Speciation Variation and Comprehensive Risk Assessment of Metal(loid)s in Surface Sediments of Intertidal Zones
Abstract
:1. Introduction
2. Materials and Methods
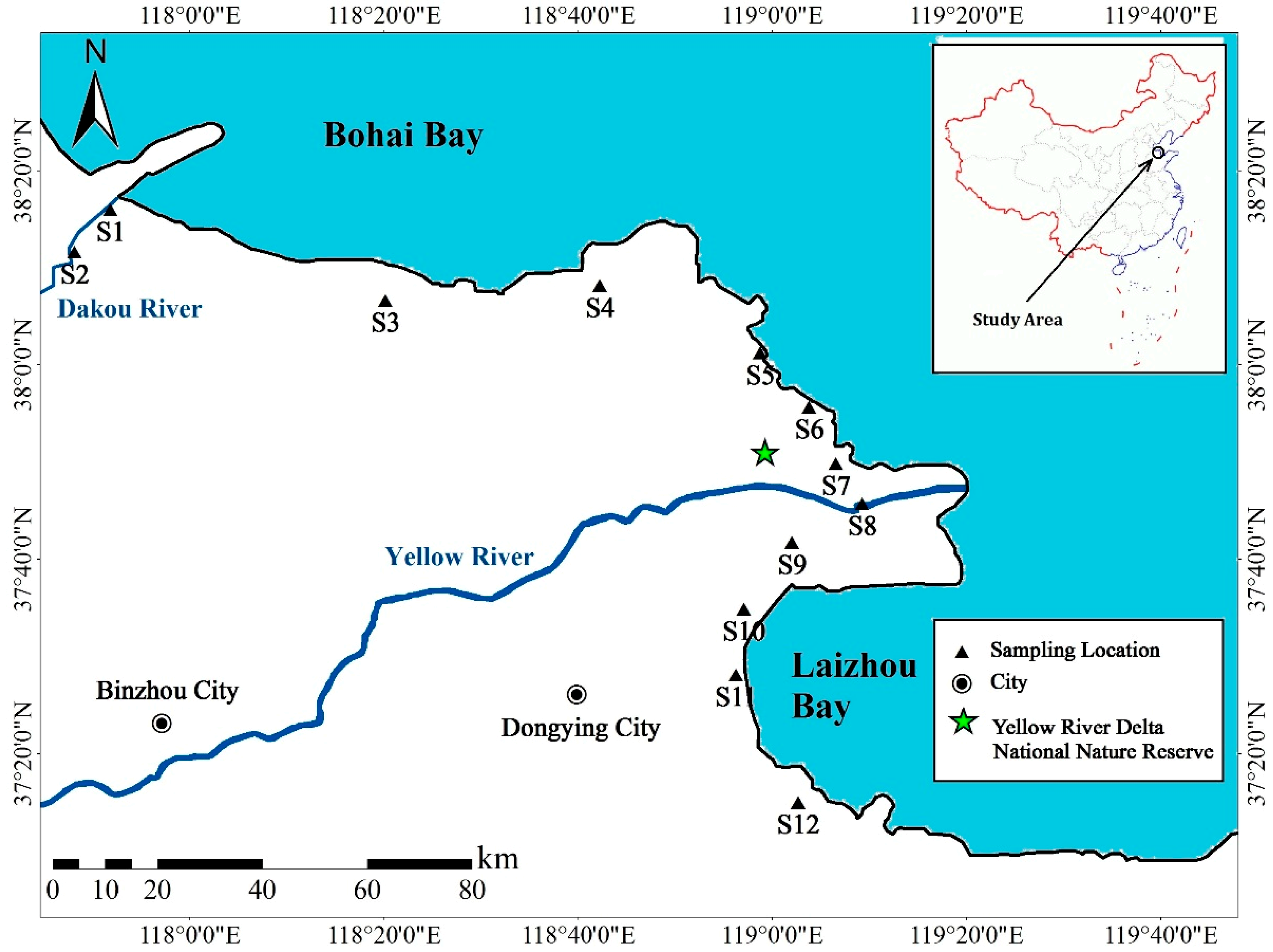
2.1. Study Area and Sample Collection
2.2. Sample Preparation and Analysis
2.3. Environmental Assessment
2.3.1. Geo-accumulation Index (Igeo)
2.3.2. Risk Assessment Code (RAC)
2.3.3. Potential Ecological Risk Index (PERI)
2.3.4. Sediment Quality Guidelines (SQGs)
2.4. Statistical Analysis
3. Results and Discussion
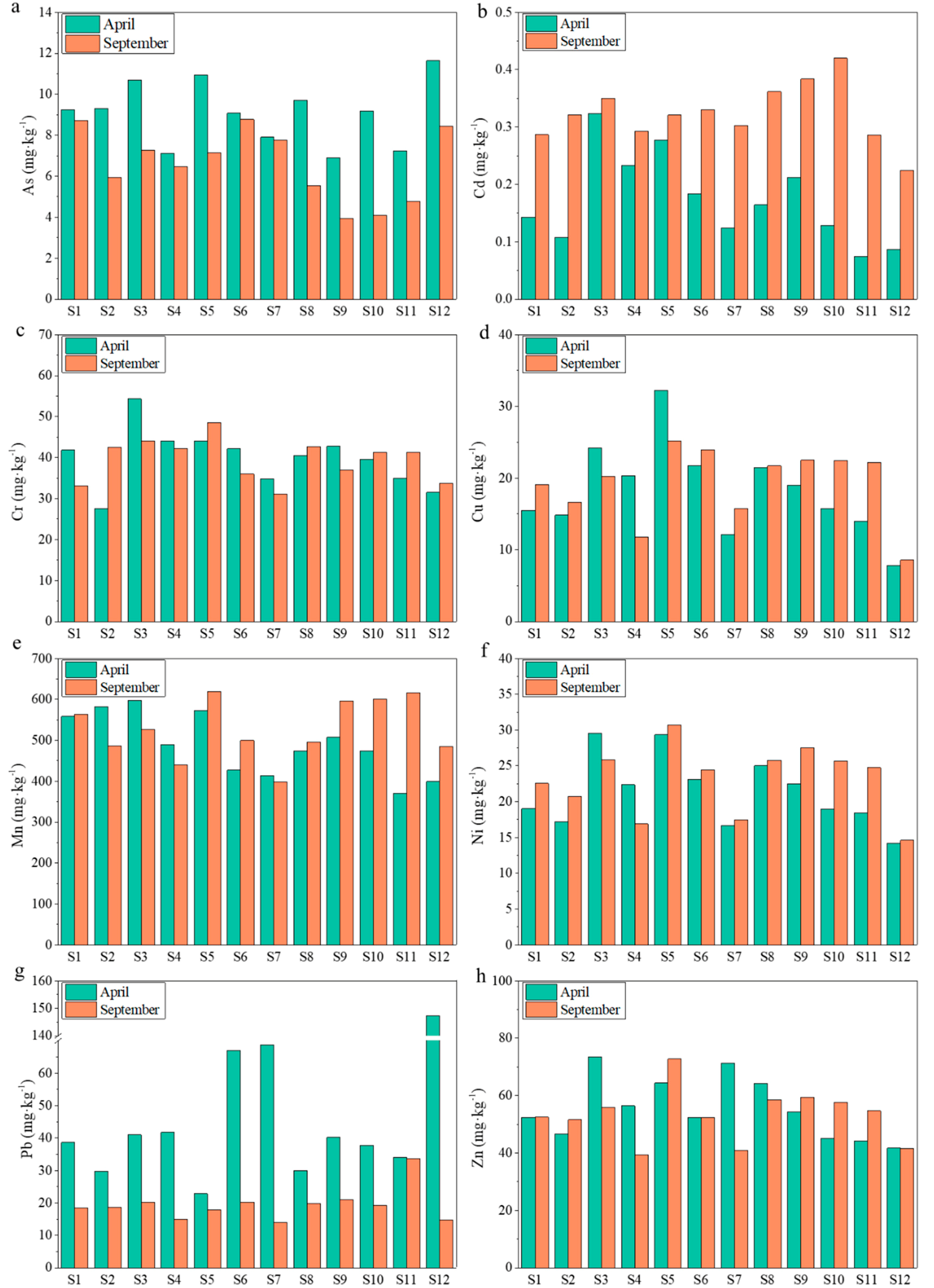
3.1. Temporal-Spatial Variation of Total Metal(loid) Concentrations
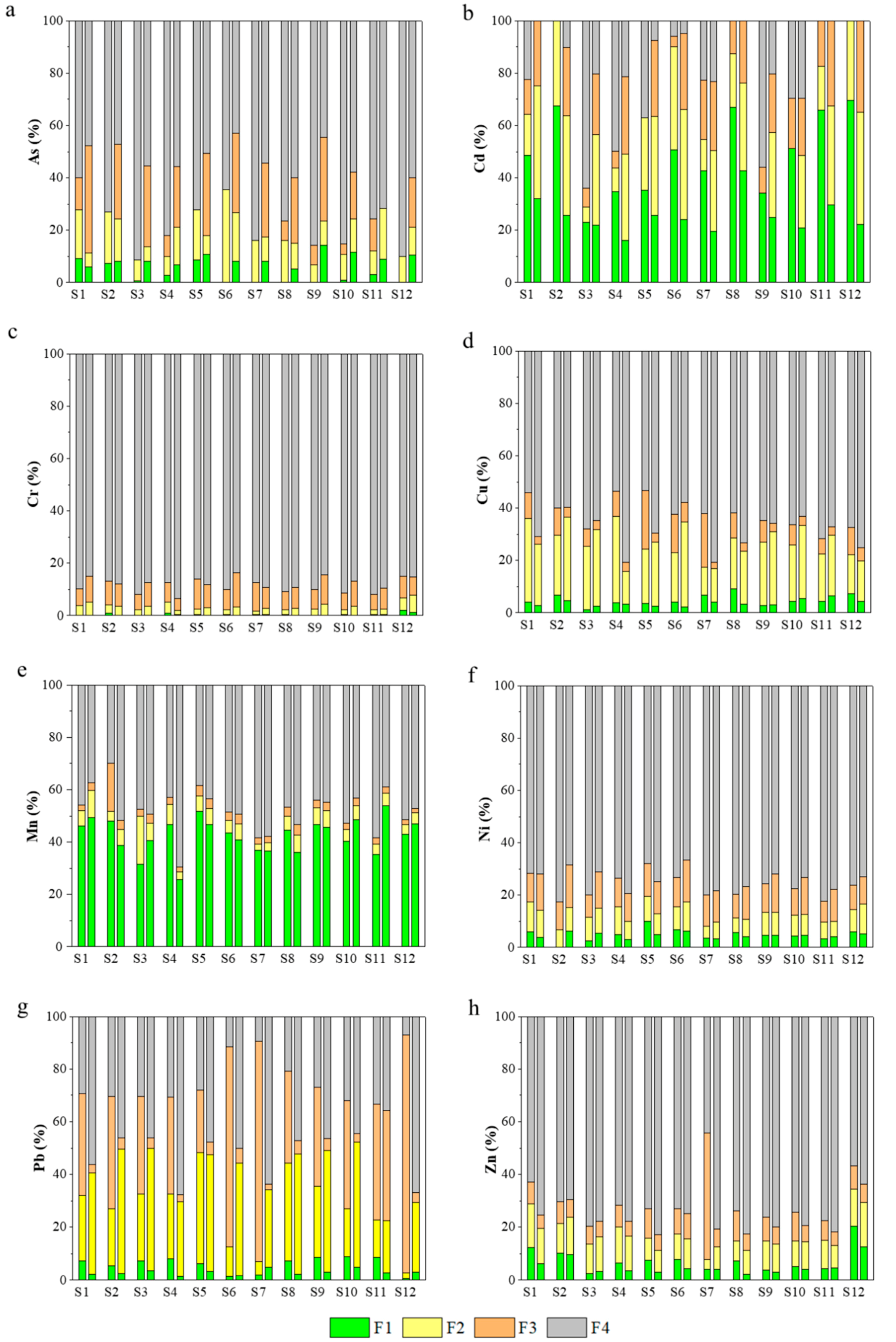
3.2. Temporal-Spatial Variation of the Metal(loid) Speciation
3.3. Environmental Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tam, N.F.; Wong, Y.S. Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ. Pollut. 2000, 110, 195–205. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Zhong, C.; Ji, J. Temporal-spatial variation and source apportionment of soil heavy metals in the representative river-alluviation depositional system. Environ. Pollut. 2016, 216, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tanner, P.A.; Lai, S.L.; Shao, M.P. Contamination of heavy metals in marine sediment cores from victoria harbour, Hong Kong. Mar. Pollut. Bull. 2000, 40, 769–779. [Google Scholar] [CrossRef]
- Chen, F.; Lin, J.; Qian, B.; Wu, Z.; Huang, P.; Chen, K.; Li, T. Geochemical assessment and spatial analysis of heavy metals in the surface sediments in the eastern Beibu Gulf: A reflection on the industrial development of the south China coast. Int. J. Environ. Res. Public Health 2018, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Zhang, E.; Hou, J.; Liu, X. Heavy metal speciation and risk assessment in dry land and paddy soils near mining areas at southern China. Environ. Sci. Pollut. Res. Int. 2016, 23, 8709–8720. [Google Scholar] [CrossRef] [PubMed]
- Atgin, R.S.; El-Agha, O.; Zararsız, A.; Kocataş, A.; Parlak, H.; Tuncel, G. Investigation of the sediment pollution in izmir bay: Trace elements. Spectrochim. Acta B 2000, 55, 1151–1164. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, W.; Le, M.; Lu, X. Temporal and spatial variations of heavy metals in urban riverine sediment: An example of Shenzhen river, pearl river delta, China. Quatern. Int. 2012, 282, 145–151. [Google Scholar] [CrossRef]
- Raju, K.V.; Somashekar, R.K.; Prakash, K.L. Spatio-temporal variation of heavy metals in Cauvery River basin. Proc. Int. Acad. Ecol. Environ. Sci. 2013, 3, 59–75. [Google Scholar]
- Singh, J.; Kalamdhad, A.S. Chemical speciation of heavy metals in compost and compost amended soil—A Review. Int. J. Environ. Eng. Res. 2013, 2, 27–37. [Google Scholar]
- Sabienë, N.; Brazauskienë, D.M.; Rimmer, D. Determination of heavy metal mobile forms by different extraction methods. Ekologija 2004, 1, 36–41. [Google Scholar]
- Alagarsamy, R. Distribution and seasonal variation of trace metals in surface sediments of the mandovi estuary, west coast of India. Estuar. Coast. Shelf Sci. 2006, 67, 333–339. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, L.; Chen, X.; Chuai, X.; Wu, Q.L. Spatial distribution and seasonal variation of heavy metals in water and sediments of Taihu Lake. Pol. J. Environ. Stud. 2012, 21, 1489–1496. [Google Scholar]
- Glasby, G.P.; Szefer, P.; Geldon, J.; Warzocha, J. Heavy-metal pollution of sediments from szczecin lagoon and the gdansk basin, Poland. Sci. Total Environ. 2004, 330, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Valdés, J.; Román, D.; Guiñez, M.; Rivera, L.; Morales, T.; Ávila, J.; Cortés, P. Distribution and temporal variation of trace metal enrichment in surface sediments of San Jorge Bay, Chile. Environ. Monit. Assess. 2010, 167, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Mohan, D.; Singh, V.K.; Malik, A. Studies on distribution and fractionation of heavy metals in Gomti river sediments—A tributary of the Ganges, India. J. Hydrol. 2005, 312, 14–27. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Smith, L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Hu, N.; Liu, J.; Huang, P.; Yan, S.; Shi, X.; Ma, D. Sources, geochemical speciation, and risk assessment of metals in coastal sediments: A case study in the Bohai sea, China. Environ. Earth Sci. 2017, 76, 309. [Google Scholar] [CrossRef]
- Metaxas, A.; Scheibling, R.E. Community structure and organization of tidepools. Mar. Ecol. Prog. 1993, 98, 187–198. [Google Scholar] [CrossRef]
- Gillman, G.P.; Sumpter, E.A. Modification to the compulsive exchange method for measuring exchange characteristics of soils. Soil Res. 1986, 24, 61–66. [Google Scholar] [CrossRef]
- Rauret, G.; Lópezsánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the bcr three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monitor. 1999, 1, 57. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Chinese Environmental Monitoring Station. Soil Background Values of China; Chinese Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified bcr sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Ruiz, E.; Alonsoazcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D. Recommended uses of empirically derived sediment quality guidelines for marine and estuarine ecosystems. Hum. Ecol. Risk Assess. 1998, 4, 1019–1039. [Google Scholar] [CrossRef]
- Huang, W.W.; Martin, J.M.; Seyler, P.; Zhang, J.; Zhong, X.M. Distribution and behaviour of arsenic in the Huang He (Yellow River) estuary and Bohai sea. Mar. Chem. 1988, 25, 75–91. [Google Scholar] [CrossRef]
- Hu, N.J.; Huang, P.; Liu, J.H.; Shi, X.F.; Ma, D.Y.; Zhu, A.M.; Zhang, J.; Zhang, H.; He, L.H. Tracking lead origin in the Yellow River Estuary and nearby Bohai Sea based on its isotopic composition. Estuar. Coast. Shelf Sci. 2015, 163, 99–107. [Google Scholar] [CrossRef]
- Dong, A.G.; Zhai, S.K.; Yu, Z.H.; Han, D.M. Evaluation on potential ecological risk of the heavy metals in the surface sediments of the Changjiang (Yangtze) estuary and its adjacent coastal area. Mar. Sci. 2010, 34, 69–75. (In Chinese) [Google Scholar]
- Li, L.; Ping, X.Y.; Wang, Y.L.; Jiang, M.; Tang, F.H.; Shen, X.Q. Spatial and temporal distribution and pollution analysis of the heavy metals in surface sediments of the Changjiang Estuary and its adjacent areas. China Environ. Sci. 2012, 32, 2245–2252. (In Chinese) [Google Scholar]
- Sillén, L.G.; Martell, A.E. Stability Constants of Metal-Ion Complexes. Soil Sci. 1965, 100, S769–S770. [Google Scholar] [CrossRef]
- Mantoura, R.F.C.; Dickson, A.; Riley, J.P. The complexation of metals with humic materials in natural waters. Estuar. Coast. Mar. Sci. 1978, 6, 387–408. [Google Scholar] [CrossRef]
- Shim, Y.S.; Kim, Y.K.; Kong, S.H.; Rhee, S.W.; Lee, W.K. The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manag. 2003, 23, 851–857. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Makino, T.; Wu, L.; Nanzyo, M. The heavy metal partition in size-fractions of the fine particles in agricultural soils contaminated by waste water and smelter dust. J. Hazard. Mater. 2013, 248–249, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Qian, X.; Gao, H.; Wang, Y.; Xia, B. Seasonal and spatial variations of heavy metals in two typical chinese rivers: Concentrations, environmental risks, and possible sources. Int. J. Environ. Res. Public Health 2014, 11, 11860–11878. [Google Scholar] [CrossRef] [PubMed]
- Teuchies, J.; Jacobs, S.; Oosterlee, L.; Bervoets, L.; Meire, P. Role of plants in metal cycling in a tidal wetland: Implications for phytoremidiation. Sci. Total Environ. 2013, 445–446, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Okbah, M.A.; Nasr, S.M.; Soliman, N.F.; Khairy, M.A. Distribution and contamination status of trace metals in the mediterranean coastal sediments, Egypt. Soil Sedim. Contam. 2014, 23, 656–676. [Google Scholar] [CrossRef]
- Lu, Q.; Bai, J.; Gao, Z.; Zhao, Q.; Wang, J. Spatial and seasonal distribution and risk assessments for metals in a tamarix chinensis, wetland, China. Wetlands 2016, 36, 125–136. [Google Scholar] [CrossRef]
- Krishna, A.K.; Mohan, K.R.; Murthy, N.N. A multivariate statistical approach for monitoring of heavy metals in sediments: A case study from Wailpalli Watershed, Nalgonda District, Andhra Pradesh, India. Res. J. Environ. Earth Sci. 2011, 3, 103–113. [Google Scholar]
- Tang, A.; Liu, R.; Ling, M.; Xu, L.; Wang, J. Distribution characteristics and controlling factors of soluble heavy metals in the Yellow River estuary and adjacent sea. Procedia Environ. Sci. 2010, 2, 1193–1198. [Google Scholar] [CrossRef]
- Velimirovic, M.B.; Prica, M.D.; Dalmacija, B.D.; Rončević, S.D.; Dalmacija, M.B.; Bečelić, M.D.; Tričković, J.S. Characterisation, availability, and risk assessment of the metals in sediment after aging. Water Air Soil Pollut. 2011, 214, 219–229. [Google Scholar] [CrossRef]
- Kalhori, A.A.; Jafari, H.; Yavari, A.; Prohic, E.; Kokya, T.A. Evaluation of anthropogenic impacts on soil and regolith materials based on BCR sequential extraction analysis. Int. J. Environ. Res. 2012, 6, 185–194. [Google Scholar]
- Jain, C.K.; Rao, V.V.S.G.; Prakash, B.A.; Kumar, K.M.; Yoshida, M. Metal fractionation study on bed sediments of Hussainsagar Lake, Hyderabad, India. Environ. Monit. Assess. 2010, 166, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Shikazono, N.; Tatewaki, K.; Mohiuddin, K.M.; Nakano, T.; Zakir, H.M. Sources, spatial variation, and speciation of heavy metals in sediments of the Tamagawa River in central Japan. Environ. Geochem. Health 2012, 34, 13–26. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.R. Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef] [PubMed]
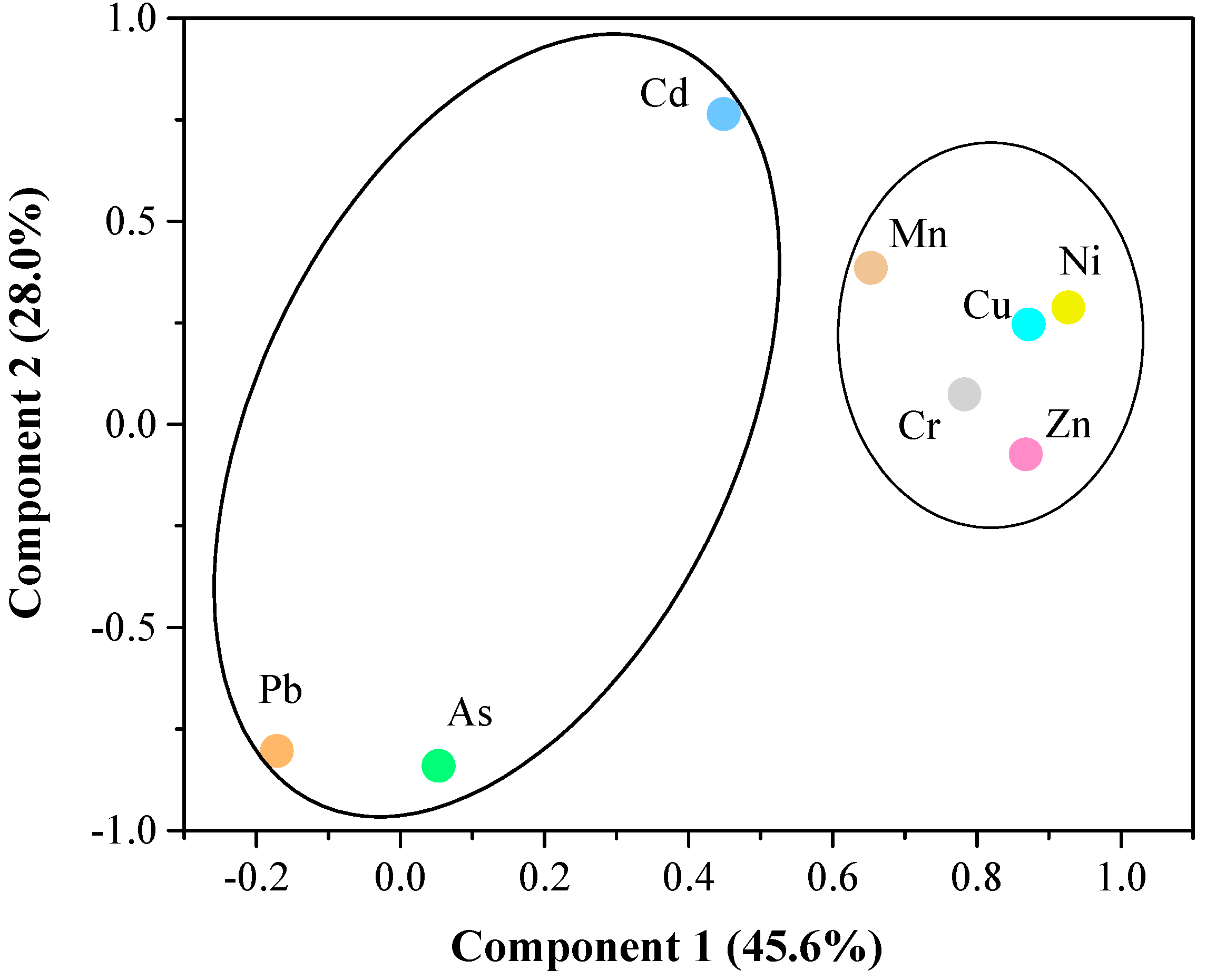
| Component | Initial Eigenvalues | Rotation Sums of Squared Loadings | ||||
|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | |
| 1 | 4.302 | 53.778 | 53.778 | 3.647 | 45.590 | 45.590 |
| 2 | 1.585 | 19.809 | 73.587 | 2.240 | 27.997 | 73.587 |
| 3 | 0.630 | 7.870 | 81.457 | |||
| 4 | 0.507 | 6.339 | 87.796 | |||
| 5 | 0.371 | 4.640 | 92.435 | |||
| 6 | 0.354 | 4.422 | 96.857 | |||
| 7 | 0.219 | 2.741 | 99.598 | |||
| 8 | 0.032 | 0.402 | 100.000 | |||
| Month | Speciation | As | Cd | Cr | Cu | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|
| April | F1 | 0.230 | 0.527 | −0.466 | 0.293 | 0.797 ** | 0.649 * | −0.524 | −0.332 |
| F2 | 0.388 | 0.282 | 0.235 | 0.842 ** | 0.636 * | 0.794 ** | −0.619 * | 0.051 | |
| F3 | −0.453 | 0.124 | 0.403 | 0.774 ** | 0.481 | 0.848 ** | 0.993 ** | 0.546 | |
| F4 | 0.818 ** | 0.893 ** | 0.992 ** | 0.951 ** | 0.236 | 0.958 ** | −0.002 | 0.716 ** | |
| September | F1 | 0.593 * | 0.529 | −0.249 | 0.439 | 0.935 ** | 0.795 ** | 0.540 | −0.214 |
| F2 | 0.370 | 0.516 | −0.184 | 0.885 ** | 0.666 * | 0.658 * | 0.351 | 0.216 | |
| F3 | 0.804 ** | 0.397 | 0.359 | 0.631 * | 0.682 * | 0.898 ** | 0.909 ** | 0.633 * | |
| F4 | 0.853 ** | 0.658 * | 0.983 ** | 0.929 ** | −0.038 | 0.969 ** | 0.673 * | 0.968 ** |
| Month | Sites | Igeo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | ||
| April | S1 | −0.59 | 0.19 | −1.24 | −1.22 | −0.79 | −1.02 | 0.00 | −0.86 |
| S2 | −0.58 | −0.23 | −1.84 | −1.28 | −0.73 | −1.17 | −0.38 | −1.03 | |
| S3 | −0.38 | 1.36 | −0.87 | −0.57 | −0.69 | −0.39 | 0.08 | −0.37 | |
| S4 | −0.97 | 0.89 | −1.17 | −0.82 | −0.98 | −0.79 | 0.11 | −0.75 | |
| S5 | −0.35 | 1.14 | −1.17 | −0.16 | −0.76 | −0.40 | −0.76 | −0.56 | |
| S6 | −0.62 | 0.54 | −1.23 | −0.73 | −1.18 | −0.75 | 0.79 | −0.86 | |
| S7 | −0.82 | −0.03 | −1.51 | −1.57 | −1.22 | −1.22 | 0.83 | −0.42 | |
| S8 | −0.52 | 0.38 | −1.29 | −0.74 | −1.03 | −0.63 | −0.37 | −0.57 | |
| S9 | −1.01 | 0.75 | −1.21 | −0.92 | −0.93 | −0.78 | 0.05 | −0.81 | |
| S10 | −0.60 | 0.03 | −1.33 | −1.20 | −1.03 | −1.03 | −0.04 | −1.08 | |
| S11 | −0.95 | −0.76 | −1.50 | −1.37 | −1.38 | −1.07 | −0.19 | −1.11 | |
| S12 | −0.26 | −0.54 | −1.65 | −2.20 | −1.28 | −1.45 | 1.93 | −1.19 | |
| September | S1 | −0.68 | 1.19 | −1.58 | −0.92 | −0.78 | −0.78 | −1.07 | −0.86 |
| S2 | −1.23 | 1.35 | −1.22 | −1.11 | −0.99 | −0.90 | −1.05 | −0.88 | |
| S3 | −0.94 | 1.47 | −1.17 | −0.83 | −0.88 | −0.58 | −0.94 | −0.77 | |
| S4 | −1.11 | 1.22 | −1.23 | −1.61 | −1.14 | −1.20 | −1.38 | −1.27 | |
| S5 | −0.97 | 1.35 | −1.03 | −0.52 | −0.64 | −0.33 | −1.11 | −0.39 | |
| S6 | −0.67 | 1.39 | −1.46 | −0.59 | −0.95 | −0.66 | −0.94 | −0.87 | |
| S7 | −0.85 | 1.26 | −1.67 | −1.19 | −1.28 | −1.15 | −1.48 | −1.22 | |
| S8 | −1.33 | 1.52 | −1.21 | −0.73 | −0.96 | −0.59 | −0.96 | −0.70 | |
| S9 | −1.82 | 1.61 | −1.42 | −0.68 | −0.70 | −0.49 | −0.89 | −0.68 | |
| S10 | −1.77 | 1.74 | −1.26 | −0.68 | −0.69 | −0.59 | −1.01 | −0.73 | |
| S11 | −1.54 | 1.18 | −1.26 | −0.70 | −0.65 | −0.64 | −0.20 | −0.80 | |
| S12 | −0.72 | 0.83 | −1.55 | −2.07 | −0.99 | −1.41 | −1.39 | −1.20 | |
| Month | Sites | RAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | ||
| April | S1 | L | H | N | L | H | L | L | M |
| S2 | L | VH | N | L | H | N | L | M | |
| S3 | N | M | N | L | H | L | L | L | |
| S4 | L | H | N | L | H | L | L | L | |
| S5 | L | H | N | L | VH | L | L | L | |
| S6 | N | VH | N | L | H | L | L | L | |
| S7 | N | H | N | L | H | L | L | L | |
| S8 | N | VH | N | L | H | L | L | L | |
| S9 | N | H | N | L | H | L | L | L | |
| S10 | N | VH | N | L | H | L | L | L | |
| S11 | L | VH | N | L | H | L | L | L | |
| S12 | N | VH | L | L | H | L | N | M | |
| September | S1 | L | H | N | L | H | L | L | L |
| S2 | L | M | N | L | H | L | L | L | |
| S3 | L | M | N | L | H | L | L | L | |
| S4 | L | M | N | L | M | L | L | L | |
| S5 | M | M | N | L | H | L | L | L | |
| S6 | L | M | N | L | H | L | L | L | |
| S7 | L | M | N | L | H | L | L | L | |
| S8 | L | H | N | L | H | L | L | L | |
| S9 | M | M | N | L | H | L | L | L | |
| S10 | M | M | N | L | H | L | L | L | |
| S11 | L | M | N | L | VH | L | L | L | |
| S12 | M | M | L | L | H | L | L | M | |
| Month | Sites | RI | Risk Grade | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | ||||
| April | S1 | 9.94 | 51.16 | 1.27 | 3.23 | 0.87 | 3.69 | 7.49 | 0.82 | 78.48 | Low |
| S2 | 10.00 | 38.35 | 0.84 | 3.09 | 0.90 | 3.33 | 5.75 | 0.73 | 63.00 | Low | |
| S3 | 11.51 | 115.43 | 1.65 | 5.04 | 0.93 | 5.72 | 7.95 | 1.16 | 149.38 | Low | |
| S4 | 7.66 | 83.32 | 1.33 | 4.24 | 0.76 | 4.33 | 8.09 | 0.89 | 110.62 | Low | |
| S5 | 11.76 | 98.93 | 1.34 | 6.71 | 0.89 | 5.68 | 4.42 | 1.01 | 130.75 | Low | |
| S6 | 9.76 | 65.63 | 1.28 | 4.52 | 0.66 | 4.47 | 12.98 | 0.82 | 100.13 | Low | |
| S7 | 8.50 | 44.16 | 1.05 | 2.53 | 0.64 | 3.23 | 13.33 | 1.12 | 74.56 | Low | |
| S8 | 10.44 | 58.76 | 1.23 | 4.48 | 0.74 | 4.85 | 5.82 | 1.01 | 87.32 | Low | |
| S9 | 7.42 | 75.78 | 1.30 | 3.96 | 0.79 | 4.36 | 7.79 | 0.85 | 102.25 | Low | |
| S10 | 9.88 | 45.93 | 1.20 | 3.27 | 0.74 | 3.67 | 7.30 | 0.71 | 72.69 | Low | |
| S11 | 7.78 | 26.49 | 1.06 | 2.91 | 0.57 | 3.57 | 6.59 | 0.70 | 49.66 | Low | |
| S12 | 12.51 | 30.86 | 0.95 | 1.63 | 0.62 | 2.75 | 28.55 | 0.66 | 78.53 | Low | |
| Mean | 9.76 | 61.23 | 1.21 | 3.80 | 0.76 | 4.14 | 9.67 | 0.87 | |||
| grade | Low | Moderate | Low | Low | Low | Low | Low | Low | |||
| September | S1 | 9.39 | 102.38 | 1.00 | 3.98 | 0.88 | 4.37 | 3.56 | 0.83 | 126.38 | Low |
| S2 | 6.37 | 114.85 | 1.29 | 3.46 | 0.75 | 4.01 | 3.62 | 0.81 | 135.17 | Low | |
| S3 | 7.81 | 125.00 | 1.33 | 4.21 | 0.82 | 5.00 | 3.92 | 0.88 | 148.98 | Low | |
| S4 | 6.94 | 104.58 | 1.28 | 2.46 | 0.68 | 3.27 | 2.88 | 0.62 | 122.73 | Low | |
| S5 | 7.67 | 114.64 | 1.47 | 5.24 | 0.96 | 5.95 | 3.47 | 1.15 | 140.54 | Low | |
| S6 | 9.43 | 117.95 | 1.09 | 4.98 | 0.78 | 4.73 | 3.92 | 0.82 | 143.70 | Low | |
| S7 | 8.33 | 107.98 | 0.94 | 3.29 | 0.62 | 3.38 | 2.69 | 0.65 | 127.88 | Low | |
| S8 | 5.97 | 129.19 | 1.29 | 4.52 | 0.77 | 4.99 | 3.85 | 0.92 | 151.50 | Moderate | |
| S9 | 4.24 | 136.94 | 1.12 | 4.69 | 0.93 | 5.33 | 4.06 | 0.94 | 158.24 | Moderate | |
| S10 | 4.40 | 149.91 | 1.25 | 4.68 | 0.93 | 4.98 | 3.72 | 0.91 | 170.78 | Moderate | |
| S11 | 5.14 | 102.29 | 1.25 | 4.62 | 0.96 | 4.80 | 6.52 | 0.86 | 126.43 | Low | |
| S12 | 9.09 | 80.07 | 1.02 | 1.78 | 0.75 | 2.83 | 2.86 | 0.65 | 99.06 | Low | |
| Mean | 7.07 | 115.48 | 1.20 | 3.99 | 0.82 | 4.47 | 3.75 | 0.84 | |||
| grade | Low | Appreciable | Low | Low | Low | Low | Low | Low | |||
| Month | As | Cd | Cr | Cu | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| April | ERLa (mg·kg−1) | 8.2 | 1.2 | 81 | 34 | - | 20.9 | 46.7 | 150 |
| ERMa (mg·kg−1) | 70 | 9.6 | 370 | 270 | - | 51.6 | 218 | 410 | |
| TELb (mg·kg−1) | 7.24 | 0.68 | 52.3 | 18.7 | - | 15.9 | 30.2 | 124 | |
| PELb (mg·kg−1) | 41.6 | 4.21 | 160 | 108 | - | 42.8 | 112 | 271 | |
| <ERL (%) | 33.33 | 100 | 100 | 100 | - | 50 | 75 | 100 | |
| ERL-ERM (%) | 66.67 | 0 | 0 | 0 | - | 50 | 25 | 0 | |
| >ERM (%) | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | |
| <TEL (%) | 16.67 | 100 | 91.67 | 50 | - | 8.33 | 25 | 100 | |
| TEL-PEL (%) | 83.33 | 0 | 8.33 | 50 | - | 91.67 | 66.67 | 0 | |
| >PEL (%) | 0 | 0 | 0 | 0 | - | 0 | 8.33 | 0 | |
| September | <ERL (%) | 75 | 100 | 100 | 100 | - | 33.33 | 100 | 100 |
| ERL-ERM (%) | 25 | 0 | 0 | 0 | - | 66.67 | 0 | 0 | |
| >ERM (%) | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 | |
| <TEL (%) | 58.33 | 100 | 100 | 33.33 | - | 8.33 | 91.67 | 100 | |
| TEL-PEL (%) | 41.67 | 0 | 0 | 66.67 | - | 91.67 | 8.33 | 0 | |
| >PEL (%) | 0 | 0 | 0 | 0 | - | 0 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Qian, X.; Peng, S.; Liu, X.; Bai, L.; Cui, B.; Bai, J. Speciation Variation and Comprehensive Risk Assessment of Metal(loid)s in Surface Sediments of Intertidal Zones. Int. J. Environ. Res. Public Health 2018, 15, 2125. https://doi.org/10.3390/ijerph15102125
Liang B, Qian X, Peng S, Liu X, Bai L, Cui B, Bai J. Speciation Variation and Comprehensive Risk Assessment of Metal(loid)s in Surface Sediments of Intertidal Zones. International Journal of Environmental Research and Public Health. 2018; 15(10):2125. https://doi.org/10.3390/ijerph15102125
Chicago/Turabian StyleLiang, Baocui, Xiao Qian, Shitao Peng, Xinhui Liu, Lili Bai, Baoshan Cui, and Junhong Bai. 2018. "Speciation Variation and Comprehensive Risk Assessment of Metal(loid)s in Surface Sediments of Intertidal Zones" International Journal of Environmental Research and Public Health 15, no. 10: 2125. https://doi.org/10.3390/ijerph15102125
APA StyleLiang, B., Qian, X., Peng, S., Liu, X., Bai, L., Cui, B., & Bai, J. (2018). Speciation Variation and Comprehensive Risk Assessment of Metal(loid)s in Surface Sediments of Intertidal Zones. International Journal of Environmental Research and Public Health, 15(10), 2125. https://doi.org/10.3390/ijerph15102125







