In Vitro and In Vivo Control of Secondary Bacterial Infection Caused by Leishmania major
Abstract
1. Introduction
2. Materials and Methods
2.1. Freezing Leishmania Major Promastigote
2.2. Preparation of the Dose of the Parasite to Inject Animals
2.3. Experimental Animals
2.4. Allicin Preparation and Concentration
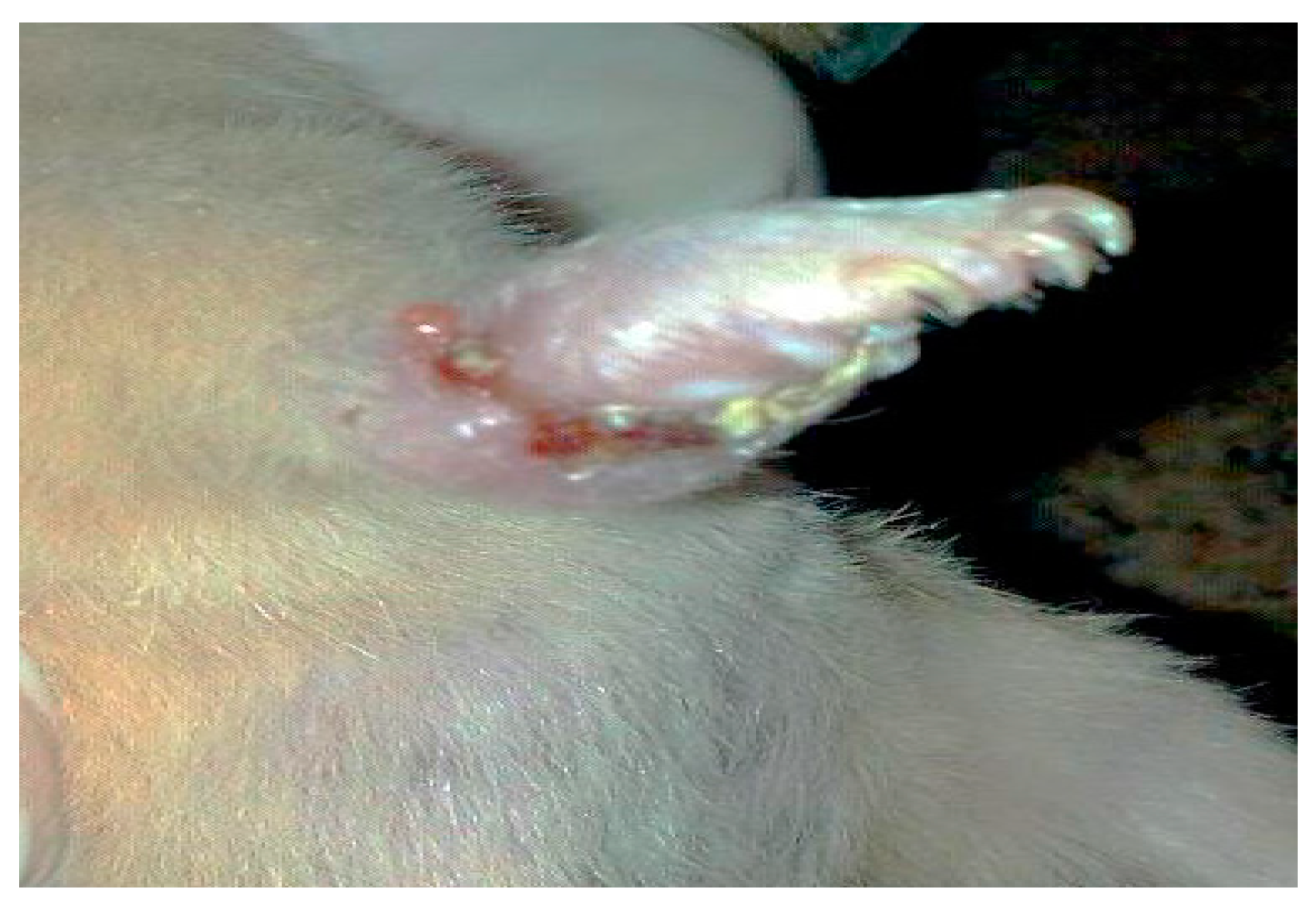
2.5. Infection in BALB/c Mice
2.6. Isolation and Identification of Bacteria and Yeast
2.7. Antimicrobial Susceptibility
2.8. Effect of Allicin Liquid on Bacterial Isolates
2.9. Experimental Protocol (In Vivo)
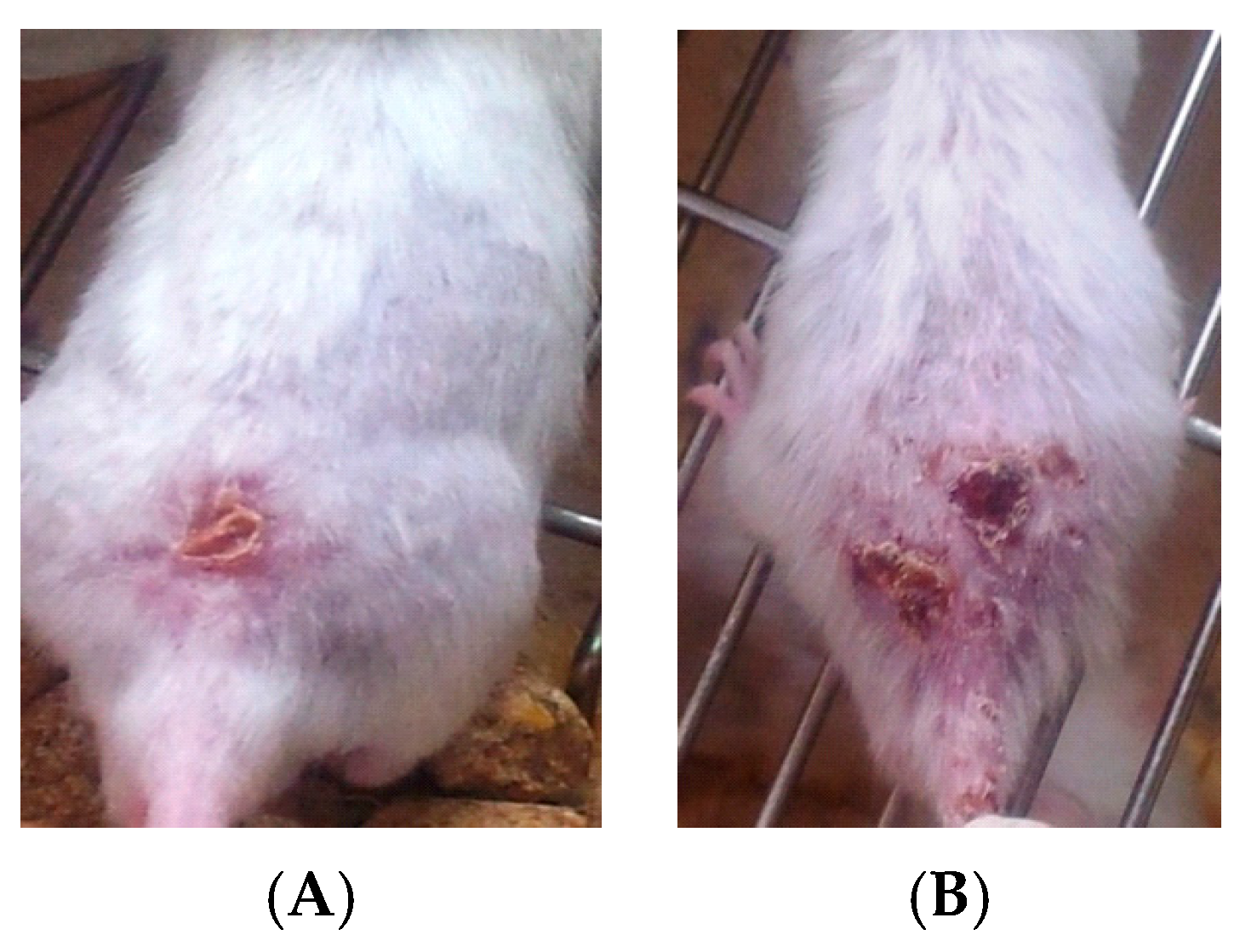
- Group l: Normal non-infected negative control group.
- Group 2: Infected non-treated positive control group: Mice were inoculated subcutaneously with a dose of 0.1 × 107 promastigotes in a shaved area above the tail.
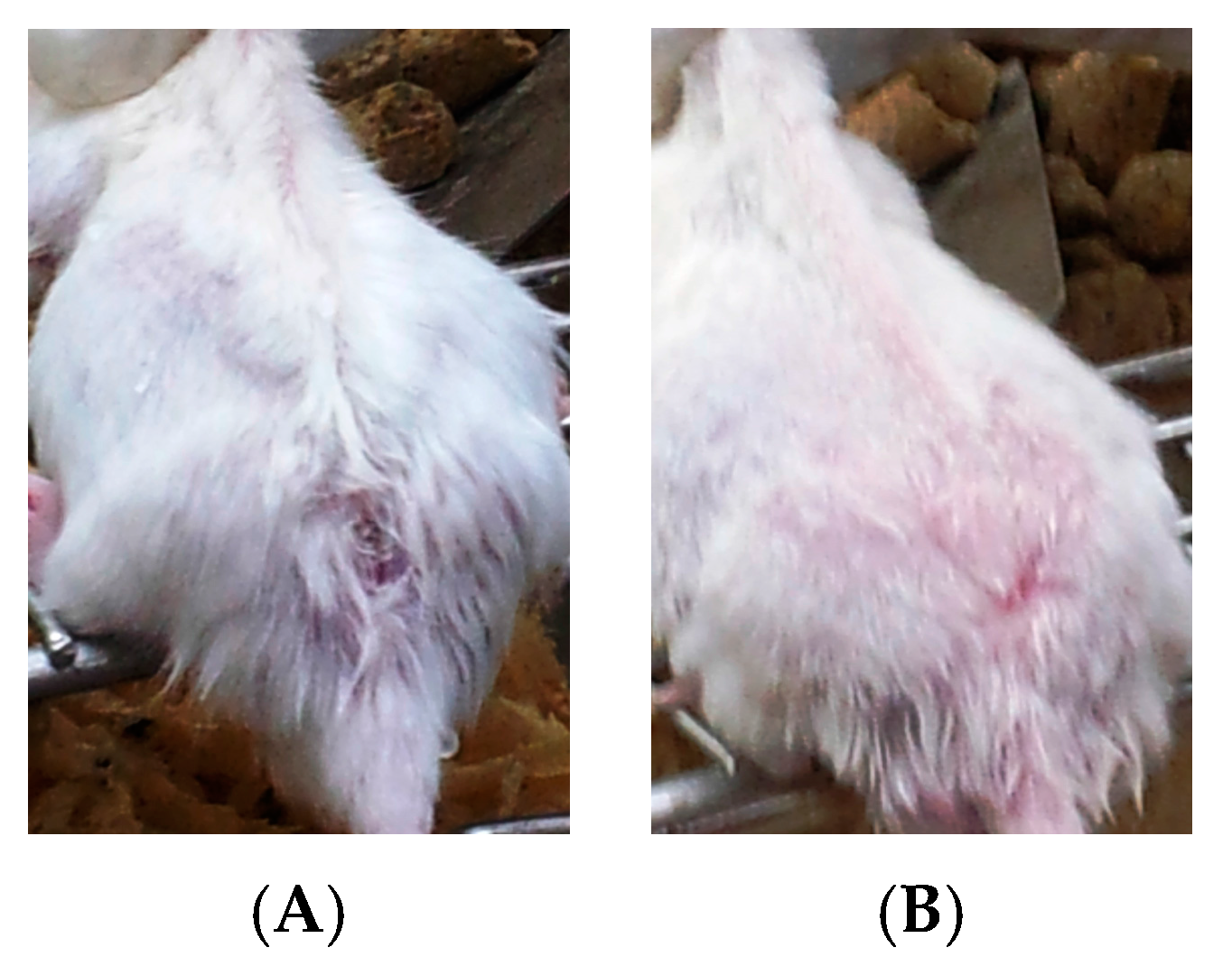
- Group 3: Infected mice treated with liquid allicin at concentrations of 0.30 μM/mouse starting with the first appearance of ulcerative lesion. Treatment was continued for four weeks.
- Group 4: Infected mice treated with ciprofloxacin (Cip, 10 mg/mL) with the first appearance of ulcerative lesion. Treatment was continued for four weeks.
- Group 5: Infected mice were treated with 0.30 μM of liquid allicin and concomitantly with the antibiotic ciprofloxacin (Cip, 10 mg/mL) with the first appearance of ulcerative lesions. Treatment was continued for four weeks.
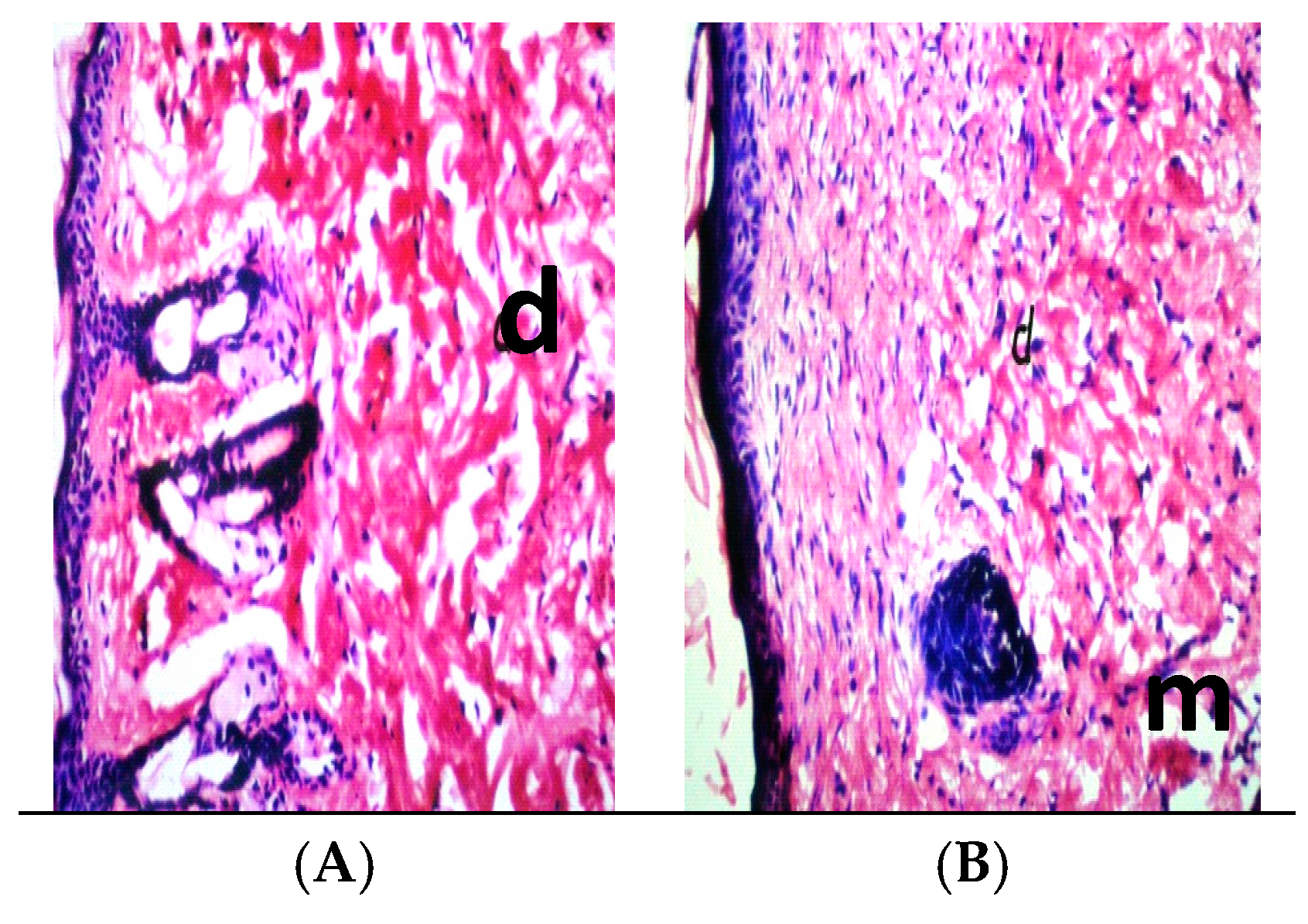
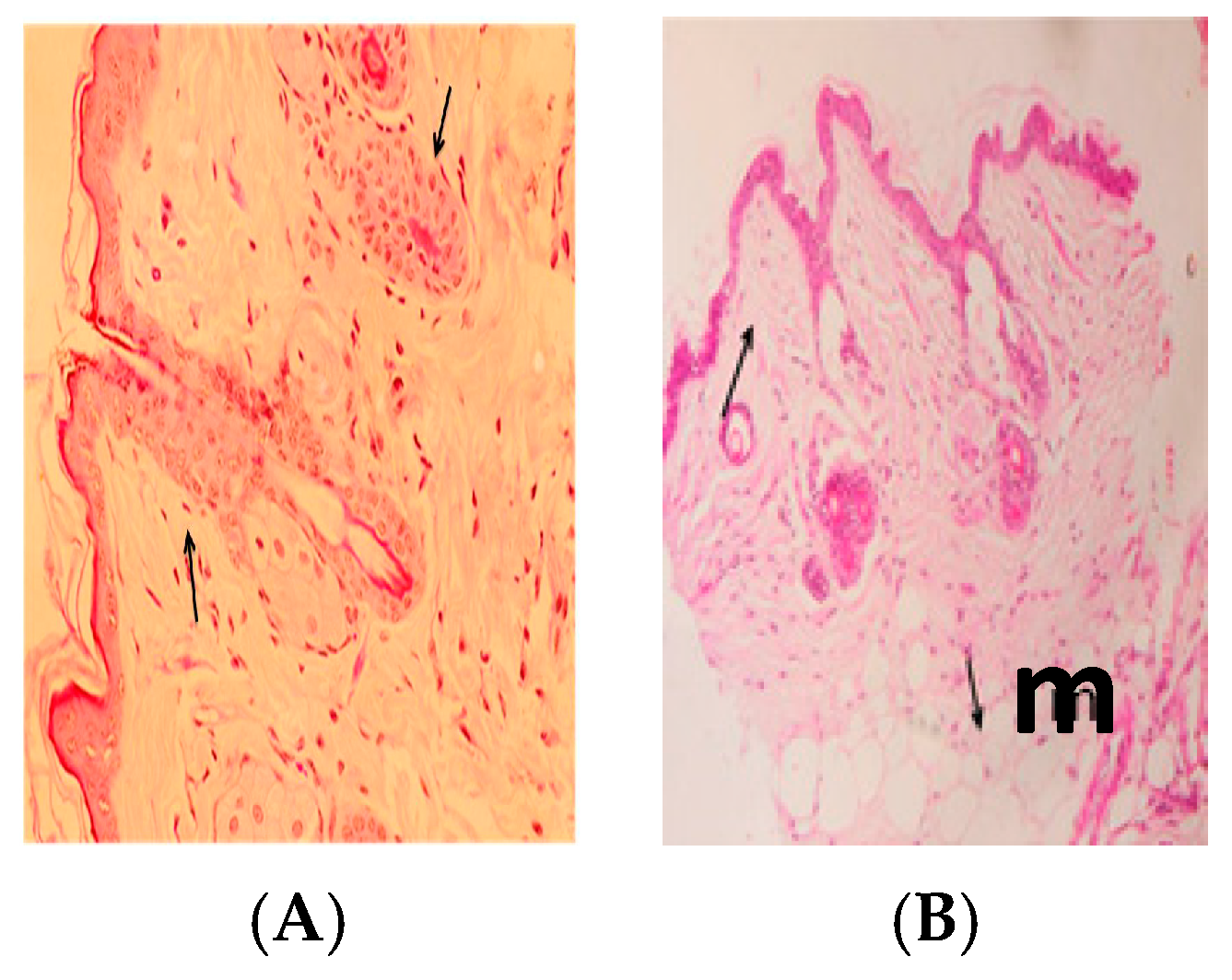
2.10. Histopathology
2.11. Ethic Statement
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Maria, V.C.; Lonardoni, M.R.; Sonia, J. Essential role of platelet-activating factor in control of Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2000, 68, 6355–6361. [Google Scholar]
- Ziaei, H.; Sadeghian, G.; Hejazi, S.H. Distribution frequency of pathogenic bacteria isolated from cutaneus leishmaniasis lesions. Korean J. Parasitol. 2008, 46, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Machado-Pinto, J.; Pinto, J.; da Costa, C.A.; Genaro, O.; Marques, M.J.; Modabber, F.; Mayrink, W. Immunochemotherapy for cutaneous leishmaniasis: A controlled trial using killed Leishmania (Leishmania) amazonensis vaccine plus antimonial. Int. J. Dermatol. 2002, 41, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.H.; Titus, R.H. T-cell and non-T cell compartments can independently determine resistance to L. major. J. Exp. Med. 1995, 181, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Lezama-Davila, C.M.; Isaac-Marquez, A.P.; Padierna-Olivos, J.; Aguilar-Torrentera, F.; Chapa-Ruiz, R. Immunomodulation of Chiclero’s Ulcer. Role of Eosinophils, T cells, Tumour Necrosis Factor and interleukin-2. Scand. J. Immunol. 1998, 47, 502–508. [Google Scholar]
- Couppie, P.; Pradinaud, R.; Grosshans, E.; Sainte-Marie, D.; Benoist, B. Rapid diagnosis of cutaneous leishmaniasis and histoplasmosis by direct microscopic tests. Ann. Dermatol. Venereol. 1997, 124, 849–851. [Google Scholar] [PubMed]
- Grasa, J.M.; Lorente, J.; Crego, F.; Naches, S.; Subirana, F.X.; Calderon, J.R.; Pollan, C.; Encarnacion, L.F.; Quesada, P. Nasal leishmaniasis in an HIV-positive patient. Acta Otorrinolaringol. Esp. 2000, 51, 169–173. [Google Scholar] [PubMed]
- Berhe, N.; Hailu, A.; Abraham, Y.; Tadesse, Y.; Breivik, K.; Abebe, Y. Inter-current and nosocomial infections among visceral leishmaniasis patients in Ethiopia: An observational study. Acta Trop. 2001, 80, 87–95. [Google Scholar] [CrossRef]
- Kerbaugh, M.A.; Evans, J.B. Aerococcus viridans in the hospital environment. Appl. Microbiol. 1968, 16, 519–523. [Google Scholar] [PubMed]
- Ruoff, K.I. Leuconostoc, pediococcus, stomatococcus, and miscellaneous Gram-positive cocci that grow aerobically. In Manual of Clinical Microbiology, 6th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; ASM Press: Washington, DC, USA, 1995; pp. 315–323. [Google Scholar]
- Dagnæs-Hansen, F.; Kilian, M.; Fuursted, K. Septicaemia associated with an Aerococcus viridans infection in immunodeficient mice. Lab. Anim. 2004, 38, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Meryem, C.N.; Sabahattin, O.K.; Devrim, E.R. An unusual case of urinary tract infection caused by Aerococcus viridians. ANKEM Derg. 2007, 21, 65–67. [Google Scholar]
- Bloch, K.C.; Nadarajah, R.; Jacobs, R. Chryseobacterium meningosepticum: An emerging pathogen among immunocompromised adults. Medicine 1997, 76, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Von Graevenitz, A. Ecology, clinical significance, and antimicrobial susceptibility of infrequently encountered glucose-nonfermenting Gram-negative rods. In Nonfermentative Gram-Negative Rods: Laboratory Identification and Clinical Aspects; Gilardi, G.L., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1985; pp. 181–232. [Google Scholar]
- Pedersen, M.M.; Marso, E.; Pickett, M.I. Nonfermentative bacilli associated with man. III. Pathogenicity and antibiotic susceptibility. Am. J. Clin. Pathol. 1970, 54, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Wafae, C.; Alaoui, A.S.; Amar, M. Chryseomonas luteola identified as the source of serious infections in a Moroccan university hospital. J. Clin. Microbiol. 2004, 42, 1837–1839. [Google Scholar]
- Burkholder, W.H. Three bacterial plant pathogens: Phytomonas earyophylli sp. n., Phytomonas alliicola sp. n., and Phytomonas manihotis (Arthaud-Berthet et Sondar) Viégas. Phytopathology 1942, 32, 141–149. [Google Scholar]
- Burkholder, W.H. Sour skin, a bacterial rot of onion bulbs. Phytopathology 1950, 64, 468–475. [Google Scholar]
- Narayan, S.; Batta, K.; Colloby, P.; Tan, C.Y. Cutaneous cryptococcus infection due to C. albidus associated with Sézary syndrome. Br. J. Dermatol. 2000, 143, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1944–1952. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1998, 13, 233–244. [Google Scholar] [CrossRef]
- Ankri, S.; David, M. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 2, 125–129. [Google Scholar] [CrossRef]
- Anthony, J.P.; Fyfe, L.; Smith, H. Plant active components—A resource for antiparasitic agents? Trends Parasitol. 2005, 21, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, D.; Monheit, D.; Varon, S. Inhibition of growth of Entamoeba histolytica by allicin, the active principle of garlic extract (Allium sativum). In Chemotherapeutic Targets in Parasites: Contemporary Strategies; Mansour, T.E., Ed.; Cambridge University Press: Cambridge, UK, 2002; p. 149. [Google Scholar]
- Ankri, S.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D. Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica. Antimicrob. Agents Chemother. 1997, 41, 2286–2288. [Google Scholar] [PubMed]
- Metwally, D.M.; Ebtesam, M.A.; Manal, F.E.; Badriah, A. Anti-Leishmanial Activity (In Vitro and In Vivo) of Allicin and Allicin Cream Using Leishmania major (Sub-Strain Zymowme LON4) and Balb/c Mice. PLoS ONE 2016, 11, e0161296. [Google Scholar] [CrossRef] [PubMed]
- Yehia, H.M. Antimicrobial resistance patterns of Enterobacteriaceae and non–Enterobacteriaceae isolated from poultry intestinal. Life Sci. J. 2013, 10, 3438–3446. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing: Twelfth Informational Supplement; NCCLS Document M100-S12; NCCLS: Wayne, PA, USA, 2002. [Google Scholar]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries; Cambridge University Press: Cambridge, UK, 2006; p. 434. [Google Scholar]
- Coyle, M.B. Manual of Antimicrobial Susceptibility Testing; American Society for Microbiology Press: Washington, DC, USA, 2005; pp. 25–39. [Google Scholar]
- Okonko, I.O.; Donbraye-Emmanuel, O.B.; Ijandipe, L.A.; Ogun, A.A.; Adedeji, A.O.; Udeze, A. Antibiotics sensitivity and resistance patterns of uropathogens to nitrofurantoin and nalidixic acid in pregnant women with urinary tract infections in Ibadan, Nigeria. Middle-East J. Sci. Res. 2009, 4, 105–109. [Google Scholar]
- Ziaie, H.; Sadeghian, G. Isolation of bacteria causing secondary bacterial infection in the lesions of cutaneous leishmaniasis. Indian J. Dermatol. 2008, 53, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Maha, M.E.; Eglal, I.A.; Shereen, F.M.; Maha, M.G.; Nahed, M.B. Miltefosine for Old World cutaneous leishmaniasis: An experimental study on Leishmania major infected mice. Alex. J. Med. 2012, 48, 261–271. [Google Scholar]
- Martins-Duarte, E.S.; Dubar, F.; Lawton, P.; França da Silva, C.; Maria de Nazaré, S.C.; de Souza, W.; Christophe, B.; Vommaro, R.C. Ciprofloxacin Derivatives Affect Parasite Cell Division and Increase the Survival of Mice Infected with Toxoplasma gondii. PLoS ONE 2015, 10, e0125705. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Nulens, E.; Bussels, B.; Bols, A.; Gordts, B.; Van Landuyt, H.W. Recurrent bacteremia by Chryseobacterium indologenes in an oncology patient with a totally implanted intravenous device. Clin. Microbiol. Infect. 2001, 7, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.G.; Wang, W.S.; Yen, C.C.; Liu, J.H.; Chiou, T.J.; Yang, M.H.; Chao, T.C.; Chen, P.M. Chryseobacterium indologenes bacteremia in a bone marrow transplant recipient with chronic graft-versus-host disease. Scand. J. Infect. Dis. 2003, 35, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Christakis, G.B.; Perlorentzou, S.P.; Chalkiopoulou, I.; Athanasiou, A.; Legakis, N.J. Chryseobacterium indologenes non-catheter-related bacteremia in a patient with a solid tumor. J. Clin. Microbiol. 2005, 43, 2021–2023. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Stassi, G.; Costa, G.B.; Crisafulli, G.; Rulli, I.; Ruggeri, C.; Iaria, C. Chryseobacterium indologenes bacteraemia in a diabetic child. J. Med. Microbiol. 2005, 54, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, M.R.; Aktas, E.; Ersay, Y.; Cicek, A.; Durmaz, R. Postoperative Chryseobacterium indologenes bloodstream infection caused by contamination of distillate water. Infect. Control Hosp. Epidemiol. 2007, 28, 368–369. [Google Scholar]
- Al-Tatari, H.; Asmar, B.I.; Ang, J.Y. Lumboperitoneal shunt infection due to Chryseobacterium indologenes. Pediatr. Infect. Dis. J. 2007, 26, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.; Singh, R.K.; Curtis, B.; Boyd, J.M.; Bouevitch, A.; Kimball, J.; Munholland, J.; Murphy, C.; Sarty, D.; Williams, J.; et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: Insights into the evolution of a fish pathogen. BMC Genom. 2008, 9, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Bhatawadekar, S.M. Community-acquired urinary tract infection by Pseudomonas oryzihabitans. J. Glob. Infect. Dis. 2013, 5, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Decker, C.F.; Simon, G.L.; Keiser, J.F. Flavimonas oryzihabitans (Pseudomonas oryzihabitans; CDC group Ve-2) bacteremia in the immunocompromised host. Intern. Med. 1991, 151, 603–604. [Google Scholar] [CrossRef]
- Figueras, M.J. Clinical relevance of Aeromonas. Rev. Med. Microbiol. 2005, 16, 145–153. [Google Scholar] [CrossRef]
- Martin-Carnahan, A.; Joseph, S.W. Aeromonadaceae. In Bergey’s Manual of Systematic Bacteriology; Garrity, G.M., Ed.; Springer: New York, NY, USA, 2005; Volume 2, pp. 556–580. [Google Scholar]
- Güngör, S.M.; Özen Akinci, A.; Durmaz, R. A Chryseobacterium miningosepticum outbreak in a neonatal ward. Infect. Control Hosp. Epidemiol. 2003, 24, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.T.; Sader, H.S.; Walsh, T.R.; Jones, R.N. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp.: Report from the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 2004, 42, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Mori, T.; Shimizu, T.; Yokoyama, A.; Takayama, N.; Ikeda, Y.; Okamoto, S. Chryseobacterium meningosepticum septicemia in a recipient of allogeneic cord blood transplantation. Scand. J. Infect. Dis. 2004, 36, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, G.L. Infrequently encountered Pseudomonas species causing infection in 42 humans. Ann. Intern. Med. 1972, 77, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Lapage, S.P.; Easterling, B.G. Distribution in clinical material and identification of Pseudomonas maltophilia. J. Clin. Pathol. 1979, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Khardori, N.; Elting, L.; Wong, E.; Schable, B.; Bodey, G.P. Nosocomial infections due to Xanthomonas maltophilia (Pseudomonas maltophilia) in patients with cancer. Rev. Infect. Dis. 1991, 12, 997–1003. [Google Scholar] [CrossRef]
- Midelfart, J.; Midelfart, A.; Bevanger, L. Microbial contamination of contact lens cases among medical students. CLAO J. 1996, 22, 21–24. [Google Scholar] [PubMed]
- Denton, M.; Kerr, K.G. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 1998, 11, 57–80. [Google Scholar] [PubMed]
- Wishart, M.M.; Riley, T.V. Infection with Pseudomonas maltophilia: Hospital outbreak due to contaminated disinfectant. Med. J. Aust. 1976, 2, 710–712. [Google Scholar] [PubMed]
- Papadakis, K.A.; Vartivarian, S.E.; Vassilaki, M.E.; Anaissie, E.J. Septic prepatellar bursitis caused by Stenotrophomonas (Xanthomonas) maltophilia. Clin. Infect. Dis. 1996, 22, 388–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ranjan, K.P.; Ranjan, N.; Bansal, S.K.; Arora, D.R. Prevalence of Pseudomonas aeruginosa in post- operative wound infection in a referral hospital in Haryana, India. J. Lab. Physicians 2010, 2, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Fefer, J.J.; Ratzan, K.R.; Sharp, S.E.; Saiz, E. Lactococcus garvieae endocarditis: Report of a case and review of the literature. Diagn. Microbiol. Infect. Dis. 1998, 32, 127–130. [Google Scholar] [CrossRef]
- Elliot, J.A.; Collins, M.D.; Pigott, N.E.; Facklam, R.R. Differentiation of Lactococcus lactis and Lactococcus garviae from humans by comparison of whole-cell protein patterns. J. Clin. Microbiol. 1991, 29, 2731–2734. [Google Scholar]
- Gluck, J.L.; Myers, J.P.; Pass, L.M. Cryptococcemia due to Cryptococcus albidus. South. Med. J. 1987, 80, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.P. Clinical problems posed by multiresistant nonfermenting Gram-negative pathogens. Clin. Infect. Dis. 1998, 27, S117–S124. [Google Scholar] [CrossRef] [PubMed]
- Vartivarian, S.E.; Anaissie, E.; Bodey, G.; Sprigg, H.; Rolston, K. A changing pattern of susceptibility of Xanthamonas maltophilia to antimicrobial agents: Implications for therapy. Antimicrob. Agents Chemother. 1994, 38, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Pankuch, G.A.; Jacobs, M.R.; Appelbaum, P.C. Susceptibilities of 123 Xanthomonas maltophilia strains to clinafloxacin, PD 131628, PD 138312, PD 140248, ciprofloxacin, and ofloxacin. Antimicrob. Agents Chemother. 1994, 38, 369–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robert, E.W.; Hancock, L.; David, P. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and impact on treatment. Drug Resist. Updates 2000, 3, 247–255. [Google Scholar]
- Nikaido, H. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 1996, 178, 5853–5859. [Google Scholar] [CrossRef] [PubMed]
- Bianca, J.L.; Shawn, D.A.; Wendy, F.; Paul, C.H.; Noni, E.M. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 2000, 161, 1206–1212. [Google Scholar]
- Kaskhedikar, M.; Chhabra, D. Multiple drug resistance in Aeromonas hydrophila isolates of fish. Vet. World 2010, 2, 76–77. [Google Scholar]
- Sarah, E.B.; Katja, S.; Thomas, W.; Joachim, F. Evidence for a type III secretion system in Aeromonas salmo nicida subsp. Salmonicida. J. Bacteriol. 2002, 184, 5966–5970. [Google Scholar]
- Stuber, K.; Burr, S.E.; Braun, M.; Wahli, T.; Frey, J. Type III secretion genes in Aeromonas salmonicida subsp. salmonicida are located on a large thermolabile virulence plasmid. J. Clin. Microbiol. 2003, 41, 3854–3856. [Google Scholar] [CrossRef] [PubMed]
- Noonan, B.; Trust, T.J. The synthesis, secretion and role in virulence of the paracrystalline surface protein layers of Aeromonas salmonicida and A. hydrophila. FEMS Microbiol. Lett. 1997, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dacanay, A.; Boyd, J.M.; Fast, M.D.; Knickle, L.C.; Reith, M.E. Aeromonas salmonicida Type I pilus system contributes to host colonization but not invasion. Dis. Aquat. Org. 2010, 88, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cynthia, L.M.; Scott, E.L.; Andrew, W.M.; Mark, S.S. An Aeromonas salmonicida type IV pilin is required for virulence in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 2002, 51, 13–25. [Google Scholar]
- Vipond, R.; Bricknell, I.R.; Durant, E.; Bowden, T.J.; Ellis, A.E.; Smith, M.; MacIntyre, S. Defined deletion mutants demonstrate that the major secreted toxins are not essential for the virulence of Aeromonas salmonicida. Infect. Immun. 1998, 66, 1990–1998. [Google Scholar]
- Lee, K.K.; Ellis, A.E. Glycerophospholipid-cholesterol acyltransferase complexed with lipopolysaccharide (Lps) is a major lethal exotoxin and cytolysin of Aeromonas salmonicida—Lps stabilizes and enhances toxicity of the enzyme. J. Bacteriol. 1990, 172, 5382–5393. [Google Scholar] [CrossRef] [PubMed]
- Hirono, I.; Aoki, T. Cloning and characterization of 3 hemolysin genes from Aeromonas salmonicida. Microb. Pathog. 1993, 15, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Larbi, E.B.; al-Khawajah, A.; al-Gindan, Y.; Jain, S.; Abahusain, A.; Al-Zayer, A. A randomized, double-blind, clinical trial of topical clotrimazole versus miconazole for treatment of cutaneous leishmaniasis in the eastern province of Saudi Arabia. Am. J. Trop. Med. Hyg. 1995, 52, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Zakai, H.A.; Zimmo, S.K. Effects of itraconazole and terbinafine on Leishmania major lesions in BALB/c mice. Ann. Trop. Med. Parasitol. 2000, 94, 787–791. [Google Scholar] [CrossRef] [PubMed]
| Doses | First Week | Four Weeks | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Control (Infected, non-treated) | 8.22 ± 0.88 | 10.8 ± 1.89 | <0.0001 |
| Liquid allicin (0.30 μM/mouse) | 7.88 ± 1.67 | 5.65 ± 1.01 | 0.107 |
| Ciprofloxacin (10 mg/mL) | 7.43 ± 1.99 | 6.79 ± 1.16 | 0.775 |
| Allicin (0.30 μM + Ciprofloxacin (10 mg/mL)) | 7.66 ± 1.97 | 3.17 ± 1.12 | 0.058 * |
| Microorganism | Gram Stain | Total Isolates (n = 48) | % |
|---|---|---|---|
| Erwinia sp. | − | 2 | 4.16 |
| Pantoea sp. | − | 2 | 4.16 |
| Chryseomonas luteola | − | 4 | 8.33 |
| Stenotrophomonas maltophilia | − | 2 | 4.16 |
| Pseudomonas aeruginosa | − | 2 | 4.16 |
| Burkholderia cepacia | − | 4 | 8.33 |
| Aeromonas salmonicida subsp. Salmonicida | − | 4 | 8.33 |
| Aeromonas hydrophila | − | 2 | 4.16 |
| Flavobacterium indologenes | − | 2 | 4.16 |
| Chryseobacterium meningosepticum | − | 4 | 8.33 |
| Bacillus sp. | + | 2 | 4.16 |
| Aerococcus viridans | + | 4 | 8.33 |
| Lactococcus lactis subsp. lactis | + | 2 | 4.16 |
| Chryseomonas indologenes | − | 2 | 4.16 |
| Flavimonas oryzihabitans | − | 6 | 12.5 |
| Comamonas acidovorans | − | 2 | 4.16 |
| Cryptococcus albidus | + | 2 | 4.16 |
| Gram-positive | + | 10 | 20.84 |
| Gram-negative | − | 38 | 79.16 |
| Bacterial Isolates | Allicin Zone of Inhibition (mm) |
|---|---|
| Aeromonas salmonicida subsp. salmonicida | 15 |
| Pantoea sp. | 15 |
| Flavimonas oryzihabitans | − |
| Erwinia sp. | − |
| Chryseomonas indologenes | 10 |
| Chryseobacterium meningosepticum | 12 |
| Stenotrophomonas maltophilia | − |
| Burkholderia cepacia | 10 |
| Pseudomonas aeruginosa | 10 |
| Burkholderia cepacia | 10 |
| Bacillus sp. | 15 |
| Chryseomonas luteola | 10 |
| Cryptococcus albidus | 15 |
| Comamonas acidovorans | − |
| Efficiency rate (%) | 71.43 |
| % | E | CFR | TE | C | K | CIP | CT | SXT | LZD | F | VA | TIC | N | AMP | AML | Antibiotic | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Resistance | 15 | 30 | 30 | 30 | 30 | 5 | 25 | 25 | 30 | 300 | 30 | 75 | 30 | 25 | 25 | ||
| ≤13 | ND | ≤11 | ≤12 | ≤13 | ≤15 | ND | ≤10 | ≤20 | ≤14 | ≤13 | ≤11 | ≤13 | ≤13 | ≤13 | R | Microorganism | |
| 14–22 | 12–14 | 13–17 | 14–17 | 16–20 | 11–15 | 21–22 | 15–16 | 14–18 | 12–14 | 14–15 | 14–16 | 14–17 | I | ||||
| ≥23 | ≥15 | ≥18 | ≥18 | ≥21 | ≥16 | ≥23 | ≥17 | ≥19 | ≥15 | ≥16 | ≥17 | ≥18 | S | ||||
| 60 | R | R | 15 | 15 | 15 | 30 | 15 | 25 | R | R | R | R | R | R | R | Comamonas acidovorans | |
| 0 | 17 | 30 | 15 | 15 | 30 | 30 | 16 | 30 | 30 | 24 | 30 | 30 | 15 | 30 | 30 | Chryseobacterium meningosepticum | |
| 0 | 25 | 30 | 20 | 18 | 30 | 30 | 10 | 25 | 24 | 20 | 20 | 30 | 20 | 30 | 20 | Bacillus | |
| 60 | R | R | 13 | R | 20 | 30 | 13 | R | R | R | R | 12 | 15 | R | R | Erwinia sp. | |
| 26.67 | 16 | ND | 25 | 15 | 25 | 25 | 10 | R | R | R | R | 30 | 20 | 20 | 20 | Pantoea sp. | |
| 26.67 | 15 | 20 | 24 | 18 | 20 | 30 | 20 | 30 | R | 26 | R | 12 | 16 | R | R | Aeromonas salmonicida subsp. salmonicida | |
| 20 | 14 | R | 20 | R | R | 20 | 10 | 30 | 30 | 18 | 20 | 25 | 16 | 26 | 25 | Chryseomonas luteola | |
| 60 | 16 | R | 14 | 30 | R | 30 | R | 30 | R | R | 15 | R | R | R | R | Chryseomonas indologenes | |
| 46.67 | 14 | R | 20 | 20 | R | R | R | 15 | R | R | 14 | 12 | R | 14 | 18 | Flavimonas oryzihabitans | |
| 66.67 | R | R | R | 15 | 20 | 30 | 12 | 20 | R | R | R | R | R | R | R | Burkholderia cepacia | |
| 66.67 | R | R | 12 | 14 | R | 30 | 15 | 25 | R | R | R | R | R | R | R | Pseudomonas aeruginosa | |
| 33.33 | R | 12 | 12 | 15 | 20 | 20 | 14 | 20 | R | 15 | R | 13 | 20 | R | R | Aeromonas hydrophila | |
| 73.33 | R | R | R | 20 | R | 25 | 14 | R | R | R | R | R | R | R | R | Stenotrophomonas maltophilia | |
| 6 | 7 | 2 | 2 | 5 | 1 | 2 | 3 | 10 | 8 | 8 | 5 | 6 | 8 | 8 | Resistance number for each antibiotic | ||
| 46.15 | 53.8 | 15.39 | 15.39 | 38.46 | 7.70 | 15.38 | 23.08 | 76.92 | 61.54 | 61.54 | 38.46 | 46.15 | 61.54 | 61.5 | Resistance rate (%) | ||
| 83.85 | 30.7 | 84.61 | 84.61 | 61.54 | 92.30 | 84.61 | 76.92 | 23.08 | 38.46 | 38.46 | 61.54 | 83.85 | 38.46 | 38.4 | Sensitivity rate (%) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yehia, H.M.; Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M. In Vitro and In Vivo Control of Secondary Bacterial Infection Caused by Leishmania major. Int. J. Environ. Res. Public Health 2017, 14, 777. https://doi.org/10.3390/ijerph14070777
Yehia HM, Al-Olayan EM, El-Khadragy MF, Metwally DM. In Vitro and In Vivo Control of Secondary Bacterial Infection Caused by Leishmania major. International Journal of Environmental Research and Public Health. 2017; 14(7):777. https://doi.org/10.3390/ijerph14070777
Chicago/Turabian StyleYehia, Hany M., Ebtesam M. Al-Olayan, Manal F. El-Khadragy, and Dina M. Metwally. 2017. "In Vitro and In Vivo Control of Secondary Bacterial Infection Caused by Leishmania major" International Journal of Environmental Research and Public Health 14, no. 7: 777. https://doi.org/10.3390/ijerph14070777
APA StyleYehia, H. M., Al-Olayan, E. M., El-Khadragy, M. F., & Metwally, D. M. (2017). In Vitro and In Vivo Control of Secondary Bacterial Infection Caused by Leishmania major. International Journal of Environmental Research and Public Health, 14(7), 777. https://doi.org/10.3390/ijerph14070777







