Characteristics of Refractive Errors in a Population of Adults in the Central Region of Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects, Eye Examinations and Definitions
2.2. Data Management and Statistical Analysis
3. Results
3.1. Subjects
3.2. Distribution of Distance Visual Acuity and Refractive Errors
3.3. Multiple Logistic Regression Modeling
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-W.; Ramamurthy, D.; Saw, S.-M. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol. Opt. 2012, 32, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Romero, A.F. The prevalence of refractive conditions in Puerto Rican adults attending an eye clinic system. J. Optom. 2014, 7, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.R.; Holden, B.A.; Wilson, D.A.; Schlenther, G.; Naidoo, K.S.; Resnikoff, S.; Frick, K.D. Global cost of correcting vision impairment from uncorrected refractive error. Bull. World Health Organ. 2012, 90, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.F.; Cotch, M.F.; Vitale, S.; Zhang, X.; Klein, R.; Friedman, D.S.; Klein, B.E.; Saaddine, J.B. Age-related eye diseases and visual impairment among U.S. adults. Am. J. Prev. Med. 2013, 45, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.S.; Smigielski, J. The prevalence and causes of visual impairment and blindness among older adults in the city of Lodz, Poland. Medicine 2015, 94, e505. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, C.; Höhn, R.; Kottler, U.; Wild, P.; Blettner, M.; Bühren, J.; Pfeiffer, N.; Mirshahi, A. Prevalence of refractive errors in the European adult population: The Gutenberg Health Study (GHS). Br. J. Ophthalmol. 2014, 98, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.J.; Jiang, Y. Epidemiology of myopia. Eye 2014, 28, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Nemesure, B.; Leske, M.C. Refractive errors in black adult population: The Barbados Eye Study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2179–2184. [Google Scholar]
- Hyman, L. Myopic and hyperopic refractive error in adults: An overview. Ophthalmic Epidemiol. 2007, 14, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Antón, A.; Andrada, M.T.; Mayo, A.; Portela, J.; Merayo, J. Epidemiology of refractive errors in an adult European population: The Segovia Study. Ophthalmic Epidemiol. 2009, 16, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.W.; Wong, T.Y.; Lavanya, R.; Wu, R.Y.; Zheng, Y.F.; Lin, X.Y.; Mitchell, P.; Aung, T.; Saw, S.M. Prevalence and risk factors for refractive errors in Indians: The Singapore Indian Eye Study (SINDI). Investig. Ophthalmol. Vis. Sci. 2011, 52, 3166–3173. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.; Ramesh, S.V.; Arvind, H.; George, R.; Baskaran, M.; Paul, P.G.; Kumaramanickavel, G.; McCarty, C.; Vijaya, L. Prevalence of refractive errors in a rural South Indian population. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Chan, Y.H.; Wong, T.Y.; Gazzard, G.; Niti, M.; Ng, T.P.; Saw, S.M. Prevalence and risk factors for refractive errors and ocular biometry parameters in an elderly Asian population: The Singapore Longitudinal Aging Study (SLAS). Eye 2011, 25, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Sawada, A.; Tomidokoro, A.; Araie, M.; Iwase, A.; Yamamoto, T.; Tajimi Study Group. Refractive errors in elderly Japanese population: The Tajimi Study. Ophthalmology 2008, 115, 363–370. [Google Scholar] [PubMed]
- Xu, L.; Li, J.; Cui, T.; Hu, A.; Fan, G.; Zhang, R.; Yang, H.; Sun, B.; Jonas, J.B. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology 2005, 112, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Hsu, W.M.; Liu, J.H.; Tsai, S.Y.; Chou, P. Refractive errors in an elderly Chinese population in Taiwan: The Shihpai Eye Study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4630–4638. [Google Scholar] [CrossRef]
- Ziaei, H.; Katibeh, M.; Solaimanizad, R.; Hosseini, S.; Gilasi, H.R.; Golbafian, F.; Javadi, M.A. Prevalence of refractive errors: The Yazd Eye Study. J. Ophthalmic Vis. Res. 2013, 8, 227–236. [Google Scholar] [PubMed]
- Kim, E.C.; Morgan, I.G.; Kakizaki, H.; Kang, S.; Jee, D. Prevalence and risk factors for refractive errors: Korean National Health and Nutrition Examination Survey 2008–2011. PLoS ONE 2013, 8, e80361. [Google Scholar] [CrossRef] [PubMed]
- Attebo, K.; Ivers, R.Q.; Mitchell, P. Refractive errors in an older population: The Blue Mountain Eye Study. Ophthalmology 1999, 106, 1066–1072. [Google Scholar] [CrossRef]
- Wensor, M.; McCarthy, C.A.; Taylor, H.R. Prevalence and risk factors of myopia in Victoria, Australia. Arch. Ophthalmol. 1999, 117, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Klein, B.E.; Klein, R.; Moss, S.E. Refractive status in Beaver Dam Eye Study. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4344–4347. [Google Scholar]
- Katz, J.; Tielsch, J.M.; Sommer, A. Prevalence and risk factors for refractive errors in an adult inner city population. Investig. Ophthalmol. Vis. Sci. 1997, 38, 334–340. [Google Scholar]
- Vitale, S.; Ellwein, L.; Cotch, M.F.; Ferris, F.L., 3rd; Sperduto, R. Prevalence of refractive error in the United States, 1999–2004. Arch. Ophthalmol. 2008, 126, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, J.C.; Khawaja, A.P.; Broadway, D.; Luben, R.; Hayat, S.; Dalzell, N.; Wareham, N.J.; Khaw, K.T.; Foster, P.J. Uncorrected refractive error in older British adults: The EPIC-Norfolk Eye Study. Br. J. Ophthalmol. 2012, 96, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, E.; Jonasson, F.; Jonsson, V.; Stefánsson, E.; Sasaki, H.; Sasaki, K. “With the rule” astigmatism is not the rule in the elderly. Reykjavik Eye Study: A population based study of refraction and visual acuity in citizens of Reykjavik 50 years and older. Iceland-Japan Co-Working Study Groups. Acta Ophthalmol. Scand. 2000, 78, 642–646. [Google Scholar] [CrossRef] [PubMed]
- The National Census of Population and Housing 1 April–30 June 2011. Zakład Wydawnictw Statystycznych: Warszawa, 2013. Available online: http://www.stat.gov.pl/gus/nsp (accessed on 1 June 2017).
- Nowak, M.S.; Smigielski, J. The prevalence of age-related eye diseases and cataract surgery among older adults in the city Lodz, Poland. J. Ophthalmol. 2015, 2015, 605814. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.S.; Goś, R.; Jurowski, P.; Śmigielski, J. Correctable and non-correctable visual impairment among young males: A 12-year prevalence study of the Military Service in Poland. Ophthalmic Physiol. Opt. 2009, 29, 443–448. [Google Scholar] [PubMed]
- Nowak, M.S.; Jurowski, P.; Goś, R.; Śmigielski, J. Ocular findings among young men: A 12 year prevalence study of military service in Poland. Acta Ophthamol. 2010, 88, 535–540. [Google Scholar] [CrossRef] [PubMed]
- European Glaucoma Society. Terminology and Guidelines for Glaucoma, 3rd ed.; Dogma: Savona, Italy, 2008; pp. 93–116. [Google Scholar]
- Pan, C.W.; Zheng, Y.F.; Anuar, A.R.; Chew, M.; Gazzard, G.; Aung, T.; Cheng, C.Y.; Wong, T.Y.; Saw, S.M. Prevalence of refractive errors in a multiethnic Asian population: The Singapore epidemiology of eye disease study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Ezelum, C.; Razavi, H.; Sivasubramaniam, S.; Gilbert, C.E.; Murthy, G.V.; Entekume, G.; Abubakar, T. Nigeria National Blindness and Visual Impairment Study Group. Refractive error in Nigerian adults: Prevalence, type, and spectacle coverage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5449–5456. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.; Dineen, B.P.; Ali, S.M.; Noorul Huq, D.M.; Johnson, G.J. Prevalence of refractive error in Bangladeshi adults: Results of the National Blindness and Low Vision Survey of Bangladesh. Ophthalmology 2004, 111, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.M.; Iribarren, R.; Latino, S.G.; Torres, V.E.; Gramajo, A.L.; Artal, M.N.; Yadarola, M.B.; Garay, P.R.; Luna, J.D.; Juarez, C.P. Prevalence of refractive errors in Villa Maria, Cordoba, Argentina. Eye Sci. 2016, 31, 68–77. [Google Scholar] [CrossRef]
- Hashemi, H.; Khabazkhoob, M.; Iribarren, R.; Emamian, M.H.; Fotouhi, A. Five-year change in refraction and its ocular components in the 40- to 64-year-old population of the Shahroud eye cohort study. Clin. Exp. Ophthalmol. 2016, 44, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, S.; Foster, P.J.; Uranchimeg, D.; Lee, P.S.; Devereux, J.G.; Alsbirk, P.H.; Machin, D.; Johnson, G.J.; Baasanhu, J. Ocular biometry and refraction in Mongolian adults. Investig. Ophthalmol. Vis. Sci. 2004, 45, 776–783. [Google Scholar] [CrossRef]
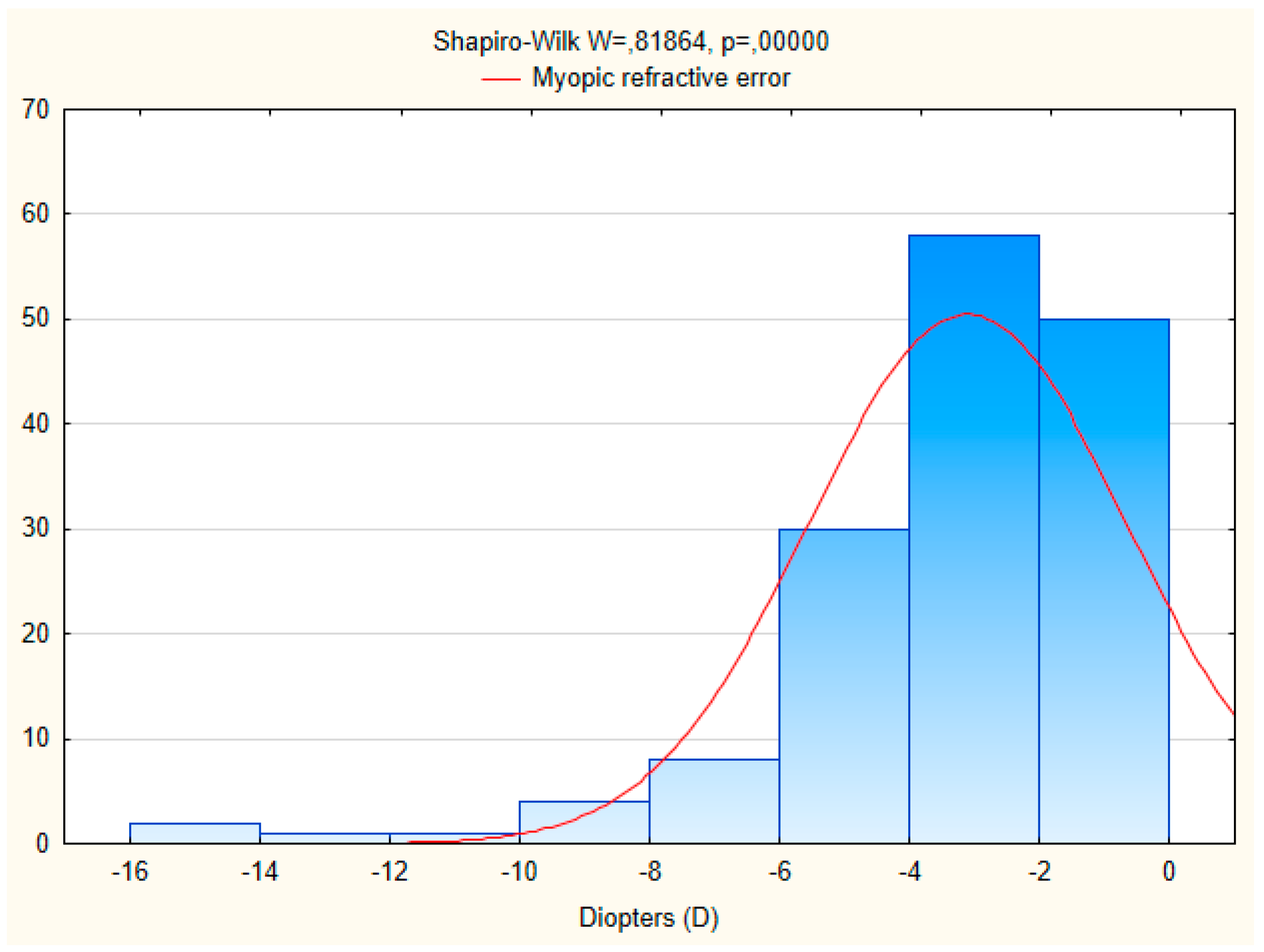
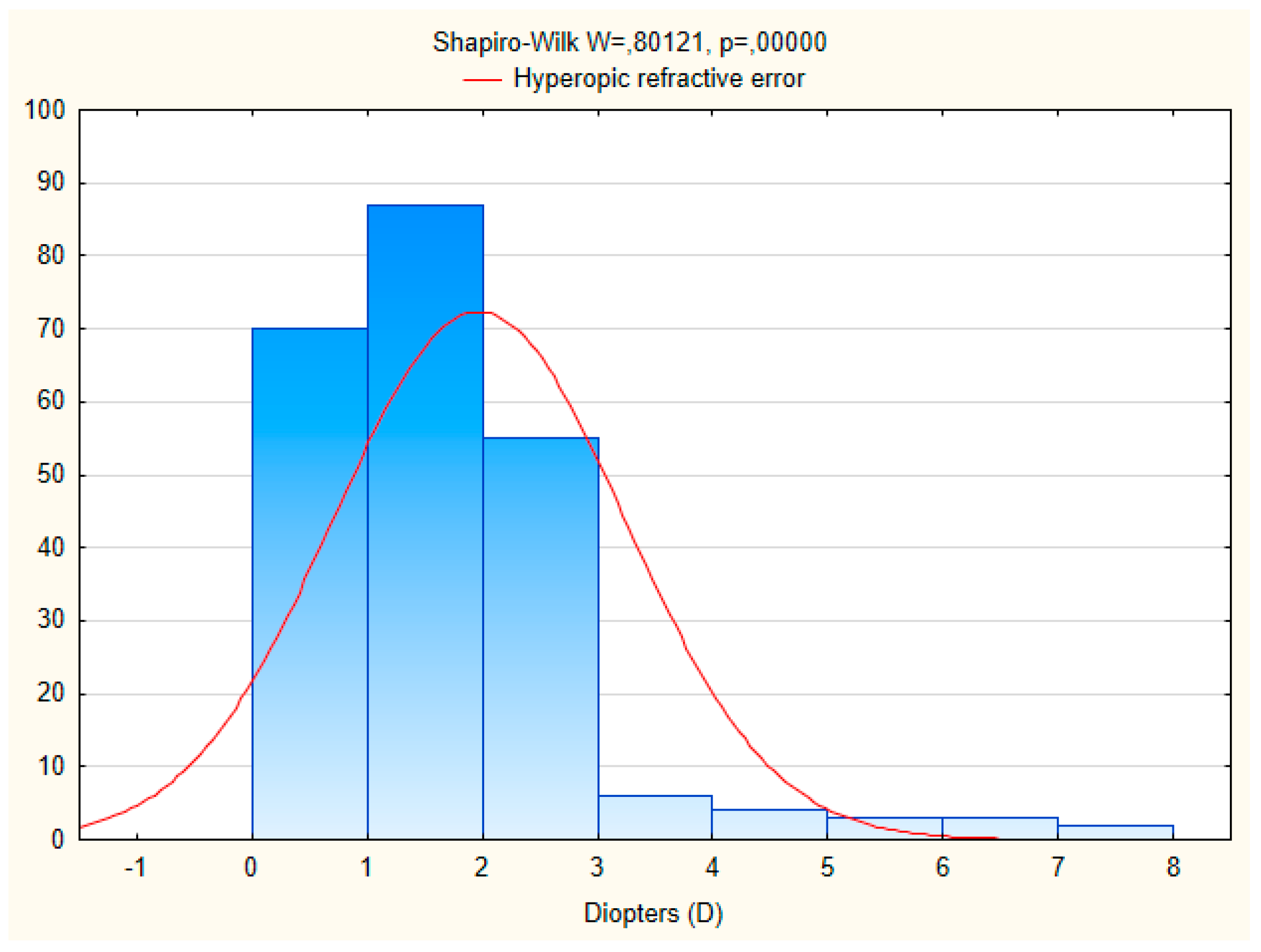
| Visual Acuity Category | Right Eyes n (%; 95% CI) | Left Eyes n (%; 95% CI) | Both Eyes n (%; 95% CI) | |
|---|---|---|---|---|
| ≥20/40 | 842 (76.0%; 73.5.5–78.6) | 846 (76.4%; 73.9–78.9) | 803 (72.5%; 69.9–75.1) | |
| >20/200 <20/40 | 209 (18.9%; 16.6–21.2) | 202 (18.3%; 16.0–20.5) | 224 (20.2%; 17.8–22.6) † | |
| ≤20/200 | 56 (5.1%; 3.8–6.3) | 59 (5.3%; 4.0–6.6) | 80 (7.3%; 5.7–8.7) † | |
| All | 1107 (100%) | 1107 (100%) | 1107 (100%) | |
| 35–59 Years | ≥60 Years | |||
| ≥20/40 | 397 (76.4%; 72.7–80.0) | 406 (69.2%; 65.4–72.9) | ||
| >20/200 <20/40 | 88 (16.9%; 13.7–20.1) † | 136 (23.2%; 19.7–26.6) † | ||
| ≤20/200 | 35 (6.7%; 4.6–8.9) † | 45 (7.6%; 5.5–9.8) † | ||
| all | 520 (100%) | 587 (100%) | ||
| χ2 test p < 0.001 | ||||
| Men | Women | |||
| ≥20/40 | 358 (77.0%; 73.2–80.8) | 445 (69.3%; 65.7–72.9) | ||
| >20/200 <20/40 | 76 (16.3%; 13.0–19.7) † | 148 (23.1%; 19.8–26.3) † | ||
| ≤20/200 | 31 (6.7%; 4.4–8.9) † | 49 (7.6%; 5.6–9.7) † | ||
| all | 465 (100%) | 642 (100%) | ||
| χ2 test, p = 0.01 | ||||
| Refractive Error | 35–59 Years (n; %; 95% CI) | ≥60 Years (n; %; 95% CI) | Men (n; %; 95% CI) | Women (n; %: 95% CI) | Totally (n; %; 95% CI) |
|---|---|---|---|---|---|
| Emmetropia (>−0.5 D, <+0.5 D, SE) | 214 (42.7%; 38.4–47.0) | 62 (12.5%; 9.6–15.4) | 142 (34.2%; 29.6–38.8) | 134 (23.0%; 19.6–26.4) | 276 (27.6%; 24.9–30.4) |
| Myopia (≤−0.5 D, SE) | 144 (28.7%; 24.8–32.7) | 96 (19.3%; 15.8–22.8) | 90 (21.7%; 17.7–25.6) | 150 (25.7%; 22.2–29.3) | 240 (24.1%; 21.4–26.7) † |
| Hyperopia (≥+0.5 D, SE) | 109 (21.8%; 18.1–25.4) | 265 (53.3%; 48.9–57.7) | 129 (31.1%; 26.6–35.5) | 245 (42.0%; 38.0–46.0) | 374 (37.5%; 34.5–40.5) † |
| Astigmatism (≥0.5 D, Cyl) | 34 (6.8%; 4.6–9.0) | 74 (14.9%; 11.8–18.0) | 54 (13.0%; 9.8–16.2) | 54 (9.3%; 6.9–11.6) | 108 (10.8%; 8.9–12.7) † |
| All | 501 (100%) | 497 (100%) | 415 (100%) | 583 (100%) | 998 (100%) |
| Variables | Myopia ≤ 0.5 D | Hyperopia ≥ 0.5 D |
|---|---|---|
| OR, 95% CI, p Value | OR, 95% CI, p Value | |
| Age, per year increase | 0.98 (0.96–1.00); p = 0.023 | 1.02 (1.00–1.04); p = 0.046 |
| Women vs. men | 1.87 (1.18–2.95); p = 0.007 | 2.16 (1.38–3.38); p = 0.001 |
| Any cataract | 2.40 (1.24–4.63); p = 0.009 | 1.68 (0.96–2.96); p = 0.070 |
| Glaucoma and ocular hypertension (OHT) | 0.36 (0.11–1.19); p = 0.094 | 0.52 (0.22–1.23); p = 0.136 |
| Socioeconomic status | 1.21 (0.35–4.14); p = 0.766 | 1.87 (0.62–5.63); p = 0.264 |
| Epidemiological Study | Sampling Technique | Age Group (Years) | Myopia (%) | Hyperopia (%) | Astigmatism (%) | Anisometropia (%) |
|---|---|---|---|---|---|---|
| The Beaver Dam Eye Study (USA) [22] † | a door to door census | ≥43 | 26.2 | 49.0 | NA | NA |
| The Blue Mountains Eye Study (Australia) [20] † | a door to door census | ≥49 | 15.5 | 56.6 | NA | NA |
| The Tajimi Study (Japan) [15] † | random sampling | ≥40 | 41.8 | 27.9 | 54.0 | 15.1 |
| The Gutenberg Health Study (Germany) [7] † | random sampling | ≥35 | 35.1 | 32.8 | 32.3 | 13.5 |
| The Barbados Eye Study (Barbados) [9] † | random sampling | ≥40 | 21.9 | 46.9 | NA | NA |
| The Singapore Indian Eye Study (Singapore) [12] † | age-stratified random sampling | ≥40 | 28.0 | 35.9 | 54.9 | 9.8 |
| The Segovia Study (Spain) [11] ‡ | age-stratified random sampling | ≥40 | 25.4 | 43.6 | 53.5 | 12.3 |
| The Yazd Eye Study (Iran) [18] ‡ | multistage random cluster sampling | ≥40 | 36.5 | 20.6 | 53.8 | 11.9 |
| Korean National Health and Nutrition Examination Survey (South Korea) [19] § | multistage stratified cluster random sampling | ≥20 | 48.1 | 24.2 | 34.0 | NA |
| The Nigerian National Blindness and Visual Impairment Study (Nigeria) [33] † | multistage stratified cluster random sampling | ≥40 | 16.2 | 50.7 | 63.5 | NA |
| The National Blindness and Low Vision Survey of Bangladesh (Bangladesh) [34] † | cluster sampling and a door to door enumeration | ≥30 | 22.1 | 20.6 | 32.4 | 7.5 |
| The Shihpai Eye Study (Taiwan) [17] † | random sampling and a door to door enumeration | ≥65 | 19.4 | 59.0 | 74.0 | 21.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, M.S.; Jurowski, P.; Grzybowski, A.; Smigielski, J. Characteristics of Refractive Errors in a Population of Adults in the Central Region of Poland. Int. J. Environ. Res. Public Health 2018, 15, 90. https://doi.org/10.3390/ijerph15010090
Nowak MS, Jurowski P, Grzybowski A, Smigielski J. Characteristics of Refractive Errors in a Population of Adults in the Central Region of Poland. International Journal of Environmental Research and Public Health. 2018; 15(1):90. https://doi.org/10.3390/ijerph15010090
Chicago/Turabian StyleNowak, Michal S., Piotr Jurowski, Andrzej Grzybowski, and Janusz Smigielski. 2018. "Characteristics of Refractive Errors in a Population of Adults in the Central Region of Poland" International Journal of Environmental Research and Public Health 15, no. 1: 90. https://doi.org/10.3390/ijerph15010090
APA StyleNowak, M. S., Jurowski, P., Grzybowski, A., & Smigielski, J. (2018). Characteristics of Refractive Errors in a Population of Adults in the Central Region of Poland. International Journal of Environmental Research and Public Health, 15(1), 90. https://doi.org/10.3390/ijerph15010090







