.; Fenwick, E.K.; Sabanayagam, C.; Chua, J.; Leong, Y.Y.; Wong, T.Y.; Lamoureux, E.L.; Cheng, C.Y.; et al. Incidence and risk factors of symptomatic dry eye disease in Asian Malays from the Singapore Malay Eye Study. Ocul. Surf. 2017, 15, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, M.M.; Shihadeh, W.A.; Haddad, M.F.; Khader, Y.S. Epidemiology of symptoms of dry eye disease (DED) in Jordan: A cross-sectional non-clinical population-based study. Cont. Lens Anterior Eye 2016, 39, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Bielory, L. Contact lenses and associated anterior segment disorders: Dry eye disease, blepharitis, and allergy. Immunol. Allergy Clin. N. Am. 2008, 28, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Ichinose, T.; Yamagami, S.; Fujishima, H.; Kamei, Y.; Goto, M.; Takada, S.; Matsubara, M. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ. 2014, 487, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhong, T.; Li, H.; Xu, J.; Ye, X.; Mu, Z.; Lu, Y.; Mashaghi, A.; Zhou, Y.; Tan, M. Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 2016, 6, 23858. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Hsieh, C.J.; Tseng, C.C.; Yiin, L.M. Association between the first occurrence of allergic rhinitis in preschool children and air pollution in Taiwan. Int. J. Environ. Res. Public Health 2016, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L.; Lin, Y.C.; Sung, F.C.; Huang, S.L.; Ko, Y.C.; Lai, J.S.; Su, H.J.; Shaw, C.K.; Lin, R.S.; Dockery, D.W. Climate, traffic-related air pollutants, and asthma prevalence in middle-school children in Taiwan. Environ. Health Perspect. 1999, 107, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shaw, C.; Su, H.; Lai, J.; Ko, Y.; Huang, S.-L.; Sung, F.-C.; Guo, Y. Climate, traffic-related air pollutants and allergic rhinitis prevalence in middle-school children in Taiwan. Eur. Respir. J. 2003, 21, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Hoek, G.; Van Vliet, P.; Meliefste, K.; Fischer, P.H.; Wijga, A.; Koopman, L.P.; Neijens, H.J.; Gerritsen, J.; Kerkhof, M. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am. J. Respir. Crit. Care Med. 2002, 166, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Choi, Y.H.; Paik, H.J.; Wee, W.R.; Kim, M.K.; Kim, D.H. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Um, S.B.; Kim, N.H.; Lee, H.K.; Song, J.S.; Kim, H.C. Spatial epidemiology of dry eye disease: Findings from South Korea. Int. J. Health Geogr. 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Maclure, M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 1991, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Gilbard, J.P. Human tear film electrolyte concentrations in health and dry-eye disease. Int. Ophthalmol. Clin. 1994, 34, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Truong, S.; Cole, N.; Stapleton, F.; Golebiowski, B. Sex hormones and the dry eye. Clin. Exp. Optom. 2014, 97, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Lanier, B. Urban eye allergy syndrome: A new clinical entity? Curr. Med. Res. Opin. 2008, 24, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Novaes, P.; Saldiva, P.H.; Matsuda, M.; Macchione, M.; Rangel, M.P.; Kara-Jose, N.; Berra, A. The effects of chronic exposure to traffic derived air pollution on the ocular surface. Environ. Res. 2010, 110, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Yenigun, A.; Dadaci, Z.; Sahin, G.O.; Elbay, A. Prevalence of allergic rhinitis symptoms and positive skin-prick test results in patients with dry eye. Am. J. Rhinol. Allergy 2016, 30, e26–e29. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.-F.; Jaakkola, J.J.; Lee, Y.-L.; Lin, Y.-C.; Guo, Y.-l.L. Relation between air pollution and allergic rhinitis in Taiwanese schoolchildren. Respir. Res. 2006, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Shen, L.; Chen, L.; Rashid, S.; Rolando, M.; Dana, M.R. The controlled-environment chamber: A new mouse model of dry eye. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Abusharha, A.A.; Pearce, E.I. The effect of low humidity on the human tear film. Cornea 2013, 32, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Abusharha, A.A.; Pearce, E.I.; Fagehi, R. Effect of ambient temperature on the human tear film. Eye Contact Lens 2016, 42, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Cable News Network (CNN). These Countries Want to Ban Gas and Diesel Cars. 2017. Available online: Https://money.cnn.com/2017/09/11/autos/countries-banning-diesel-gas-cars/index.html (accessed on 30 August 2018).
- Beggs, P.J.; Bambrick, H.J. Is the global rise of asthma an early impact of anthropogenic climate change? Environ. Health Perspect. 2005, 113, 915–919. [Google Scholar] [CrossRef] [PubMed]
| Study Subjects | All Patients | p-Value | |
|---|---|---|---|
| Total | 25,818 | 46,907 | |
| Age (Mean ± SD) | 51.1 ± 17.7 | 51.3 ± 17.5 | 0.300 a |
| n (%) | n (%) | ||
| Age | 0.285 b | ||
| <18 | 472 (1.8) | 867 (1.9) | |
| 18~49 | 11,520 (44.6) | 20,644 (44.0) | |
| ≥50 | 13,826 (53.6) | 25,396 (54.1) | |
| Gender | 0.687 b | ||
| Male | 8021 (31.1) | 14,505 (30.9) | |
| Female | 17,797 (68.9) | 32,402 (69.1) | |
| Season | 0.521 b | ||
| Spring | 6684 (25.9) | 12,282 (26.2) | |
| Summer | 6561 (25.4) | 11,931 (25.4) | |
| Fall | 6407 (24.8) | 11,711 (25.0) | |
| Winter | 6166 (23.9) | 10,983 (23.4) |
| CO | NO2 | O3 (8h) | PM2.5 | PM10 | SO2 | RH | Temperature | |
|---|---|---|---|---|---|---|---|---|
| CO | 1 | |||||||
| NO2 | 0.828 | 1 | ||||||
| O3 (8h) | 0.025 | 0.074 | 1 | |||||
| PM2.5 | 0.429 | 0.533 | 0.503 | 1 | ||||
| PM10 | 0.372 | 0.473 | 0.461 | 0.870 | 1 | |||
| SO2 | 0.346 | 0.529 | 0.160 | 0.480 | 0.455 | 1 | ||
| RH | –0.123 | –0.198 | –0.297 | –0.281 | –0.328 | –0.232 | 1 | |
| Temperature | –0.186 | –0.216 | 0.115 | –0.164 | –0.163 | 0.019 | –0.108 | 1 |
| Model 1 a | Model 2 b | Model 3 c | Model 4 d | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| CO (ppm) | 1.116 (1.026, 1.214) | 0.010 | - | - | 1.105 (1.004, 1.216) | 0.042 | - | - |
| NO2 (10 ppb) | - | - | 1.068 (1.037, 1.100) | <0.001 | - | - | 1.075 (1.040, 1.111) | <0.001 |
| O3 8h (10 ppb) | 1.000 (0.990, 1.011) | 0.932 | 0.998 (0.998, 1.009) | 0.725 | 1.000 (0.989, 1.011) | 0.960 | 0.997 (0.986, 1.008) | 0.616 |
| PM10 (10 μg/m3) | 1.001 (0.994, 1.009) | 0.717 | 1.000 (0.992, 1.007) | 0.920 | - | - | - | - |
| PM2.5 (10 μg/m3) | - | - | - | - | 1.006 (0.991, 1.022) | 0.422 | 1.001 (0.986, 1.016) | 0.900 |
| SO2 (ppb) | 1.004 (0.996, 1.012) | 0.284 | 0.998 (0.990, 1.006) | 0.621 | 1.006 (0.998, 1.015) | 0.148 | 1.000 (0.991, 1.008) | 0.923 |
| RH (10%) | 0.935 (0.916, 0.954) | <0.001 | 0.930 (0.910, 0.949) | <0.001 | 0.936 (0.916, 0.956) | <0.001 | 0.929 (0.909, 0.949) | <0.001 |
| Temperature (°C) | 1.008 (1.002, 1.013) | 0.004 | 1.010 (1.005, 1.015) | <0.001 | 1.008 (1.003, 1.014) | 0.003 | 1.011 (1.005, 1.016) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.-Y.; Lee, Y.-C.; Hsieh, C.-J.; Tseng, C.-C.; Yiin, L.-M. Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 2269. https://doi.org/10.3390/ijerph15102269
Zhong J-Y, Lee Y-C, Hsieh C-J, Tseng C-C, Yiin L-M. Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan. International Journal of Environmental Research and Public Health. 2018; 15(10):2269. https://doi.org/10.3390/ijerph15102269
Chicago/Turabian StyleZhong, Jia-Yu, Yuan-Chieh Lee, Chia-Jung Hsieh, Chun-Chieh Tseng, and Lih-Ming Yiin. 2018. "Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan" International Journal of Environmental Research and Public Health 15, no. 10: 2269. https://doi.org/10.3390/ijerph15102269
APA StyleZhong, J.-Y., Lee, Y.-C., Hsieh, C.-J., Tseng, C.-C., & Yiin, L.-M. (2018). Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan. International Journal of Environmental Research and Public Health, 15(10), 2269. https://doi.org/10.3390/ijerph15102269







