Abstract
Aiming to investigate the efficacy of different materials as bio-sorbents for the purification of As-polluted waters, batch-type experiments were employed to study As(V) sorption and desorption on oak ash, pine bark, hemp waste, mussel shell, pyritic material, and soil samples, as a function of the As(V) concentration added. Pyritic material and oak ash showed high sorption (90% and >87%) and low desorption (<2% and <7%). Alternatively, hemp waste showed low retention (16% sorption and 100% desorption of the amount previously sorbed), fine shell and pine bark sorbed <3% and desorbed 100%, the vineyard soil sample sorbed 8% and released 85%, and the forest soil sample sorbed 32% and desorbed 38%. Sorption data fitted well to the Langmuir and Freundlich models in the case of both soil samples and the pyritic material, but only to the Freundlich equation in the case of the various by-products. These results indicate that the pyritic material and oak ash can be considered efficient As(V) sorbents (thus, useful in remediation of contaminated sites and removal of that pollutant), even when As(V) concentrations up to 6 mmol L−1 are added, while the other materials that were tested cannot retain or remove As(V) from polluted media.
1. Introduction
Arsenic pollution is a public hazard, mainly in relation to drinking water. As(V) species are predominant in aerated surface waters, whereas the presence of As(III) is more prevalent in groundwater. Forest areas can suffer arsenic pollution due to wood preservative compounds that include As [1], and vineyard soils can be affected by As-based herbicides [2], resulting in increased risks of pollution [3].
Although different methods can be used to remove As from liquid media, adsorption has been considered as a relatively simplistic and safer alternative [4]. Previous studies have focused on As retention and release on soils and different sorbent materials [5,6,7], and the use of bio-sorbents has been considered as a low-cost alternative to remove As from polluted media, similar to that showed for other pollutants [8,9]. In fact, many low-cost sorbents have been previously investigated regarding their As removal potential, some of them showing encouraging results [10]. We have previously studied As(V) retention on soils and wastes [11,12,13,14,15,16,17], using low As(V) concentrations (<1.5 mmol L−1). In recent works, we used different sorbent materials to study As(V) and Cr(VI) competitive sorption [18,19], but non-competitive As(V) sorption/release was not investigated.
As indicated by Núñez-Delgado et al. [20], for As(V)-polluted media, it would be interesting to determine the retention potential of sorbent materials candidate to remove As(V), covering a wide As(V)-concentrations interval.
In view of that, the objective of the present work is to investigate non-competitive As(V) sorption/desorption on oak ash, hemp waste, pine bark, fine mussel shell, pyritic material, and on forest and vineyard soil samples, for different As(V) concentrations added (up to 6 mmol L−1). The results of this research could aid in programming the appropriate management of the studied soils and waste materials when focusing on As(V) retention and/or removal, thus aiding to fight As(V) water pollution.
2. Materials and Methods
2.1. Materials
We studied the following materials: oak ash, hemp waste, pine bark, finely ground mussel shell, pyritic material, forest and vineyard soil samples. The forest and vineyard soil, as well as the mussel shell samples were previously described [13], as the oak ash and the hemp waste [18], the pyritic material [21], and the pine bark [22]. Furthermore, Rivas-Pérez et al. [17] previously studied some of the materials here assayed.
Full details regarding analytical methods [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] and results corresponding to chemical characteristics of each material are included in the Supplementary Materials (see Table S1, as well as Figures S1–S7).
2.2. Methods
2.2.1. Sorption/Desorption Experiments
As(V) sorption/desorption experiments were performed as per Arnesen and Krogstrad [46], working at 20 ± 1 °C.
To carry out the procedure, individual samples (3 g of each soil or waste material) were added with 30 mL of 0.01 M NaNO3 dissolutions containing 0, 0.5, 1.5, 3, and 6 mmol As(V) L−1, prepared from analytical grade Na2HAsO4 (Panreac, Barcelona, Spain). Each suspension was shaken for 24 h, centrifuged for 15 min (6167× g), and filtered through acid-washed paper (2.5 µm pore size). The following parameters were determined in the equilibrium dissolutions: pH (glass electrode, Crison, Barcelona, Spain), dissolved organic carbon (DOC) (UV-visible spectroscopy -UV-1201, Shimadzu, Kyoto, Japan), and As (ICP-mass -Varian 800-NS, Denver, CO, USA). Sorbed As(V) was estimated as the difference between the concentration of As(V) added and As remaining in the equilibrium solution.
After the end of the sorption trials, 30 mL of 0.01 M NaNO3 were added to each individual sample to desorb As(V). They were then shaken, centrifuged and filtered as above, and desorbed As, DOC and pH were quantified. The difference between the concentration retained in the sorption phase and that released in the desorption phase, was used to calculate desorbed As, which was expressed as a percentage of the concentration sorbed.
Triplicate trials were carried out for each of the sorption and desorption tests.
2.2.2. Data Analyses
Statistical analyses were carried out by means of SPSS 21 (IBM, New York, NY, USA). Specifically, it was used for descriptive statistics, stepwise linear regression and correlation analyses, considering statistical significance for p < 0.05. It was also used to fit As sorption data to the Freundlich and Langmuir models.
The Freundlich equation is formulated as:
with qe being the As(V) sorbed per unit of mass of the sorbent, Ce the equilibrium concentration of the dissolved As, KF a constant related to the sorption capacity, and n a constant related to the sorption intensity.
qe = KF·Cen
The Langmuir equation is formulated as:
with Xm being the maximum sorption capacity and KL a constant related to the sorption energy.
qe = Xm·KL·Ce/(1 + KL·Ce)
3. Results
3.1. As Sorption for Different As(V) Concentrations Added
As(V) sorption results corresponding to the forest and vineyard soil samples, pyritic material, and the other waste materials, for different As(V) concentrations added, are shown in Figure 1.
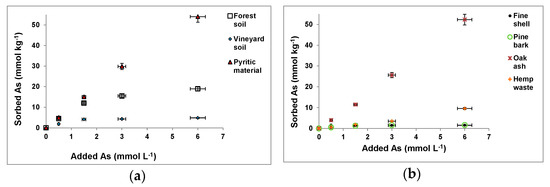
Figure 1.
As(V) sorption for different As(V) concentrations added to the soil and pyritic samples (a) and to the waste materials (b) tested. Mean values for 3 replicates, with error bars (coefficients of variation always <5%).
For the forest and vineyard soil samples, mussel shell and pine bark, As(V) sorption clearly increased, meanwhile, added As(V) was 1.5 mmol L−1 or lower, then showing a much lower sorption increase (Figure 1a,b). However, the pyritic material, oak ash, and hemp waste showed increased As(V) sorption for all As(V) concentrations added.
Osorio-López et al. [12], Seco-Reigosa et al. [16], and Rivas-Pérez et al. [17] also showed increased As(V) sorption as a function of the As(V) concentration added for most of the soil and waste samples here used, although clearly lower As(V) concentrations (<1.3 mmol L−1) were added in these previous studies.
Figure 1a,b show that maximum As(V) sorption when 6 mmol As(V) L−1 were added corresponded to the pyritic material (54 mmol kg−1, representing 90% of the As(V) added), followed by oak ash (52.29 mmol kg−1, 87% of added As(V)), forest soil sample (32% of added As(V)), hemp waste (16% of added As(V)), vineyard soil sample (8% of added As(V)), and pine bark and fine mussel shell (both 3% of the As(V) added).
Taking into account that pointed out by Liu and Zhang [47], the high As(V) sorption on the pyritic material may be due to the fact that at the acid pH prevailing the dominant As(V) species is H2AsO4−, which could sorb on positively charged Fe and Al sites on the pyritic material (Table S1, Supplementary Materials). Oak ash also showed high As(V) sorption, although having alkaline pH, situation where HAsO42− is dominant [47], and the variable charge compounds are not protonated. In fact, these ashes have notable concentrations of Feo and Alo (Table S1, Supplementary Materials), which are negatively charged at alkaline pH, favoring sorption of anionic As(V) by means of cationic bridges. In the case of the forest soil sample, sorption was 90% when 0.5 mmol L−1 As(V) was added, decreasing to 80% when the added concentration was 1.5 mmol L−1, which was similar to that previously found by Osorio-López et al. [12]. However, in the present study, much higher As(V) concentrations were added, which caused clearly lower sorption results (33% when a 6 mmol As(V) L−1 concentration was added). The vineyard soil, fine shell, and pine bark samples also showed a progressive decrease in percentage sorption as As(V) concentration added increased, indicating progressive saturation of sorption sites when high As(V) concentrations are present. See below further comments regarding pH evolution in the equilibrium solution as a function of As(V) concentration added, which could affect sorption.
The pyritic material showed the highest As(V) sorption (always >90%), and also high in the case of the oak ash (always >76%, and >87% when 6 mmol As(V) L−1 was added). Taking into account that the pyritic material showed a pH of 2.97, and that it was 11.31 for oak ash, this fact was in line with the wide pH range (4–11) signaled by Stanic et al. [48] for As(V) sorption on zeolites. It was also coincident with findings by Williams [49], and Smedley and Kinniburgh [50], although differing in narrower ranges found for impregnated alumina (6–8) [51], or for impregnated chitosan (2–4) [52].
For the forest soil sample, sorption was relevant (>80%) only when added As(V) concentrations were low (1.5 mmol L−1 or lower), which can be related to the presence of organo-aluminum complexes.
As(V) sorption was low on hemp waste (<16%), pine bark (<9.9%), vineyard soil sample (<39%), and fine mussel shell (<31%). The latter has alkaline pH, with HAsO42− being dominant [47,53], which can bind to calcite (abundant in mussel shell) by inner sphere complex with octahedral Ca [54], although carbonates have much lower As(V) sorption capacity than Fe oxides [55].
Regarding pH evolution in the equilibrium solution, it increased as a function of increased As(V) sorption for forest soil sample (from 3.62 to 5.5), vineyard soil sample (from 3.84 to 6.02), pyritic material (from 3.07 to 3.61), and fine mussel shell (from 6.40 to 7.97), which can be related to anionic exchange of As(V) species and OH− groups [46,56]. This effect could have influenced adsorption, especially for materials with low buffer abilities, such as the pyritic residue. For the pyritic material, a significant correlation was found between pH in the equilibrium and sorbed As(V) for each As(V) concentration added (r = 0.968, p < 0.01). However, pH remained almost unchanged in the case of pine bark (3.46–3.74), oak ash (13.11–13.13), and hemp waste (8.37–8.25), suggesting the absence of anionic exchange with OH− in these materials.
However, other possible As(V) sorption mechanisms do not cause release of OH− [57], and it is also possible that anionic exchange processes cause SO42−, PO43−, or organic anions release. In relation to the latter, DOC (mg L−1) increased in the equilibrium solution as a function of As(V) sorption for the forest soil sample (from 5.5 to 42.4), vineyard soil sample (6.9 to 32.4), pyritic material (0.7 to 2.0), fine shell (6.3 to 11.6), pine bark (15.9 to 23.7), oak ash (92.0 to 102.0), and hemp waste (from 383.4 to 491.7). Significant correlations were found between sorbed As(V) and DOC for forest soil sample (r = 0.908, p < 0.05), pyritic material (r = 0.934, p < 0.05), and hemp waste (r = 0.894 p < 0.05), suggesting a release of organic anions during As(V) sorption [58,59].
3.2. As Sorption Curves
Figure 2 shows As(V) sorption curves for the soil samples (Figure 2a) and waste materials assayed (Figure 2b,c), showing a clearly higher sorption on the pyritic material and the oak ash samples (Figure 2b), followed by the forest soil sample (Figure 2a) and the hemp waste (Figure 2c).
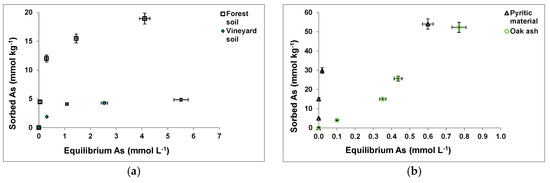
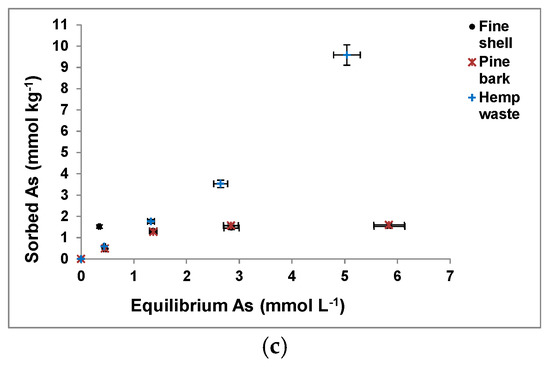
Figure 2.
As(V) sorption curves for the soil samples and waste materials tested (a–c). Mean values for 3 replicates, with error bars (coefficients of variation always <5%).
Table 1 shows that As(V) sorption presented good adjustment to the Langmuir and Freundlich models for both soil samples and the pyritic material, but fine shell, pine bark, oak ash, and hemp waste cannot be adjusted to the Langmuir model. Maji et al. [60] found good fitting to both Langmuir and Freundlich models when studying As(V) sorption on lateritic soils, whereas Yolcubal and Akyol [61] found better adjustment to the Freundlich equation for As sorption on carbonate-rich soils.

Table 1.
Constants and R2−coefficients for fitting of As(V) sorption data to the Freundlich and Langmuir models in the soil samples and waste materials studied. Error values into brackets. -: too high error values avoid fitting.
Although utilizable data regarding the Langmuir model was too scarce, it can be commented that a positive correlation was found between Langmuir’s KL and FeT (r = 0.950, p < 0.01) and Feo (r = 0.991, p < 0.01), as well as between Langmuir’s Xm and Alo (r = 0.858, p < 0.05). This can be due to the influence of amorphous Fe and Al compounds on As(V) sorption. In addition, positive correlations were also found between KL and Al saturation (r = 0.847, p < 0.05), and between Xm and Mge (r = 0.923, p < 0.01), Nae (r = 0.954, p < 0.01), Ke (r = 0.987, p < 0.01), eCEC (r = 0.970, p < 0.01), MgT (r = 0.960, p < 0.01), and KT (r = 9.991, p < 0.01).
Regarding the Freundlich constants, KF correlated with MgT (r = 0.851, p < 0.05) and MnT (r = 0.771, p < 0.05), and the n parameter correlated with Mge (r = 0.878, p < 0.01), Ke (r = 0.773, p < 0.05), eCEC (r = 0.827, p < 0.05) and KT (r = 0.762, p < 0.05). These correlations can be in relation to As(V) binding through cationic bridges in some cases. Furthermore, some of these cations can be in the form of oxides, thus favoring surface bindings [62].
3.3. As(V) Desorption
Figure 3 shows As(V) desorption and sorption for each material and for any As(V) concentration added, showing that forest soil sample released 38% of the As(V) previously sorbed when 6 mmol As(V) L−1 was added (Figure 3a). Desorption was clearly higher from the vineyard soil sample (85%) (Figure 3b), and even higher from the fine shell, pine bark, and hemp waste (100%) (Figure 3d,e,g). However, the pyritic material desorbed just 1.6% (Figure 3c), and oak ash desorbed 6.5% (Figure 3f), indicating a notable degree of irreversibility in the sorption process taking place on both materials.
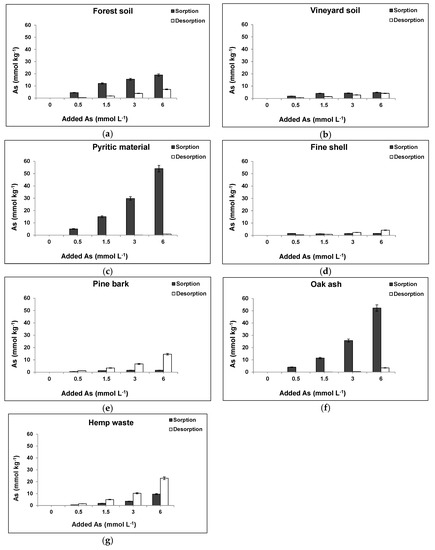
Figure 3.
As(V) sorption and desorption for each soil sample and waste material and different As(V) concentrations added. Mean values for 3 replicates, with error bars (coefficients of variation always <5%). (a) Forest soil; (b) Vineyard soil; (c) Pyritic material; (d) Fine mussel shell; (e) Pine bark; (f) Oak ash; (g) Hemp waste.
Strong As(V) bonds on Fe oxides at acid pH could explain its low release from the pyritic material, showing that pH reaches alkalinity to significantly increase desorption [55]. For oak ash, low As(V) release can be due to the high cation concentrations characterizing this material (Table S1, Supplementary Materials), including those in oxides which can retain As(V), as previously noted by Rahman et al. [62] studying wood ash.
4. Conclusions
The pyritic material and the oak ash here studied can be considered effective As(V) sorbents, in view of their high sorption and low desorption capabilities. However, the studied hemp waste, fine shell, pine bark, and the vineyard soil sample did not act as appropriate bio-sorbents for As(V) removal, whereas the forest soil sample showed slightly better results. Sorption data adjusted better to the Freunlich than to the Langmuir model, indicating a low probability of easy saturation of sorption sites. These results indicate that oak ash and the pyritic material are the best As(V) sorbents among those here studied, being useful to fight As(V) pollution, even against As(V) concentrations up to 6 mmol L−1.
Supplementary Materials
The following are available online at www.mdpi.com/1660-4601/14/7/803/s1, Table S1: General characteristics of the sorbent materials (average values for 3 replicates, with coefficients of variation always <5%), Figure S1: Infrared spectrum of forest soil, Figure S2: Infrared spectrum of vineyard soil, Figure S3: Infrared spectrum of pyritic material, Figure S4: Infrared spectrum of fine mussel shell, Figure S5: Infrared spectrum of pine bark, Figure S6: Infrared spectrum of oak ash, Figure S7: Infrared spectrum of hemp waste.
Acknowledgments
Funds received to perform this study: grant numbers CGL2012-36805-C02-01 and CGL2012-36805-C02-02 (Ministerio de Economía y Competitividad, Government of Spain). Also the European Regional Development Fund (ERDF) partially financed it. We have not received funds for covering the costs to publish in open access.
Author Contributions
David Fernández-Calviño, Juan Carlos Nóvoa-Muñoz, Manuel Arias-Estévez, María J. Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez and Avelino Núñez-Delgado conceived and designed the experiments; Ana Quintáns-Fondo and David Fernández-Calviño performed the experiments; David Fernández-Calviño, Juan Carlos Nóvoa-Muñoz, Manuel Arias-Estévez, María J. Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez and Avelino Núñez-Delgado analyzed the data; Ana Quintáns-Fondo, David Fernández-Calviño, Juan Carlos Nóvoa-Muñoz, Manuel Arias-Estévez, María J. Fernández-Sanjurjo, Esperanza Álvarez-Rodríguez and Avelino Núñez-Delgado wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Smith, E.; Naidu, R.; Alston, A.M. Arsenic in the soil environment, a review. Adv. Agron. 1998, 64, 149–195. [Google Scholar] [CrossRef]
- Gur, A.; Gil, Y.; Bravd, B. The efficacy of several herbicides in the vineyard and their toxicity to grapevines. Weed. Res. 1979, 19, 109–116. [Google Scholar] [CrossRef]
- Clothier, B.E.; Green, S.R.; Vogeler, I.; Greven, M.M.; Agnew, R.; van den Dijssel, C.W.; Neal, S.; Robinson, B.H.; Davidson, P. CCA transport in soil from treated-timber posts, pattern dynamics from the local to regional scale. Hydrol. Earth Syst. Sci. Discuss. 2006, 3, 2037–2061. [Google Scholar] [CrossRef]
- Gerard, N.; Krishnan, R.S.; Ponnusamy, S.K.; Cabana, H.; Vaidyanathan, V.K. Adsorptive potential of dispersible chitosan coated iron-oxide nanocomposites toward the elimination of arsenic from aqueous solution. Process. Saf. Environ. Prot. 2016, 104A, 185–195. [Google Scholar] [CrossRef]
- Bowell, R.J. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 1994, 9, 279–286. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, G.; Li, H. Efficient removal of arsenic from water using a granular adsorbent: Fe–Mn binary oxide impregnated chitosan bead. Bioresour. Technol. 2015, 193, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Selim, H.M. Kinetics of Arsenate Adsorption-Desorption in Soils. Environ. Sci. Technol. 2005, 39, 6101–6108. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, E.; Moharem, M.; Mahdy, A.; Mesalem, M. Sorption, Release and Forms of Mercury in Contaminated Soils Stabilized with Water Treatment Residual Nanoparticles. Land Degrad. Dev. 2016, 28, 752–761. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Cutillas-Barreiro, L.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cu Immobilization and Lolium perenne Development in an Acid Vineyard Soil Amended With Crushed Mussel Shell. Land Degrad. Dev. 2016, 28, 762–772. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Garrido-Rodríguez, B.; Cutillas-Barreiro, L.; Araújo-Nespereira, P.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Influence of mussel shell on As and Cr competitive and non-competitive sorption—Desorption kinetics in a mine soil: Stirred flow chamber experiments. Geoderma 2014. [Google Scholar] [CrossRef]
- Osorio-López, C.; Seco-Reigosa, N.; Garrido-Rodríguez, B.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: Kinetics and fractionation. J. Taiwan Inst. Chem. Eng. 2014, 45, 1007–1014. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Bermúdez-Couso, A.; Garrido-Rodríguez, B.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) retention on soils and forest by-products and other waste materials. Environ. Sci. Pollut. Res. 2013, 20, 6574–6583. [Google Scholar] [CrossRef] [PubMed]
- Seco-Reigosa, N.; Peña-Rodriguez, S.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Arsenic, chromium and mercury removal using mussel shell ash or a sludge/ashes waste mixture. Environ. Sci. Pollut. Res. 2013b, 20, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Seco-Reigosa, N.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Mixtures including wastes from the mussel shell processing industry: Retention of arsenic, chromium and mercury. J. Clean. Prod. 2014, 84, 680–690. [Google Scholar] [CrossRef]
- Seco-Reigosa, N.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Adsorption, desorption and fractionation of As(V) on untreated and mussel shell-treated granitic material. Solid Earth 2015, 6, 337–346. [Google Scholar] [CrossRef]
- Rivas-Pérez, I.M.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As (V) and P Competitive Sorption on Soils, By-Products and Waste Materials. Int. J. Environ. Res. Public Health 2015, 12, 15706–15715. [Google Scholar] [CrossRef] [PubMed]
- Quintáns-Fondo, A.; Ferreira-Coelho, G.; Paradelo-Núñez, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V)/Cr(VI) pollution control in soils, hemp waste and other by-products: Competitive sorption trials. Environ. Sci. Pollut. Res. 2016, 23, 19182–19192. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Pérez, I.M.; Conde-Cid, M.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V)/Cr(VI) retention on un-amended and waste-amended soil samples: Competitive experiments. Environ. Sci. Pollut. Res. 2017, 24, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Calviño, D. Perspectives on the use of by-products to treat soil and water pollution. Microporous Mesoporous Mater. 2015, 210, 199–201. [Google Scholar] [CrossRef]
- Otero, M.; Cutillas-Barreiro, L.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Cr(VI) sorption/desorption on untreated and mussel-shell-treated soil materials: fractionation and effects of pH and chromium concentration. Solid Earth 2015, 6, 373–382. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Ansias-Manso, L.; Fernandez Calviño, D.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Pine bark as bio-adsorbent for Cd, Cu, Ni, Pb and Zn: batch-type and stirred flow chamber experiments. J. Environ. Manag. 2014, 144, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Alejano, L.R.; Perucho, A.; Olalla, C.; Jiménez, R. Rock Engineering and Rock Mechanics: Structures in and on Rock Masses; CRC Press: London, UK, 2014; p. 372. [Google Scholar]
- Álvarez, E.; Fernández-Sanjurjo, M.J.; Núñez, A.; Seco, N.; Corti, G. Aluminium fractionation and speciation in bulk and rhizosphere of a grass soil amended with mussel shells or lime. Geoderma 2012. [Google Scholar] [CrossRef]
- Brás, I.; Teixeira-Lemos, L.; Alves, A.; Pereira, M.F.R. Application of pine bark as a sorbent for organic pollutants in effluents. Manag. Environ. Qual. 2004, 15, 491–501. [Google Scholar] [CrossRef]
- Chatterjee, A.; Lal, R.; Wielopolski, L.; Martin, M.Z.; Ebinger, M.H. Evaluation of Different Soil Carbon Determination Methods. Crit. Rev. Plant Sci. 2009, 28, 164–178. [Google Scholar] [CrossRef]
- Coelho, G.F.; ConÇalves, A.C., Jr.; Tarley, C.R.T.; Casarin, J.; Nacke, N.; Francziskowski, M.A. Removal of metal ions Cd (II), Pb (II) and Cr (III) from water by the cashew nut shell Anarcadium occidentale L. Ecol. Eng. 2014, 73, 514–525. [Google Scholar] [CrossRef]
- Dlapa, P.; Bodí, M.B.; Mataix-Solera, J.; Cerdà, A.; Doerr, S.H. FT-IR spectroscopy reveals that ash water repellency is highly dependent on ash chemical composition. Catena 2013, 108, 35–43. [Google Scholar] [CrossRef]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. Localisation and characterisation of incipient brown-rot decay within spruce wood cell walls using FT-IR imaging microscopy. Enzyme Microb. Technol. 2010, 47, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Haberhauer, G.; Gerzabek, M.H. Drift and transmission FT-IR spectroscopy of forest soils: An approach to determine decomposition processes of forest litter. Vib. Spectrosc. 1999, 19, 413–417. [Google Scholar] [CrossRef]
- Kamprath, E.J. Exchangeable aluminium as a criterion for liming leached mineral soils. Soil Sci. Soc. Am. Proc. 1970, 34, 252–254. [Google Scholar] [CrossRef]
- Margenot, A.J.; Calderón, F.J.; Goyne, K.W.; Mukome, F.N.D.; Parikh, S.J. IR Spectroscopy, Soil Analysis Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J., Tranter, G., Koppenaal, D., Eds.; Oxford Academic Press: London, UK, 2017; pp. 448–454. [Google Scholar]
- McLean, E.O. Soil pH and Lime Requirement. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA: Madison, WI, USA, 1982; pp. 199–223. [Google Scholar]
- Mimura, A.M.S.; Vieira, T.V.A.; Martinelli, P.B.; Gorgulho, H.F. Utilization of rice husk to remove Cu2+, Al3+, Ni2+ and Zn2+ from wastewater. Quím. Nova 2010, 33, 1279–1284. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Nóbrega, J.A.; Pirola, C.; Fialho, L.L.; Rota, G.; de Campos, C.E.; Pollo, F. Microwave-assisted digestion of organic samples: How simple can it become? Talanta 2012, 98, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introdução à Espectroscopia, 4th ed.; Cengage Learning: São Paulo, Brazil, 2010; p. 700. [Google Scholar]
- Rubio, F.; GonÇalves, A.C., Jr.; Meneghel, A.P.; Tarley, C.R.T.; Schwantes, D.; Coelho, G.F. Removal of cadmium from water using by-product Crambe abyssinica Hochst seeds as biosorbent material. Water Sci. Technol. 2013, 68, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 1, 206–210. [Google Scholar] [CrossRef]
- Sila, A.M.; Shepherd, K.D.; Pokhariyal, G.P. Evaluating the utility of mid-infrared spectral subspaces for predicting soil properties. Chemometr. Intell. Lab. Syst. 2016, 153, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Smidt, W.; Meissl, K. The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag. 2007, 27, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis, Part 3, Chemical Methods; Page, D.L., Ed.; ASA: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Tan, K.H. Soil Sampling, Preparation, and Analysis; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Tarley, C.R.T.; Arruda, M.A.Z. Biosorption of heavy metals using rice milling by-products. Characterisation and application for removal of metals from aqueous effluents. Chemosphere 2004, 54, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Tinti, A.; Tugnoli, V.; Bonora, S.; Francioso, O. Recent applications of vibrational mid-Infrared (IR) spectroscopy for studying soil components: A review. J. Cent. Eur. Agric. 2015, 16, 1–22. [Google Scholar] [CrossRef]
- Arnesen, A.K.M.; Krogstad, T. Sorption and desorption of fluoride in soil polluted from the aluminium smelter at Ardal in Western Norway. Water Air Soil Pollut. 1998, 103, 357–373. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H. The adsorption of arsenic on magnetic iron-manganese oxide in aqueous medium. In Proceedings of the International MultiConference of Engineers and Computer Scientists (IMECS 2008), Hong Kong, China, 19–21 March 2008; pp. 19–21. [Google Scholar]
- Stanic, T.; Dakovic, A.; Zivanovic, A.; Tomasevic-Canovic, M.; Dondur, V.; Milicevic, S. Adsorption of arsenic (V) by iron (III) modified natural zeolitic tuff. Environ. Chem. Lett. 2009, 7, 161–166. [Google Scholar] [CrossRef]
- Williams, M. Arsenic in mine waters: An international study. Environ. Geol. 2001, 40, 267–278. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinninburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Wasay, S.A.; Tokunaga, S.; Park, S.W. Removal of hazardous anions from aqueous solution by La (III)-and Y(III)-impregnated alumina. Sep. Sci. Technol. 1996, 31, 1501–1514. [Google Scholar] [CrossRef]
- Dambies, L.; Guibai, E.; Roze, A. Arsenic (V) sorption on molybdate-impregnated chitosan beads. Colloids Surf. A: Physicochem. Eng. Asp. 2000, 170, 19–31. [Google Scholar] [CrossRef]
- Yan, X.P.; Kerrich, R.; Hendry, M.J. Distribution of arsenic(III), arsenic(V) and total inorganic arsenic in pore-waters from a thick till and clay-rich aquitard sequence, Saskatchewan, Canada. Geochim. Cosmochim. Acta 2000, 64, 2637–2648. [Google Scholar] [CrossRef]
- Alexandratos, V.G.; Elzinga, E.J.; Reeder, R.J. Arsenate uptake by calcite: Macroscopic and spectroscopic characterization of adsorption and incorporation mechanisms. Geochim. Cosmochim. Acta 2007, 71, 4172–4187. [Google Scholar] [CrossRef]
- Goldberg, S.; Glaubig, R.A. Anion sorption on a calcareous, montmorillonitic soil-arsenic. Soil Sci. Soc. Am. J. 1988, 52, 1297–1300. [Google Scholar] [CrossRef]
- Gago, C.; Romar, A.; Fernández-Marcos, M.L.; Álvarez, E. Fluorine sorption by soils developed from various parent materials in Galicia (NW Spain). J. Colloid Interface Sci. 2012, 374, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Boddu, V.; Krishnaiah, A.; Talbot, J.; Smith, E. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Blodau, C. Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci. Total Environ. 2006, 354, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Blodau, C. Arsenic distribution in the dissolved, colloidal and particulate size fraction of experimental solutions rich in dissolved organic matter and ferric iron. Geochim. Cosmochim. Acta 2009, 73, 529–542. [Google Scholar] [CrossRef]
- Maji, S.K.; Pal, A.; Pal, T.; Adak, A. Adsorption thermodynamics of arsenic on laterite soil. Surf. Sci. Technol. 2007, 22, 161–176. [Google Scholar] [CrossRef]
- Yolcubal, I.; Akyol, N.H. Adsorption and transport of arsenate in carbonate-rich soils: Coupled effects of nonlinear and rate-limited sorption. Chemosphere 2008, 73, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Wasiuddin, N.M.; Islam, M.R. Experimental and numerical modeling studies of arsenic removal with wood ash from aqueous streams. Can. J. Chem. Eng. 2004, 82, 968–977. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).