Ecological Niche Modeling Identifies Fine-Scale Areas at High Risk of Dengue Fever in the Pearl River Delta, China
Abstract
:1. Introduction
2. Materials and Methods
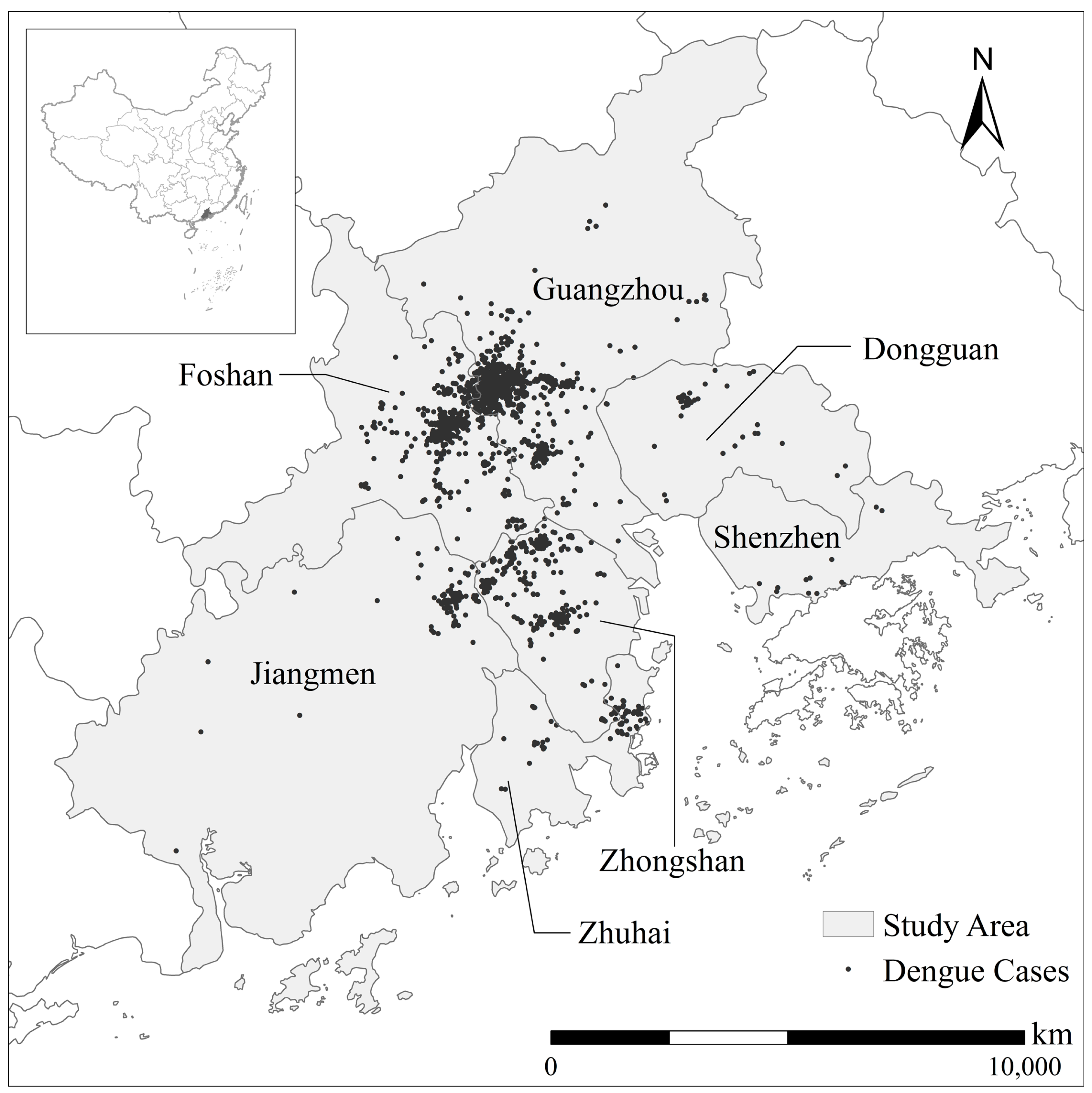
2.1. Study Area
2.2. Data Collection and Preprocessing
2.2.1. Dengue Cases
2.2.2. Environmental Conditions
2.2.3. Socioeconomic Factors
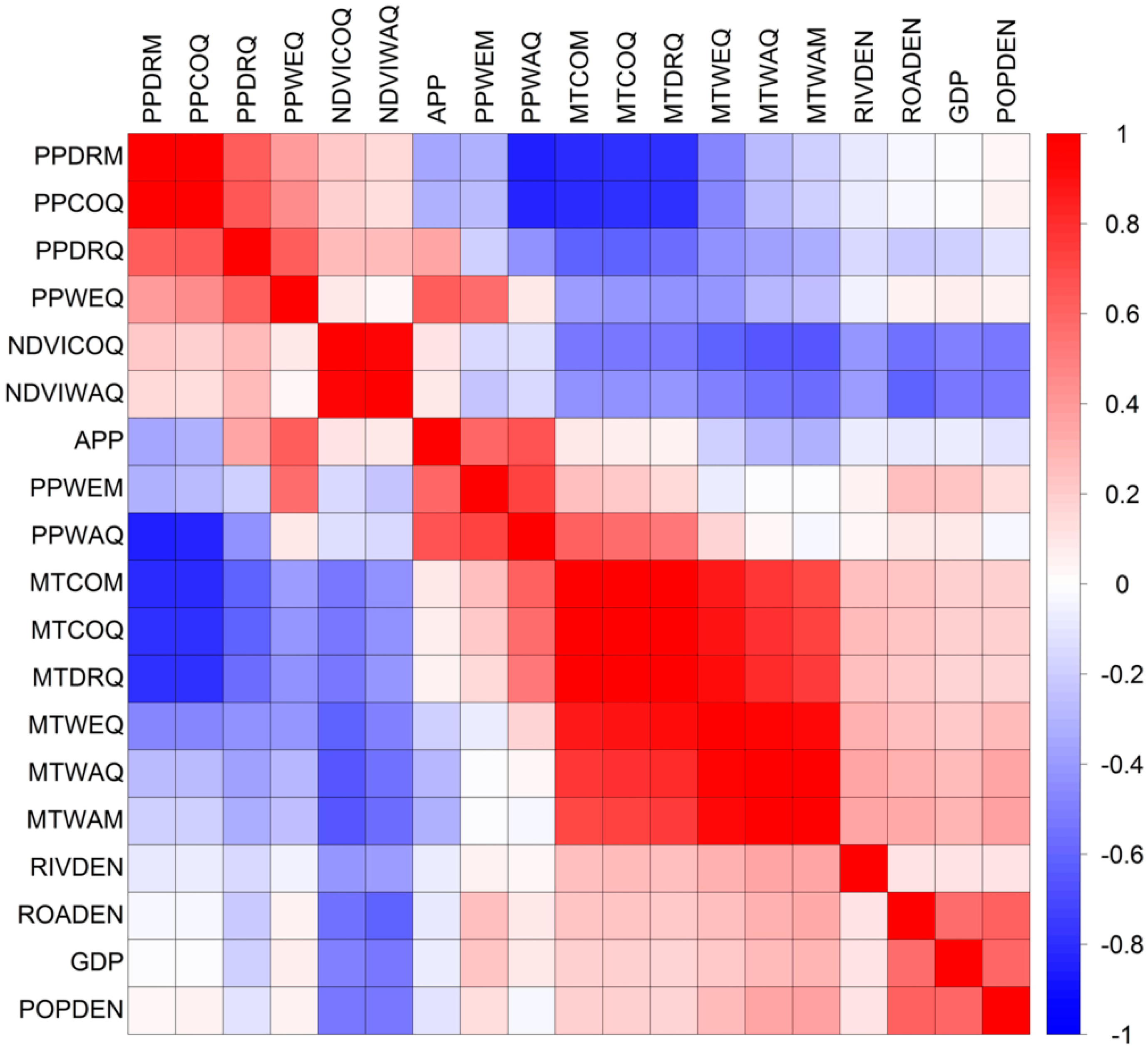
2.2.4. Combination of Variables
2.3. Modeling and Validation
3. Results
3.1. Model Performance
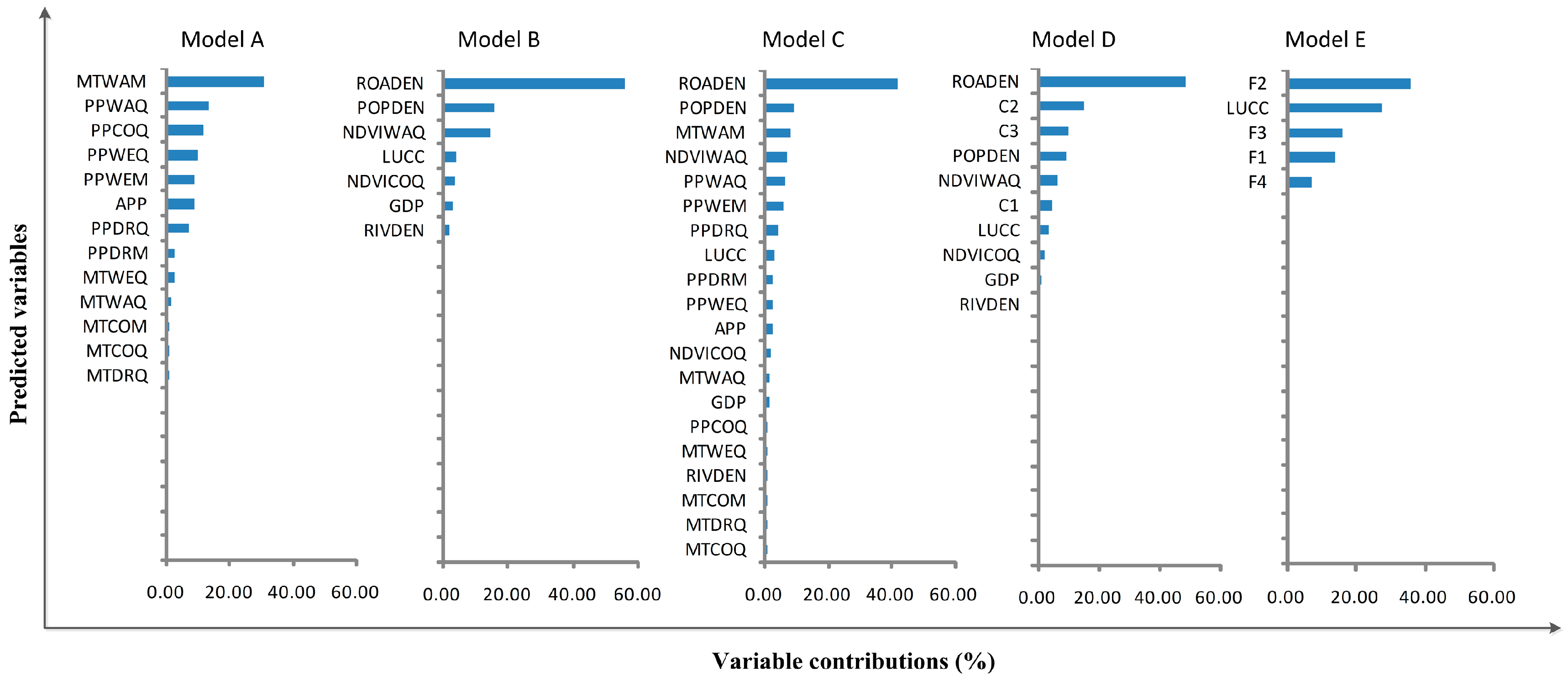
3.2. Variable Contributions
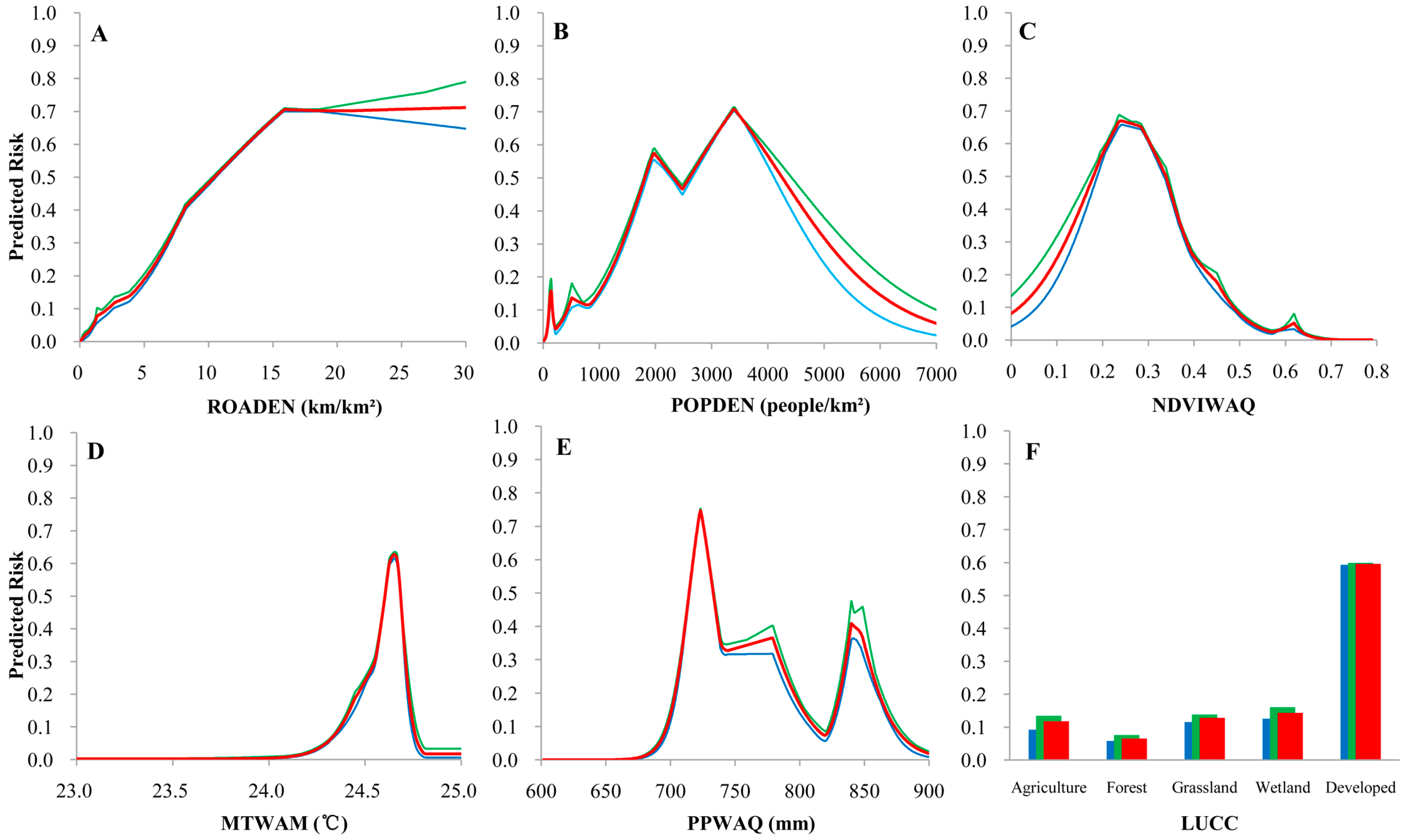
3.3. Risk Responseto Major Predictions
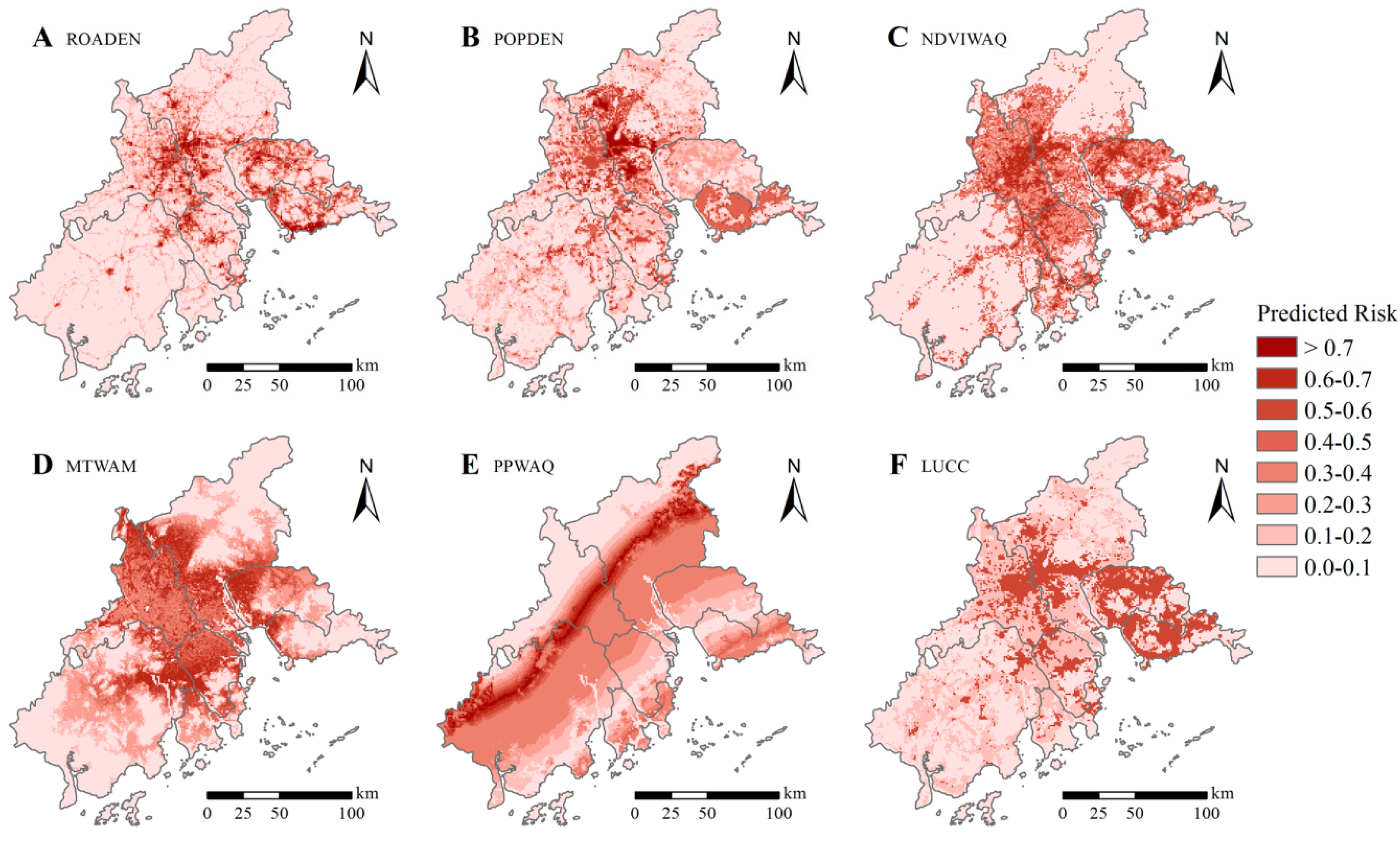
3.4. Spatial Patterns of DF-Epidemic Risk
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sang, S.; Wang, S.; Lu, L.; Bi, P.; Lv, M.; Liu, Q. The epidemiological characteristics and dynamic transmission of dengue in China, 2013. PLoS Negl. Trop. Dis. 2016, 10, e0005095. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Wu, F. A Study on the Potential Distribution of Aedes Albopictus and Risk Forecasting for Future Epidemics of Dengue in China; National Institute for Communicable Disease Control and Prevention, China CDC: Beijing, China, 2009. [Google Scholar]
- Wu, J.Y.; Lun, Z.R.; James, A.A.; Chen, X.G. Dengue fever in mainland China. Am. J. Trop. Med. Hyg. 2010, 83, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Chen, B.; Wu, H.; Yang, Z.; Di, B.; Wang, L.; Tao, X.; Liu, X.; Liu, Q. Dengue is still an imported disease in China: A case study in Guangzhou. Infect. Genet. Evolut. J. Mol. Epidemiol. Evolut. Genet. Infect. Dis. 2015, 32, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, Q. Dengue fever in China. Lancet 2015, 385, 1621–1622. [Google Scholar] [CrossRef]
- Ren, H.; Ning, W.; Lu, L.; Zhuang, D.; Liu, Q. Characterization of dengue epidemics in mainland China over the past decade. J. Infect. Dev. Ctries 2015, 9, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jing, Q.; Spear, R.C.; Marshall, J.M.; Yang, Z.; Gong, P. Climate and the timing of imported cases as determinants of the dengue outbreak in Guangzhou, 2014: Evidence from a mathematical model. PLoS Negl. Trop. Dis. 2016, 10, e0004417. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, W.; Clements, A.; Williams, G.; Lai, S.; Zhou, H.; Zhao, D.; Guo, Y.; Zhang, Y.; Wang, J. Spatiotemporal analysis of indigenous and imported dengue fever cases in Guangdong province, China. BMC Infect. Dis. 2012, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Q.; Lin, H.; Xin, B.; Nie, J. Spatial analysis of dengue fever in Guangdong province, China, 2001–2006. Asia-Pac. J. Public Health 2014, 26, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Meng, Y.J.; Li, Y.; Qi, X.P. Frequency, duration and intensity of dengue fever epidemic risk in townships in Pearl River Delta and Yunnan in China, 2013. Biomed. Environ. Sci. BES 2015, 28, 388–395. [Google Scholar] [PubMed]
- Fan, J.; Lin, H.; Wang, C.; Bai, L.; Yang, S.; Chu, C.; Yang, W.; Liu, Q. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong province, China from 2005 to 2011. Epidemiol. Infect. 2014, 142, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Liu, K.; Xia, Y.; Lu, Y.; Jing, Q.; Yang, Z.; Hu, W.; Lu, J. Developing a time series predictive model for dengue in Zhongshan, China based on weather and Guangzhou dengue surveillance data. PLoS Negl. Trop. Dis. 2016, 10, e0004473. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lin, H.; Tian, L.; Yang, W.; Sun, J.; Liu, Q. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liu, J.; Tan, Q.; Shi, B. Inferring the spatio-temporal patterns of dengue transmission from surveillance data in Guangzhou, China. PLoS Negl. Trop. Dis. 2016, 10, e0004633. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T. Ecological niche modelling and understanding the geography of disease transmission. Vet. Ital. 2007, 43, 393–400. [Google Scholar] [PubMed]
- Peterson, A.T. Ecologic niche modeling and spatial patterns of disease transmission. Emerg. Infect. Dis. 2006, 12, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Machado-Machado, E.A. Empirical mapping of suitability to dengue fever in Mexico using species distribution modeling. Appl. Geogr. 2012, 33, 82–93. [Google Scholar] [CrossRef]
- Peterson, A.T.; Martinez, C.C.Y.; Martinez, M.E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Leite, R.; Vilarinho, A.C.; Novaes, M.C.; Tonetto, A.F.; Vilardi, G.C.; Guillermo-Ferreira, R. Recent and future environmental suitability to dengue fever in Brazil using species distribution model. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; He, J.F.; Deng, A.P.; Lin, H.L.; Song, T.; Peng, Z.Q.; Wu, X.C.; Liu, T.; Li, Z.H.; Rutherford, S.; et al. Characterizing a large outbreak of dengue fever in Guangdong province, China. Infect. Dis. Poverty 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E. The re-emergence of dengue in China. BMC Med. 2015, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, Y.; Li, Y.; Meng, Y.; Chen, Q.; Ma, J.; Gao, G.F. The effects of socioeconomic and environmental factors on the incidence of dengue fever in the Pearl River Delta, China, 2013. PLoS Negl. Trop. Dis. 2015, 9, e0004159. [Google Scholar] [CrossRef] [PubMed]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived ndvi to assess ecological responses to environmental change. Trends Ecol. Evolut. 2005, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of maxent for ecologists. Divers. Distrib. 2010, 17, 43–57. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Cruz-Cárdenas, G.; López-Mata, L.; Villaseñor, J.L.; Ortiz, E. Potential species distribution modeling and the use of principal component analysis as predictor variables. Revis. Mex. Biodivers. 2014, 85, 189–199. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Miroslav, K.; Schapire, R.E. A Maximum Entropy Approach to Species Distribution Modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; p. 83. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evolut. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Qian, Q.; Wang, Z.Q.; Glass, G.E.; Song, S.X.; Zhang, W.Y.; Li, X.J.; Yang, H.; Wang, X.J.; Fang, L.Q.; et al. Using geographic information system-based ecologic niche models to forecast the risk of hantavirus infection in Shandong province, China. Am. J. Trop. Med. Hyg. 2011, 84, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. Application of roc curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15. [Google Scholar] [CrossRef]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef]
- Vanagas, G. Receiver operating characteristic curves and comparison of cardiac surgery risk stratification systems. Interact. Cardiovasc. Thorac. Surg. 2004, 3, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, R.S.; Severson, D.W.; Chadee, D.D. Impact of road networks on the distribution of dengue fever cases in Trinidad, West Indies. Acta Trop. 2012, 123, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, K.-T.; Han, F.; Karacsonyi, D.; Qian, Q. Investigating urbanization and its spatial determinants in the central districts of Guangzhou, China. Habitat Int. 2016, 51, 59–69. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.G. Urbanization increases aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X. Influence of temporary migration on the transmission of infectious diseases in a migrants’ home village. J. Theor. Biol. 2012, 300, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.P.; Suzuki, M.; Thiem, V.D.; White, R.G.; Tsuzuki, A.; Yoshida, L.M.; Yanai, H.; Haque, U.; Tho, L.H.; Anh, D.D.; et al. Population density, water supply, and the risk of dengue fever in Vietnam: Cohort study and spatial analysis. PLoS Med. 2011, 8, e1001082. [Google Scholar] [CrossRef] [PubMed]
- Troyo, A.; Fuller, D.O.; Calderon-Arguedas, O.; Solano, M.E.; Beier, J.C. Urban structure and dengue fever in Puntarenas, Costa Rica. Singap. J. Trop. Geogr. 2009, 30, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Teurlai, M.; Menkes, C.E.; Cavarero, V.; Degallier, N.; Descloux, E.; Grangeon, J.P.; Guillaumot, L.; Libourel, T.; Lucio, P.S.; Mathieu-Daude, F.; et al. Socio-economic and climate factors associated with dengue fever spatial heterogeneity: A worked example in New Caledonia. PLoS Negl. Trop. Dis. 2015, 9, e0004211. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Lay, J.G.; Guo, H.R.; Lin, C.Y.; Lung, S.C.; Su, H.J. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 2009, 407, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Bangs, M.J.; Larasati, R.P.; Corwin, A.L.; Wuryadi, S. Climatic factors associated with epidemic dengue in Palembang, Indonesia: Implications of short-term meteorological events on virus transmission. Southeast. Asian J. Trop. Med. Public Health 2006, 37, 1103–1116. [Google Scholar] [PubMed]
- Hii, Y.L.; Rocklov, J.; Ng, N.; Tang, C.S.; Pang, F.Y.; Sauerborn, R. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Glob. Health Action 2009, 2. [Google Scholar] [CrossRef]

| Abbreviation | Description | Type |
|---|---|---|
| Climatic Variables | ||
| MTWAM | Mean temperature of the warmest month (°C) | Continuous |
| MTCOM | Mean temperature of the coldest month (°C) | Continuous |
| MTWEQ | Mean temperature of the wettest quarter (°C) | Continuous |
| MTDRQ | Mean temperature of the driest quarter (°C) | Continuous |
| MTWAQ | Mean temperature of the warmest quarter (°C) | Continuous |
| MTCOQ | Mean temperature of the coldest quarter (°C) | Continuous |
| APP | Annual precipitation (mm) | Continuous |
| PPWEM | Precipitation in the wettest month (mm) | Continuous |
| PPDRM | Precipitation in the driest month (mm) | Continuous |
| PPWEQ | Precipitation in the wettest quarter (mm) | Continuous |
| PPDRQ | Precipitation in the driest quarter (mm) | Continuous |
| PPWAQ | Precipitation in the warmest quarter (mm) | Continuous |
| PPCOQ | Precipitation in the coldest quarter (mm) | Continuous |
| Environmental Variables | ||
| NDVIWAQ | Normalized difference vegetation index (NDVI) of the warmest quarter | Continuous |
| NDVICOQ | Normalized difference vegetation index (NDVI) of the coldest quarter | Continuous |
| RIVDEN | River density (km/km2) | Continuous |
| Socioeconomic Variables | ||
| ROADEN | Road density (km/km2) | Continuous |
| GDP | Gross domestic product (CNY) | Continuous |
| POPDEN | Population density (people/km2) | Continuous |
| LUCC | Land use and land cover change (LUCC) | Categorical |
| a. Full Principal Components | b. Climatic Principal Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | C1 | C2 | C3 | C4 | C5 | |
| Eigenvalue | 8.185 | 3.618 | 2.842 | 1.427 | 0.941 | 7.212 | 3.245 | 1.717 | 0.744 | 0.063 |
| variance | 43.079 | 19.043 | 14.958 | 7.512 | 4.951 | 55.48 | 24.962 | 13.204 | 5.722 | 0.486 |
| Cumulative | 43.079 | 62.122 | 77.08 | 84.592 | 89.544 | 55.48 | 80.443 | 93.647 | 99.369 | 99.855 |
| Variable Loadings | ||||||||||
| MTWAM | 0.786 | 0.446 | −0.114 | 0.329 | 0.154 | 0.705 | −0.502 | 0.488 | −0.103 | −0.013 |
| MTCOM | 0.962 | −0.207 | −0.079 | 0.101 | 0.090 | 0.991 | 0.038 | 0.096 | 0.042 | 0.055 |
| MTWEQ | 0.888 | 0.211 | −0.238 | 0.283 | 0.159 | 0.864 | −0.392 | 0.292 | 0.112 | −0.047 |
| MTDRQ | 0.955 | −0.173 | −0.155 | 0.134 | 0.105 | 0.988 | −0.034 | 0.085 | 0.121 | 0.011 |
| MTWAQ | 0.829 | 0.380 | −0.146 | 0.316 | 0.156 | 0.763 | −0.471 | 0.438 | −0.050 | −0.010 |
| MTCOQ | 0.964 | −0.181 | −0.112 | 0.113 | 0.095 | 0.992 | −0.003 | 0.094 | 0.066 | 0.033 |
| APP | −0.036 | −0.660 | 0.603 | 0.338 | 0.087 | 0.019 | 0.883 | 0.307 | 0.344 | −0.083 |
| PPWEM | 0.231 | −0.422 | 0.742 | 0.018 | −0.045 | 0.211 | 0.754 | 0.344 | −0.507 | 0.108 |
| PPDRM | −0.712 | 0.658 | 0.071 | 0.150 | 0.032 | −0.823 | −0.437 | 0.321 | −0.154 | 0.029 |
| PPWEQ | −0.404 | −0.052 | 0.779 | 0.426 | 0.089 | −0.474 | 0.520 | 0.694 | −0.093 | −0.114 |
| PPDRQ | −0.652 | 0.168 | 0.261 | 0.522 | 0.205 | −0.690 | 0.058 | 0.482 | 0.516 | 0.151 |
| PPWAQ | 0.505 | −0.769 | 0.375 | −0.090 | −0.050 | 0.587 | 0.802 | −0.079 | −0.068 | 0.034 |
| PPCOQ | −0.709 | 0.647 | 0.119 | 0.196 | 0.039 | −0.824 | −0.401 | 0.375 | −0.137 | 0.009 |
| NDVIWAQ | −0.629 | −0.456 | −0.420 | 0.059 | 0.243 | |||||
| NDVICOQ | −0.700 | −0.452 | −0.318 | −0.027 | 0.197 | |||||
| RIVDEN | 0.349 | 0.220 | 0.060 | 0.308 | −0.782 | |||||
| ROADEN | 0.402 | 0.418 | 0.500 | −0.358 | 0.098 | |||||
| GDP | 0.353 | 0.390 | 0.489 | −0.390 | 0.115 | |||||
| POPDEN | 0.355 | 0.501 | 0.436 | −0.285 | 0.204 | |||||
| Model | Description of Models | Number of Variables |
|---|---|---|
| A | Original climatic variables | 13 |
| B | Original environmental and socioeconomic variables | 7 |
| C | Full original variables | 20 |
| D | Climatic principle components (C1, C2, C3) plus original environmental and socioeconomic variables | 10 |
| E | Full principle components (F1, F2, F3, F4) plus LUCC variable | 5 |
| Model | Mean AUC Value | DF Cases in Given Risk Tiers (% of Cases/% Area of the PRD) | |||
|---|---|---|---|---|---|
| Training AUC | Test AUC | Low | Moderate | High | |
| A | 0.907 | 0.904 | 5.13/73.47 | 12.02/17.92 | 82.92/8.61 |
| B | 0.883 | 0.882 | 2.25/64.45 | 16.01/26.08 | 81.74/9.47 |
| C | 0.910 | 0.906 | 3.42/75.85 | 13.22/15.96 | 83.35/8.19 |
| D | 0.900 | 0.896 | 3.80/73.75 | 12.30/17.88 | 83.89/8.36 |
| E | 0.896 | 0.893 | 3.88/65.62 | 14.77/23.86 | 81.35/10.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Ren, H.; Zheng, L.; Cao, W.; Zhang, A.; Zhuang, D.; Lu, L.; Jiang, H. Ecological Niche Modeling Identifies Fine-Scale Areas at High Risk of Dengue Fever in the Pearl River Delta, China. Int. J. Environ. Res. Public Health 2017, 14, 619. https://doi.org/10.3390/ijerph14060619
Li Q, Ren H, Zheng L, Cao W, Zhang A, Zhuang D, Lu L, Jiang H. Ecological Niche Modeling Identifies Fine-Scale Areas at High Risk of Dengue Fever in the Pearl River Delta, China. International Journal of Environmental Research and Public Health. 2017; 14(6):619. https://doi.org/10.3390/ijerph14060619
Chicago/Turabian StyleLi, Qiaoxuan, Hongyan Ren, Lan Zheng, Wei Cao, An Zhang, Dafang Zhuang, Liang Lu, and Huixian Jiang. 2017. "Ecological Niche Modeling Identifies Fine-Scale Areas at High Risk of Dengue Fever in the Pearl River Delta, China" International Journal of Environmental Research and Public Health 14, no. 6: 619. https://doi.org/10.3390/ijerph14060619
APA StyleLi, Q., Ren, H., Zheng, L., Cao, W., Zhang, A., Zhuang, D., Lu, L., & Jiang, H. (2017). Ecological Niche Modeling Identifies Fine-Scale Areas at High Risk of Dengue Fever in the Pearl River Delta, China. International Journal of Environmental Research and Public Health, 14(6), 619. https://doi.org/10.3390/ijerph14060619









