Enhanced Adsorption Performance of Oxytetracycline by Desugared Reed Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Collection and Pretreatment
2.3. Adsorption Experiment Methods
2.4. Analysis
3. Results and Discussion
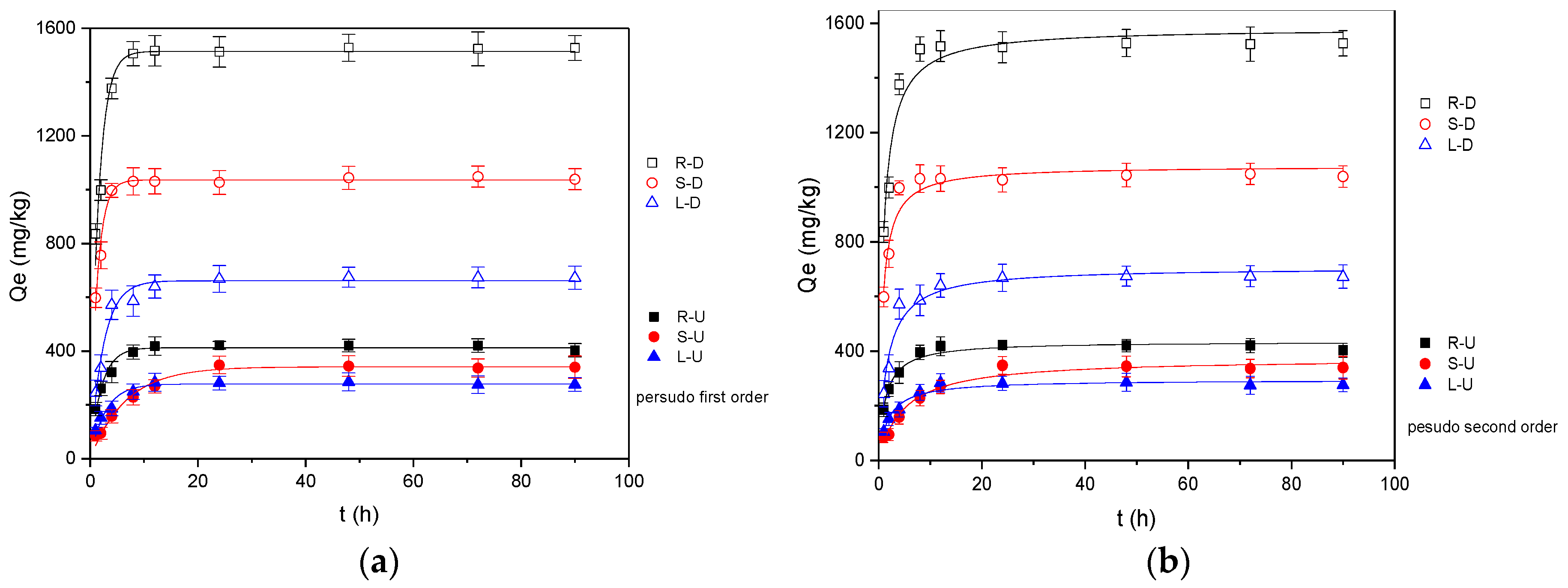
3.1. Adsorption Kinetics
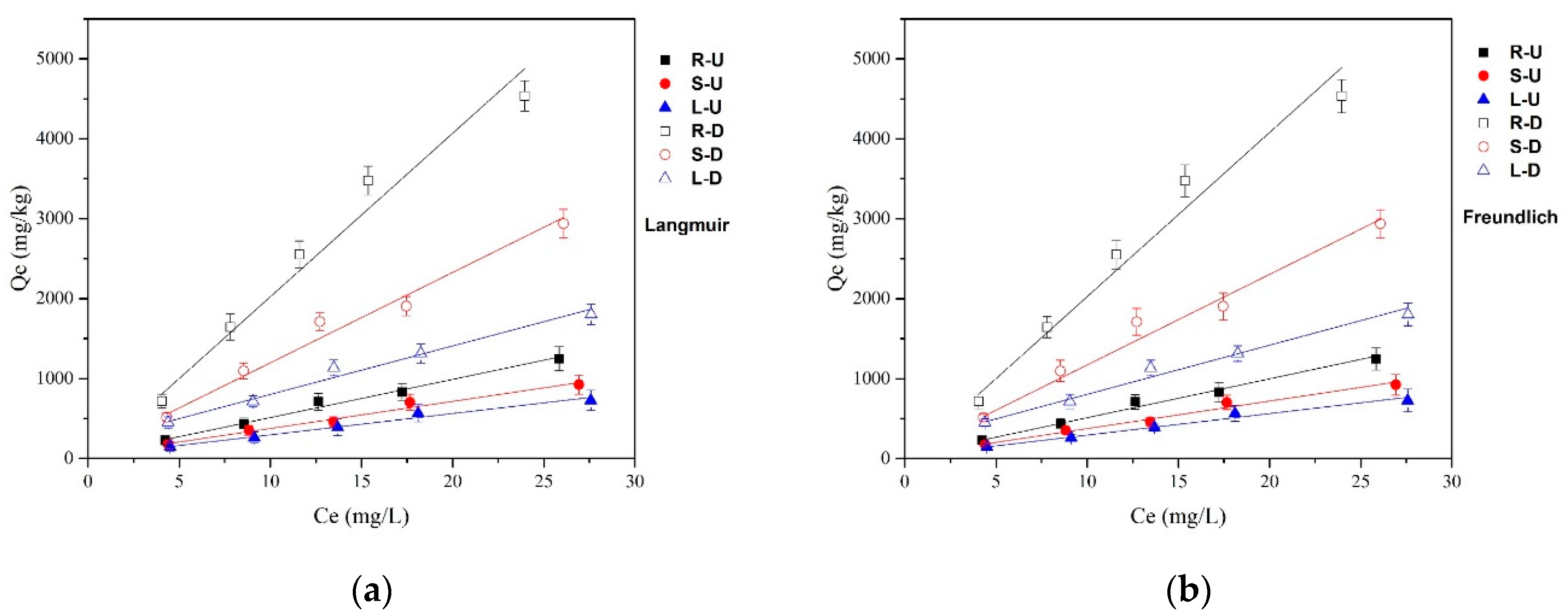
3.2. Adsorption Isotherms
3.3. Thermodynamics
3.4. Analysis of the Adsorption Mechanism of OTC Adsorption onto Reed Residues
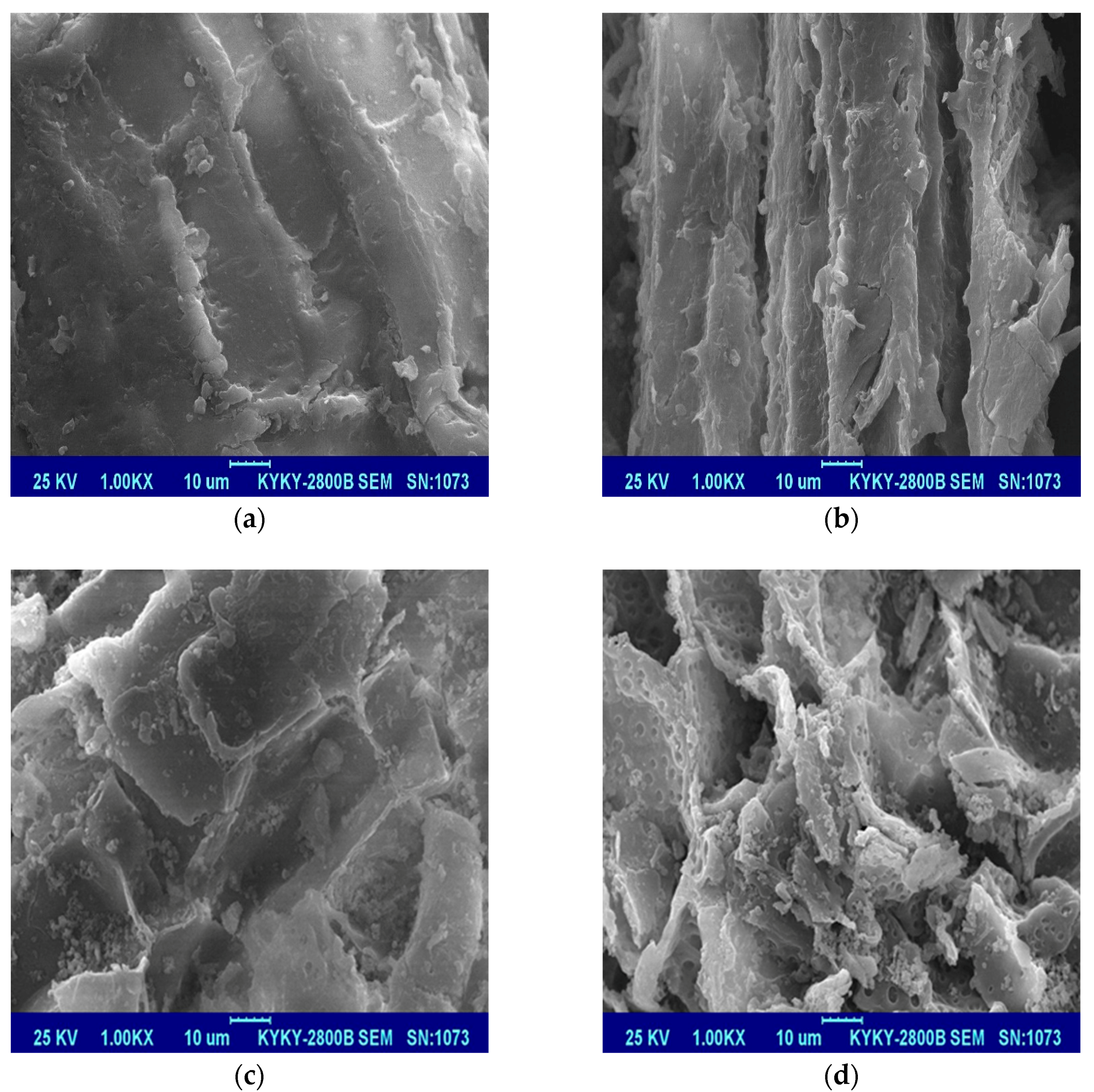
3.4.1. SEM Analysis
3.4.2. Elemental Composition Analysis
3.4.3. Analysis of FTIR
3.4.4. Analysis of Surface Area
4. Conclusions
- (1)
- The adsorption amounts of oxytetracycline by untreated reed residues were 416.35 mg/kg for roots, 341.92 mg/kg for stems and 280.21 mg/kg for leaves. The desugarization process can significantly enhance the OTC adsorption by 8–12 times (roots 1525.71 mg/kg, stems 1043.53 mg/kg and leaves 671.94 mg/kg).
- (2)
- The desugared reed tissues had a larger surface area and smaller pore area, and the aromaticity of reed residues increased. On the other hand, the polarity and hydrophilicity decreased after desugarization, which thus revealed the enhanced OTC adsorption mechanism by desugared reed residues. Therefore, protecting the natural reed residue and letting it naturally rot rather than artificially cleaning it up may be effective at promoting the adsorption of pollutants in the environment and protecting environmental and public health.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Aust, M.-O.; Godlinski, F.; Travis, G.R.; Hao, X.; McAllister, T.A.; Leinweber, P.; Thiele-Bruhn, S. Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environ. Pollut. 2008, 156, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Briones, R.M.; Sarmah, A.K.; Padhye, L.P. A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Paget, M.S.B.; Buttner, M.J. A signal transduction system in Streptomyces coelicolor that activates the expression of a putative cell wall glycan operon in response to vancomycin and other cell wall-specific antibiotics. Mol. Microbiol. 2002, 44, 1199–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colomerlluch, M.; Calerocáceres, W.; Jebri, S.; Hmaied, F.; Muniesa, M.; Jofre, J. Antibiotic resistance genes in bacterial and bacteriophage fractions of Tunisian and Spanish wastewaters as markers to compare the antibiotic resistance patterns in each population. Environ. Int. 2014, 73, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.L. Antibiotics and Antibiotic Resistance Genes in Natural Environments. Science 2008, 321, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, E.; Chiriac, C.M.; Baricz, A.; Szőke-Nagy, T.; Lung, I.; Soran, M.-L.; Rudi, K.; Dragos, N.; Coman, C. Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas. Environ. Pollut. 2018, 236, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, E.; Cummins, E. Antibiotic resistance in surface water ecosystems: Presence in the aquatic environment, prevention strategies, and risk assessment. Hum. Ecol. Risk Assess. Int. J. 2016, 23, 299–322. [Google Scholar] [CrossRef]
- WHO. Review on Antibiotic Resistance. Antibiotic Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: http://www.jpiamr.eu/wp-content/uploads/2014/12/AMRReview-Paper-Tackling-a-crisis-for-the-health-and-wealth-of-nations_1-2.pdf (accessed on 19 September 2018).
- Chen, H.; Liu, S.; Xu, X.R.; Zhou, G.J.; Liu, S.S.; Yue, W.Z.; Sun, K.F.; Ying, G.G. Antibiotics in the coastal environment of the Hailing Bay region, South China Sea: Spatial distribution, source analysis and ecological risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.; Blackwell, P.A.; Boxall, A.B.A. Fate of veterinary antibiotics in a macroporous tile drained clay soil. Environ. Toxicol. Chem. 2004, 23, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Buxton, H.T.; Kolpin, D.W. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams. In Water Encyclopedia; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Boxall, A. Targeted Monitoring Study for Veterinary Medicines in the Environment; Environment Agency: Bristol, UK, 2006. [Google Scholar]
- Gao, L.; Yang, S.; Wang, D.; Gao, C.; Wang, G. Influence of humic acid colloid on adsorption of oxytetracycline in sediment. Asian J. Chem. 2014, 26, 8303–8308. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Q.; Wang, R.; Yuan, X.; Yang, S.; Wang, W.; Zhao, Y. Effects of Dissolved Organic Matter on Sorption of Oxytetracycline to Sediments. Geofluids 2018, 2018, 1254529. [Google Scholar] [CrossRef]
- Hu, L.; Martin, H.M.; Strathmann, T.J. Oxidation kinetics of antibiotics during water treatment with potassium permanganate. Environ. Sci. Technol. 2010, 44, 6416–6422. [Google Scholar] [CrossRef] [PubMed]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Fasani, E.; Albini, A. Photochemical degradation of marbofloxacin and enrofloxacin in natural waters. Environ. Sci. Technol. 2010, 44, 4564–4569. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Sirés, I.; Oturan, N.; Ozcan, A.; Oturan, M.A. Electrochemical treatment of the antibiotic sulfachloropyridazine: Kinetics, reaction pathways, and toxicity evolution. Environ. Sci. Technol. 2012, 46, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; Mcardell, C.S. Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination. Environ. Sci. Technol. 2012, 46, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Li, X.N.; Zhao, N.M.; Quan, X.; Chen, S.; Yu, H.T. Enhanced adsorption of ionizable antibiotics on activated carbon fiber under electrochemical assistance in continuous-flow modes. Water Res. 2018, 134, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.P.; Zeng, G.M.; Yang, Z.H.; Zhou, Y.Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.Y.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Xu, S.P.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B.Y. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Liu, X.H.; Wu, L.Y.; Li, A.M. Adsorption Behaviors of Oxytetracycline on Synthetic Resins. Adv. Mater. Res. 2013, 726, 573–576. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Y.; Wang, W.; Yue, Q.; Yang, T. Comparative study on characterization of activated carbons prepared by microwave and conventional heating methods and application in removal of oxytetracycline (OTC). Chem. Eng. J. 2011, 171, 1446–1453. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.K.; Kadirvelu, K.; Sillanpaa, M. Adsorption of heavy metals from multi-metal aqueous solution by sunflower plant biomass-based carbons. Int. J. Environ. Sci. Technol. 2016, 13, 493–500. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, C.T.; Bao, Q.H.; Zheng, J.; Deng, D.; Duan, Y.Q.; Shen, L.Q. The physicochemical characterization, equilibrium, and kinetics of heavy metal ions adsorption from aqueous solution by arrowhead plant (Sagittaria trifolia L.) stalk. J. Food Biochem. 2018, 42, e12448. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Xiao, W.H.; Shen, G.H.; Ji, G.Y.; Zhang, Y.; Gao, C.F.; Han, L.J. Mechanical Fragmentation of Wheat Straw at Different Plant Scales: Pb2+ Adsorption Behavior and Mechanism. BioResources 2018, 13, 6613–6630. [Google Scholar]
- Pongener, C.; Kibami, D.; Rao, K.S.; Goswamee, R.L.; Sinha, D. Adsorption Studies of Fluoride by Activated Carbon Prepared From Mucuna Prurines Plant. J. Water Chem. Technol. 2017, 39, 108–115. [Google Scholar] [CrossRef]
- Song, R.; Yang, S.; Xu, H.; Wang, Z.; Chen, Y.; Wang, Y. Adsorption Behavior and Mechanism for the Uptake of Fluoride Ions by Reed Residues. Int. J. Environ. Res. Public Health 2018, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hameed, K.S.; Muthirulan, P.; Sundaram, M.M. Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: Equilibrium and kinetics studies. Arab. J. Chem. 2017, 10, S2225–S2233. [Google Scholar] [CrossRef]
- Boving, T.B.; Zhang, W. Removal of aqueous-phase polynuclear aromatic hydrocarbons using aspen wood fibers. Chemosphere 2004, 54, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yuan, M.; Hao, L. Removal of polycyclic aromatic hydrocarbons from aqueous solution using plant residue materials as a biosorbent. J. Hazard. Mater. 2011, 188, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Chen, B. Removal of polycyclic aromatic hydrocarbons from aqueous solution by raw and modified plant residue materials as biosorbents. J. Environ. Sci. 2014, 26, 737–748. [Google Scholar] [CrossRef]
- Lin, D.; Pan, B.; Zhu, L.; Xing, B. Characterization and phenanthrene sorption of tea leaf powders. J. Agric. Food Chem. 2007, 55, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Y.; Guo, Y.; Zhu, L.; Schnoor, J.L. Role of the Extractable Lipids and Polymeric Lipids in Sorption of Organic Contaminants onto Plant Cuticles. Environ. Sci. Technol. 2008, 42, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y.; Zhu, Z.; Xing, Y. Adsorption of tannic acid from aqueous solution onto surfactant-modified zeolite. J. Hazard. Mater. 2011, 193, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liang, L.P.; Pu, L.J.; Wang, L.P. Adsorption characteristics of Cr (VI) by wheat straw including kinetic and thermodynamics analysis. Res. Environ. Sci. 2010, 23, 1546–1552. [Google Scholar]
- Cheng, D.H.; Yang, S.K.; Zhao, Y.; Chen, J. Adsorption Behaviors of Oxytetracycline onto Sediment in the Weihe River, Shaanxi, China. J. Chem. 2013, 2013, 719–723. [Google Scholar] [CrossRef]
- Wahab, M.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kaur, H. Sugarcane bagasse for the removal of erythrosin B and methylene blue from aqueous waste. Appl. Water Sci. 2011, 1, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhuang, Y.Y.; Wang, Z.H.; Qiu, M.Q. Adsorption of water-soluble dyes onto modified resin. Chemosphere 2004, 54, 425–430. [Google Scholar] [CrossRef]
- Li, Y.H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Johnson, E.J.; Chefetz, B.; Zhu, L.; Xing, B. Sorption of Polar and Nonpolar Aromatic Organic Contaminants by Plant Cuticular Materials: Role of Polarity and Accessibility. Environ. Sci. Technol. 2005, 39, 6138–6146. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, H.; Yang, S.; Wang, W.; Wang, Y. Adsorption Property and Mechanism of Oxytetracycline onto Willow Residues. Int. J. Environ. Res. Public Health 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
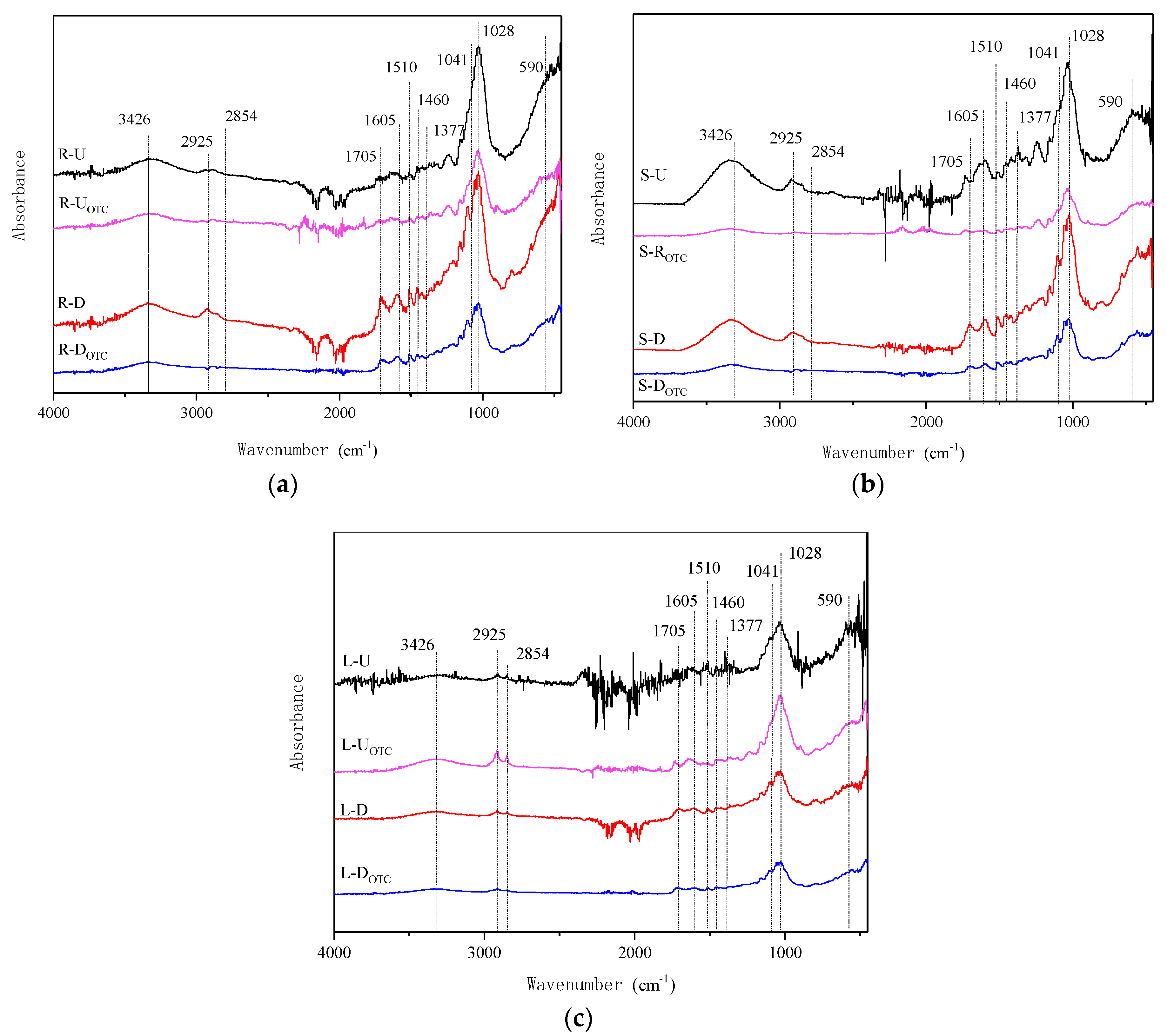
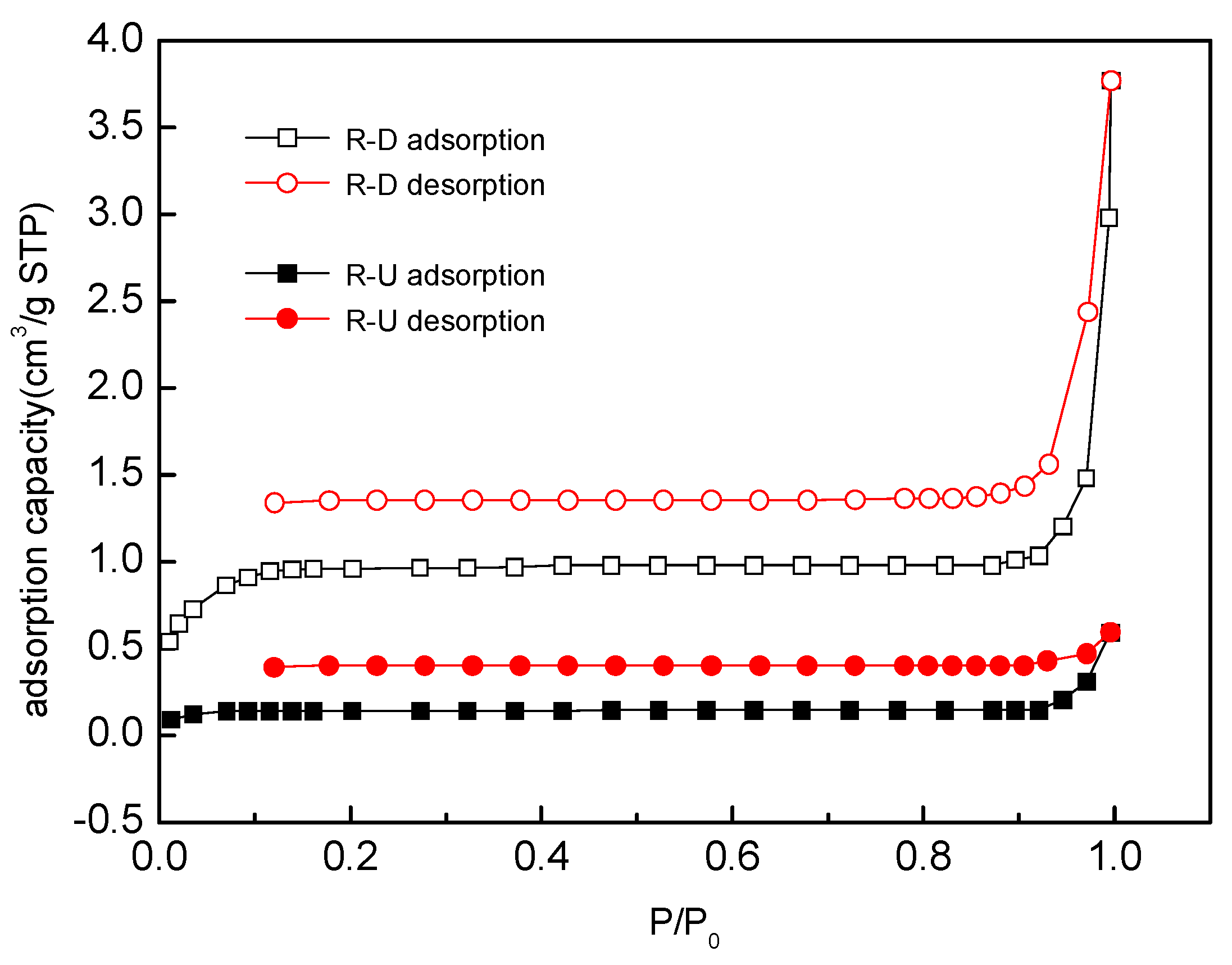
| Samples | Qe,exp (mg/kg) | Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | K1 (1/h) | Qe,cal (mg/kg) | RSS/dof | K2 (kg/mg·h) | R2 | Qe,cal (mg/kg) | RSS/dof | ||
| R-U | 416.35 | 0.9865 | 0.5392 | 416.25 | 26.50 | 0.9847 | 0.0018 | 439.09 | 29.84 |
| R-D | 1525.71 | 0.9515 | 0.6551 | 1512.88 | 359.70 | 0.9514 | 0.0007 | 1578.57 | 360.44 |
| S-U | 341.92 | 0.9855 | 0.1465 | 338.83 | 32.25 | 0.9849 | 0.0006 | 358.22 | 33.47 |
| S-D | 1043.53 | 0.9767 | 0.8094 | 1032.53 | 75.81 | 0.9757 | 0.0012 | 1085.46 | 79.00 |
| L-U | 280.21 | 0.9699 | 0.3671 | 281.25 | 29.13 | 0.9269 | 0.0017 | 306.85 | 39.06 |
| L-D | 671.94 | 0.9421 | 0.4402 | 658.52 | 145.39 | 0.9053 | 0.0010 | 705.78 | 237.96 |
| Samples | Qm (mg/kg) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | R2 | RSS/dof | 1/n | R2 | RSS/dof | ||
| R-U | 8238.81 | 0.0069 | 0.9897 | 4717.84 | 0.91 | 0.9892 | 4937.68 |
| R-D | 22,468.68 | 0.0109 | 0.9782 | 146,622.09 | 0.89 | 0.9701 | 201,419.04 |
| S-U | 5374.83 | 0.0078 | 0.9784 | 5565.49 | 0.89 | 0.9768 | 5984.34 |
| S-D | 15,848.97 | 0.0086 | 0.9788 | 52,851.38 | 0.88 | 0.9784 | 53,796.60 |
| L-U | 3573.84 | 0.0095 | 0.9823 | 2867.64 | 0.88 | 0.9782 | 3520.16 |
| L-D | 5013.84 | 0.0202 | 0.9870 | 10,800.92 | 0.76 | 0.9866 | 11,125.39 |
| Samples | T/K | ∆G/kJ·mol−1 | ∆H/kJ·mol−1 | ∆S/J·mol−1·K−1 |
|---|---|---|---|---|
| R-U | 298 | −10.36 | 10.20 | 69.00 |
| 308 | −11.05 | |||
| 318 | −11.74 | |||
| R-D | 298 | −13.74 | 4.23 | 60.30 |
| 308 | −14.34 | |||
| 318 | −14.95 | |||
| S-U | 298 | −9.62 | 24.25 | 113.66 |
| 308 | −10.76 | |||
| 318 | −11.89 | |||
| S-D | 298 | −12.53 | 16.46 | 97.27 |
| 308 | −13.50 | |||
| 318 | −14.46 | |||
| L-U | 298 | −9.47 | 46.97 | 189.38 |
| 308 | −11.36 | |||
| 318 | −13.25 | |||
| L-D | 298 | −11.17 | 39.62 | 170.43 |
| 308 | −12.87 | |||
| 318 | −14.58 |
| Samples | C (%) | H (%) | O (%) | H/C | (N + O)/C | O/C | Kd (L/kg) | Koc |
|---|---|---|---|---|---|---|---|---|
| R-U | 42.46 | 6.06 | 44.59 | 1.71 | 0.81 | 0.79 | 51.63 | 149.95 |
| R-D | 51.49 | 5.46 | 38.28 | 1.27 | 0.57 | 0.56 | 204.66 | 917.52 |
| S-U | 44.93 | 6.12 | 44.88 | 1.63 | 0.76 | 0.75 | 37.88 | 60.43 |
| S-D | 50.96 | 5.91 | 43.43 | 1.39 | 0.64 | 0.64 | 120.87 | 514.95 |
| L-U | 42.13 | 6.10 | 40.43 | 1.74 | 0.76 | 0.72 | 29.66 | 62.95 |
| L-D | 50.15 | 6.26 | 39.18 | 1.50 | 0.60 | 0.59 | 80.58 | 516.13 |
| Samples | Surface Area (m2/g) | Pore Area (m2/g) | Micropore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| R-U | 0.1844 | 2.2274 | 0.0009 | - |
| R-D | 2.6321 | 3.0050 | 0.0013 | 8.8610 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Zhu, T.; Fei, X. Enhanced Adsorption Performance of Oxytetracycline by Desugared Reed Residues. Int. J. Environ. Res. Public Health 2018, 15, 2229. https://doi.org/10.3390/ijerph15102229
Zhou M, Zhu T, Fei X. Enhanced Adsorption Performance of Oxytetracycline by Desugared Reed Residues. International Journal of Environmental Research and Public Health. 2018; 15(10):2229. https://doi.org/10.3390/ijerph15102229
Chicago/Turabian StyleZhou, Min, Tao Zhu, and Xiaohua Fei. 2018. "Enhanced Adsorption Performance of Oxytetracycline by Desugared Reed Residues" International Journal of Environmental Research and Public Health 15, no. 10: 2229. https://doi.org/10.3390/ijerph15102229
APA StyleZhou, M., Zhu, T., & Fei, X. (2018). Enhanced Adsorption Performance of Oxytetracycline by Desugared Reed Residues. International Journal of Environmental Research and Public Health, 15(10), 2229. https://doi.org/10.3390/ijerph15102229




