Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High
Abstract
:1. Introduction
2. .Material and Methods
2.1. Sample Collection
2.2. Isolation of Acinetobacter
2.3. Susceptibility Testing
2.4. Determination of β-Lactamase Genes
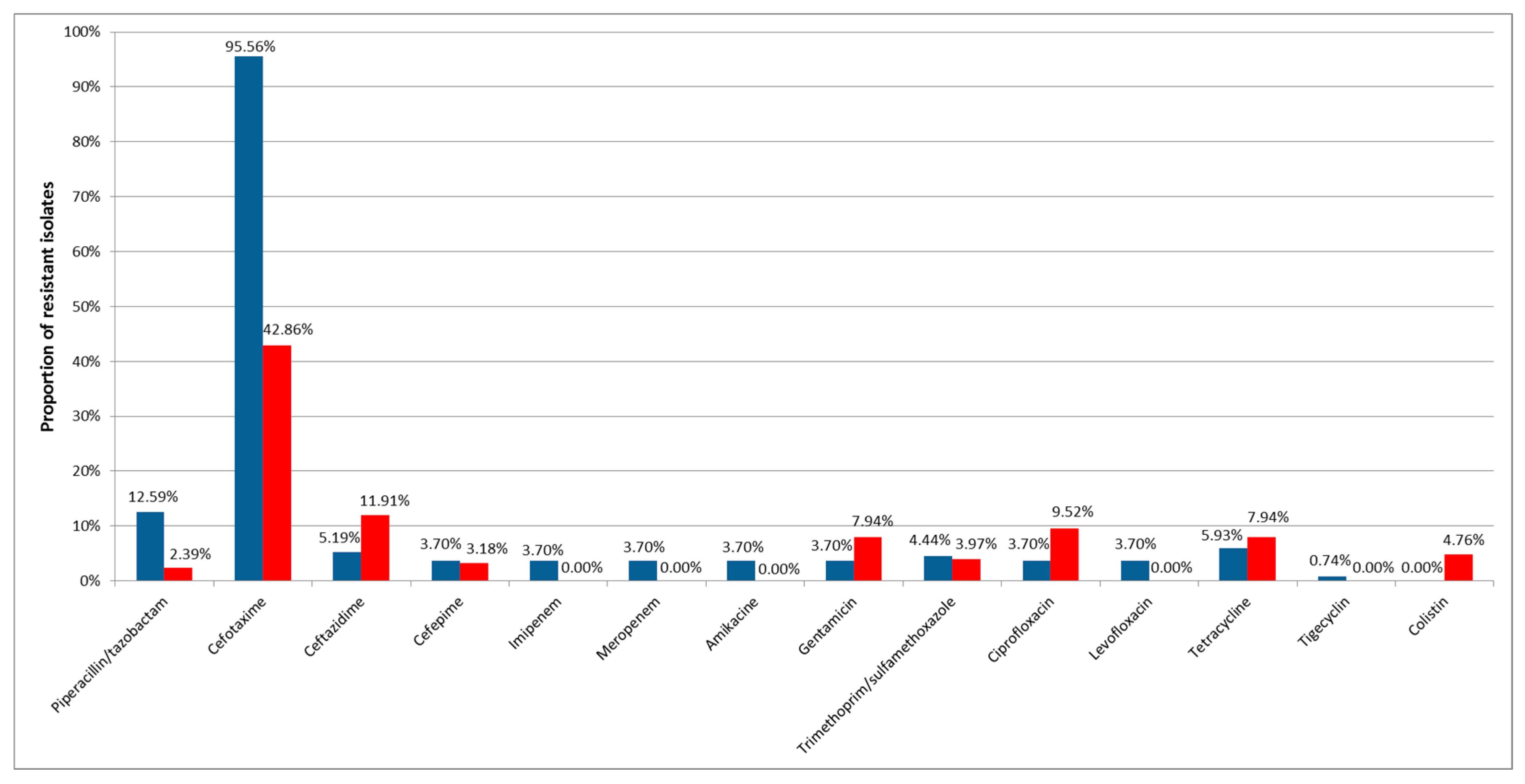
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- Towner, K.J. Acinetobacter: An Old Friend, but a New Enemy. J. Hosp. Infect. 2009, 73, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Alsan, M.; Klompas, M. Acinetobacter baumannii: An Emerging and Important Pathogen. J. Clin. Outcomes Manag. 2010, 17, 363–369. [Google Scholar] [PubMed]
- Zhao, W.H.; Hu, Z.Q. Acinetobacter: A Potential Reservoir and Dispenser for Beta-Lactamases. Crit. Rev. Microbiol. 2012, 38, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.A.; Amyes, S.G. OXA Beta-Lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Ambler, R.P.; Coulson, A.F.W.; Frere, J.M.; Ghuysen, J.M.; Joris, B.; Forsman, M.; Levesque, R.C.; Tiraby, G.; Waley, S.G. A Standard Numbering Scheme for the Class-a Beta-Lactamases. Biochem. J. 1991, 276, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Carbapenem Resistance in Acinetobacter baumannii: Mechanisms and Epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Maravic, A.; Skocibusic, M.; Fredotovic, Z.; Samanic, I.; Cvjetan, S.; Knezovic, M.; Puizina, J. Urban Riverine Environment is a Source of Multidrug-Resistant and ESBL-Producing Clinically Important Acinetobacter spp. Environ. Sci. Pollut. Res. Int. 2016, 23, 3525–3535. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Seifert, H.; Snelling, A.M.; Heritage, J.; Hawkey, P.M. Survival of Acinetobacter baumannii on Dry Surfaces: Comparison of Outbreak and Sporadic Isolates. J. Clin. Microbiol. 1998, 36, 1938–1941. [Google Scholar] [PubMed]
- Fournier, P.E.; Vallenet, D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C.; et al. Comparative Genomics of Multidrug Resistance in Acinetobacter Baumannii. PLoS Genet. 2006, 2, e7. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.F.; Lan, C.Y. Antimicrobial Resistance in Acinetobacter baumannii: From Bench to Bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhu, M.H.; Li, J.J.; Bi, S.; Sheng, Z.K.; Hu, F.S.; Zhang, J.J.; Chen, W.; Xue, X.W.; Sheng, J.F.; et al. Molecular Epidemiology and Mechanisms of Tigecycline Resistance in Clinical Isolates of Acinetobacter baumannii from a Chinese University Hospital. Antimicrob. Agents Chemother. 2014, 58, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Seruga Music, M.; Hrenovic, J.; Goic-Barisic, I.; Hunjak, B.; Skoric, D.; Ivankovic, T. Emission of Extensively-Drug-Resistant Acinetobacter baumannii from Hospital Settings to the Natural Environment. J. Hosp. Infect. 2017, 96, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Osinska, A.; Harnisz, M.; Korzeniewska, E. Prevalence of Plasmid-Mediated Multidrug Resistance Determinants in Fluoroquinolone-Resistant Bacteria Isolated from Sewage and Surface Water. Environ. Sci. Pollut. Res. Int. 2016, 23, 10818–10831. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, G.; Skiebe, E.; Higgins, P.G.; Poppel, M.T.; Blaschke, U.; Leser, S.; Heider, C.; Heindorf, M.; Brauner, P.; Jackel, U.; et al. Relatedness of Wildlife and Livestock Avian Isolates of the Nosocomial Pathogen Acinetobacter baumannii to Lineages Spread in Hospitals Worldwide. Environ. Microbiol. 2017, 19, 4349–4364. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Hamze, M.; Pailhories, H.; Eveillard, M.; Marsollier, L.; Joly-Guillou, M.L.; Dabboussi, F.; Kempf, M. Extrahuman Epidemiology of Acinetobacter baumannii in Lebanon. Appl. Environ. Microbiol. 2015, 81, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.; Albert, M.J.; Rotimi, V.O. Real-Time Comparative Evaluation of bioMerieux VITEK MS Versus Bruker Microflex MS, Two Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry Systems, for Identification of Clinically Significant Bacteria. BMC Microbiol. 2014, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 3.1, EU. 2013. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.xls (accessed on 29 December 2017).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 18th Informational Supplement; CLSI Document M100-S18; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Altun, H.U.; Yagci, S.; Bulut, C.; Sahin, H.; Kinikli, S.; Adiloglu, A.K.; Demiroz, A.P. Antimicrobial Susceptibilities of Clinical Acinetobacter baumannii Isolates with Different Genotypes. Jundishapur J. Microbiol. 2014, 7, e13347. [Google Scholar] [CrossRef] [PubMed]
- Biglari, S.; Alfizah, H.; Ramliza, R.; Rahman, M.M. Molecular Characterization of Carbapenemase and Cephalosporinase Genes among Clinical Isolates of Acinetobacter baumannii in a Tertiary Medical Centre in Malaysia. J. Med. Microbiol. 2015, 64, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Dallenne, C.; Da Costa, A.; Decre, D.; Favier, C.; Arlet, G. Development of a Set of Multiplex PCR Assays for the Detection of Genes Encoding Important Beta-Lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Zarfel, G.; Lipp, M.; Gurtl, E.; Folli, B.; Baumert, R.; Kittinger, C. Troubled Water Under the Bridge: Screening of River Mur Water Reveals Dominance of CTX-M Harboring Escherichia coli and for the First Time an Environmental VIM-1 Producer in Austria. Sci. Total Environ. 2017, 593–594, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, M.; Phee, L.; Wareham, D.W. A Novel Variant, NDM-5, of the New Delhi Metallo-Beta-Lactamase in a Multidrug-Resistant Escherichia coli ST648 Isolate Recovered from a Patient in the United Kingdom. Antimicrob. Agents Chemother. 2011, 55, 5952–5954. [Google Scholar] [CrossRef] [PubMed]
- Potron, A.; Nordmann, P.; Lafeuille, E.; Al Maskari, Z.; Al Rashdi, F.; Poirel, L. Characterization of OXA-181, a Carbapenem-Hydrolyzing Class D Beta-Lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011, 55, 4896–4899. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A.; Bratu, S.; Urban, C.; Visalli, M.; Mariano, N.; Landman, D.; Rahal, J.J.; Brooks, S.; Cebular, S.; Quale, J. Emergence of Carbapenem-Resistant Klebsiella Species Possessing the Class A Carbapenem-Hydrolyzing KPC-2 and Inhibitor-Resistant TEM-30 Beta-Lactamases in New York City. Clin. Infect. Dis. 2004, 39, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Marrs, C.F.; Simon, C.; Xi, C. Wastewater Treatment Contributes to Selective Increase of Antibiotic Resistance among Acinetobacter spp. Sci. Total Environ. 2009, 407, 3702–3706. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Bonnin, R.A.; Bogaerts, P.; De Laveleye, M.; Huang, D.T.; Dortet, L.; Glaser, P.; Glupczynski, Y.; Naas, T. Chromosomal Amplification of the blaOXA-58 Carbapenemase Gene in a Proteus Mirabilis Clinical Isolate. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Kittinger, C.; Lipp, M.; Baumert, R.; Folli, B.; Koraimann, G.; Toplitsch, D.; Liebmann, A.; Grisold, A.J.; Farnleitner, A.H.; Kirschner, A.; et al. Antibiotic Resistance Patterns of Pseudomonas Spp. Isolated from the River Danube. Front. Microbiol. 2016, 7, 586. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2015. Annual Report of the European Antimicrobial Resistance Surveilance Network (EARS-Net); ECDC: Solna Municipality, Sweden, 2016.
| SP | Name of SP | River (km) | Country |
|---|---|---|---|
| JDS2 | Kelheim, gauging station | 2415 | DE |
| JDS3 | Geisling power plant | 2354 | DE |
| JDS8 | Oberloiben | 2008 | AT |
| JDS10 | Wildungsmauer (Vienna) | 1895 | AT |
| JDS22 | ds Budapest | 1632 | HU |
| JDS28 | us Drava | 1384 | HR/RS |
| JDS36 | ds Tisa/us Sava | 1200 | RS |
| JDS38 | us Pancevo (Belgrade) | 1159 | RS |
| JDS49 | Pristol/Novo Salo | 834 | RO/BG |
| JDS57 | ds Ruse | 488 | RO/BG |
| JDS59 | ds Arges (Bucharest) | 429 | RO/BG |
| JDS63 | Siret | 154 | RO |
| JDS67 | Sulina Arm | 26 | RO |
| JDS68 | St. Gheorge Arm | 104 | RO |
| Antibiotic | Concentration | Antibioti Classes |
|---|---|---|
| piperacillin/tazobactam | 100 µg/10 µg | β-lactam |
| cefotaxime | 30 µg | β-lactam |
| ceftazidime | 30 µg | β-lactam |
| cefepime | 30 µg | β-lactam |
| imipenem | 10 µg | β-lactam |
| meropenem | 10 µg | β-lactam |
| amikacin | 30 µg | aminoglycoside |
| gentamicin | 10 µg | aminoglycoside |
| trimethoprim/sulfamethoxazole | 1.25 µg/23.75 µg | folate synthesis inhibitors |
| ciprofloxacin | 5 µg | quinolone |
| levofloxacin | 5 µg | quinolone |
| tigecycline | Etest | tetracyclin |
| tetracycline | 30 µg | tetracycline |
| colistin | Etest | polypeptide antibiotic |
| Isolate | Site of Isolation | Susceptible Antibiotics | Detected β-Lactamases |
|---|---|---|---|
| JDS38AC017 | us Pancevo (Belgrade) | colistin, tigecycline | OXA-23, OXA-51, VIM-2 |
| JDS38AC018 | us Pancevo (Belgrade) | colistin | OXA-23, OXA-51, VIM-2, TEM-1 |
| JDS38AC020 | us Pancevo (Belgrade) | colistin, tigecycline | OXA-24, OXA-51, |
| JDS59AC001 | ds Arges (Bucharest) | colistin, tigecycline | OXA-23, OXA-51 |
| JDS59AC007 | ds Arges (Bucharest) | colistin, tigecycline | OXA-23, OXA-51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittinger, C.; Kirschner, A.; Lipp, M.; Baumert, R.; Mascher, F.; Farnleitner, A.H.; Zarfel, G.E. Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High. Int. J. Environ. Res. Public Health 2018, 15, 52. https://doi.org/10.3390/ijerph15010052
Kittinger C, Kirschner A, Lipp M, Baumert R, Mascher F, Farnleitner AH, Zarfel GE. Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High. International Journal of Environmental Research and Public Health. 2018; 15(1):52. https://doi.org/10.3390/ijerph15010052
Chicago/Turabian StyleKittinger, Clemens, Alexander Kirschner, Michaela Lipp, Rita Baumert, Franz Mascher, Andreas H. Farnleitner, and Gernot E. Zarfel. 2018. "Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High" International Journal of Environmental Research and Public Health 15, no. 1: 52. https://doi.org/10.3390/ijerph15010052
APA StyleKittinger, C., Kirschner, A., Lipp, M., Baumert, R., Mascher, F., Farnleitner, A. H., & Zarfel, G. E. (2018). Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High. International Journal of Environmental Research and Public Health, 15(1), 52. https://doi.org/10.3390/ijerph15010052





