The Use of Chemoprophylaxis after Floods to Reduce the Occurrence and Impact of Leptospirosis Outbreaks
Abstract
:1. Introduction
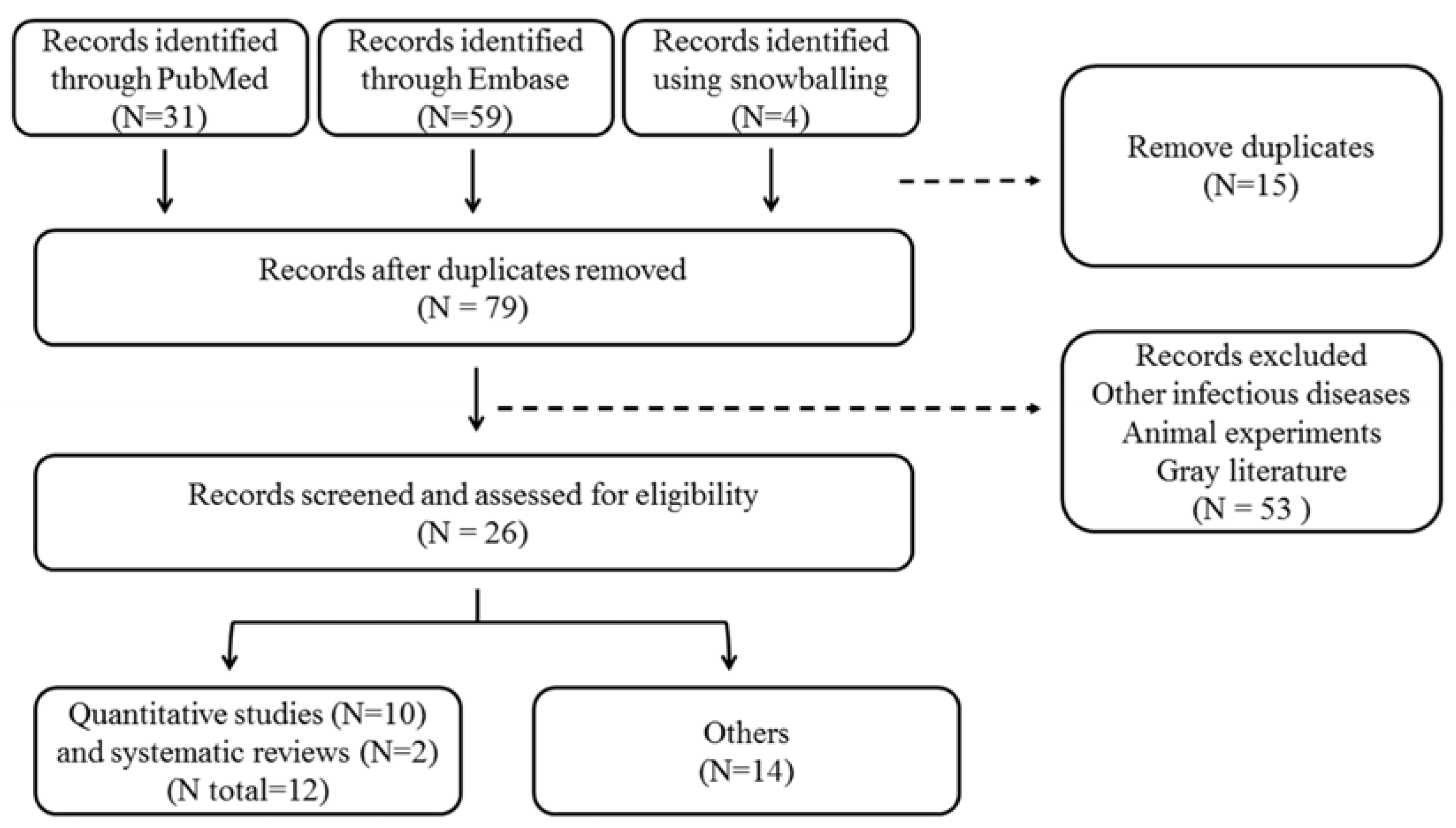
2. Materials and Methods
2.1. Literature Review on Chemoprophylaxis Use for Leptospirosis
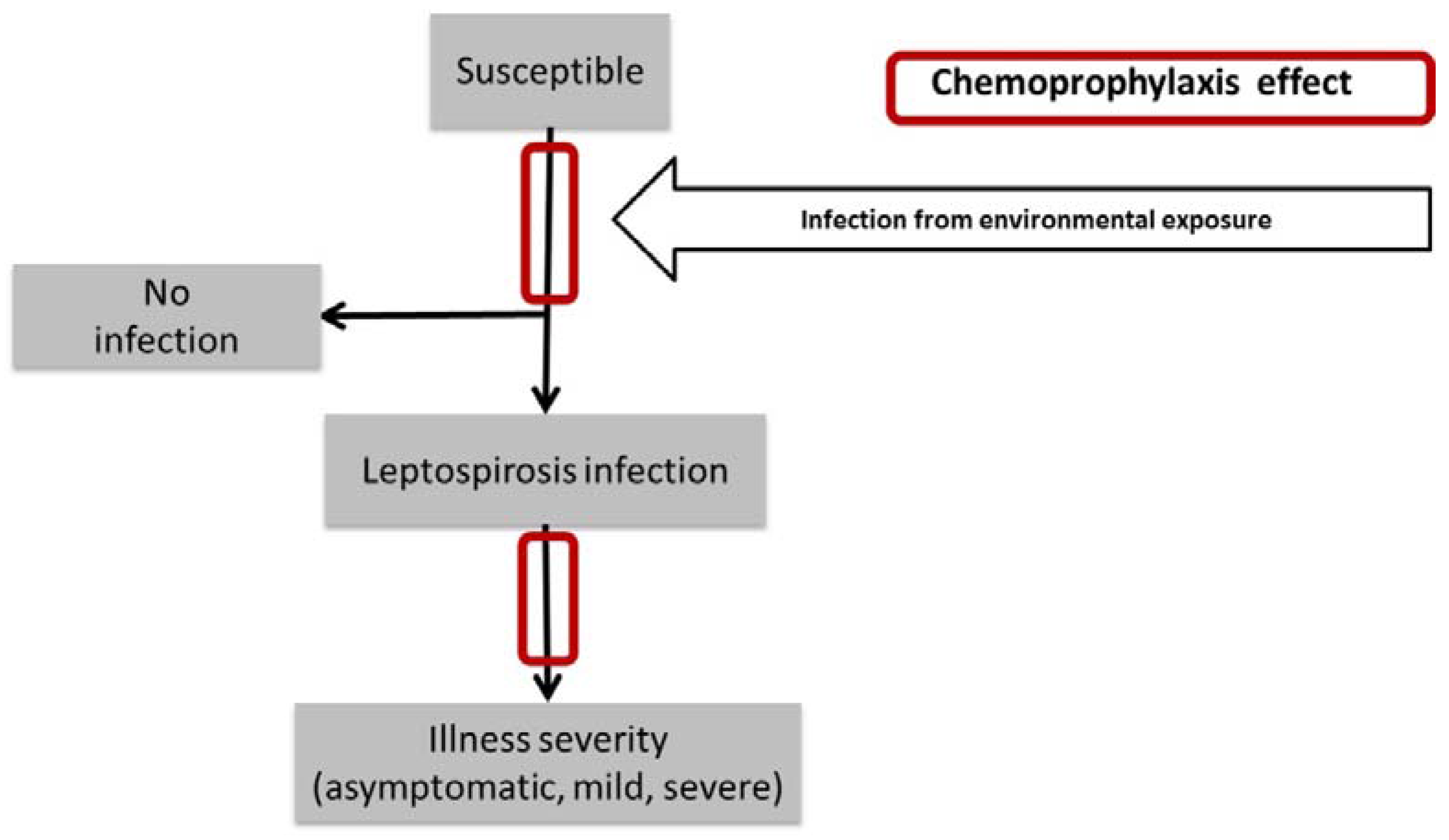
2.2. The Mathematical Model
- (1)
- (2)
- (3)
- (4)
- (5)
- (6)
- (7)
- (8)
- (9)
- (10)
- (11)
- (12)
- (13)
- (14)
- (15)
- (16)
3. Results
3.1. Literature Review on Chemoprophylaxis Use for Leptospirosis
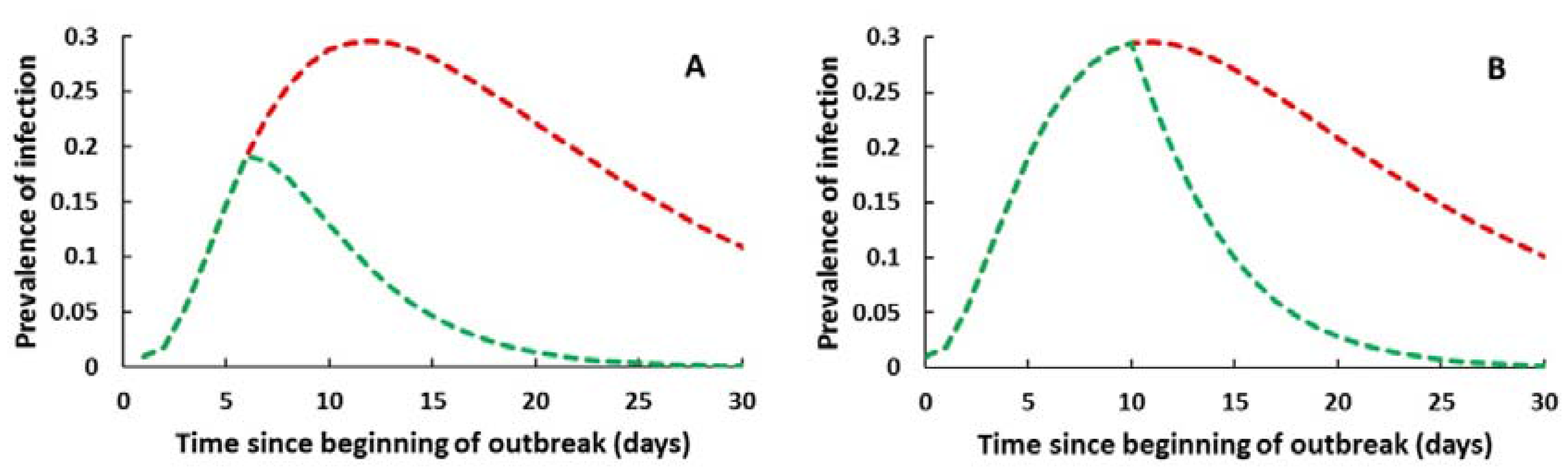
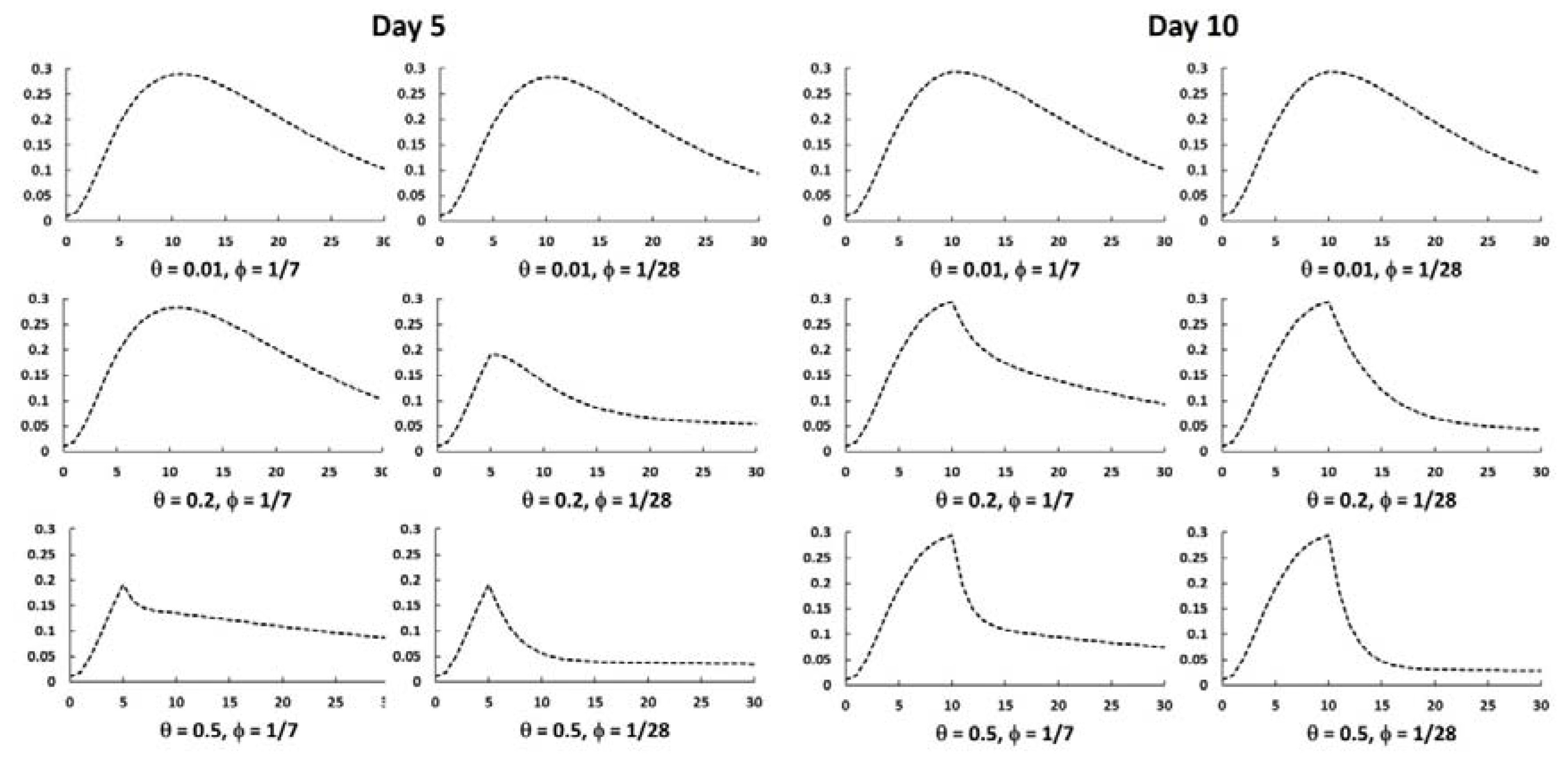
3.2. Mathematical Simulation of the Outbreak and Effect of Chemoprophylaxis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Type | Subtype | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drought | Drought | 2 | 4 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 2 | 1 | 3 | 3 | 0 | 22 |
| Earthquake | Ground movement | 2 | 3 | 0 | 4 | 1 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 3 | 1 | 4 | 0 | 0 | 26 |
| Epidemic | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bacterial disease | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Viral disease | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 4 | 1 | 1 | 0 | 15 | |
| Extreme temperature | Cold wave | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 10 |
| Severe winter conditions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Flood | - | 3 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 15 |
| Coastal flood | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| Flash flood | 1 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 10 | |
| Riverine flood | 4 | 0 | 9 | 4 | 5 | 9 | 4 | 9 | 12 | 9 | 13 | 12 | 4 | 2 | 5 | 6 | 1 | 108 | |
| Landslide | Landslide | 1 | 0 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 3 | 1 | 0 | 0 | 0 | 1 | 1 | 18 |
| Mass movement (dry) | Landslide | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Storm | - | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Convective storm | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 5 | |
| Tropical cyclone | 4 | 10 | 6 | 2 | 0 | 16 | 3 | 9 | 6 | 4 | 15 | 6 | 2 | 4 | 7 | 1 | 8 | 103 | |
| Volcanic activity | Ash fall | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 9 |
| Wildfire | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Forest fire | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | |
| Land fire | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 24 | 22 | 29 | 19 | 12 | 30 | 14 | 22 | 22 | 23 | 35 | 25 | 12 | 13 | 21 | 18 | 15 | 356 |



Appendix B
| Author | Year | Journal | Type of the Study | Prophylactic Antibiotic | Recommendations/Conclusions |
|---|---|---|---|---|---|
| Devishree [55] | 2015 | J. Pharm. Sci. Res. | Review | Doxycycline | Pre-exposure: doxycycline (200 mg/week). Post exposure prophylaxis with doxycycline based on the degree of exposure (low, moderate, high) |
| Charan [56] | 2012 | Nat. J. Physiol. Pharm. Pharmacol. | Review | Doxycycline and penicillin | The role of antibiotics in chemoprophylaxis of leptospirosis is uncertain due to lack of large scale trials. More evidence based studies are required to generate evidence for antibiotics being used as chemoprophylaxis. |
| Dechet [35] | 2012 | PloS ONE | Chemoprophylaxis campaign description | Doxycycline | The effectiveness of the massive chemoprophylaxis campaign was inconclusive. |
| McBride [57] | 2010 | Pharmaceuticals | Review | Doxycycline | May only act to reduce clinical illness rather than infection. May cause nausea and vomiting |
| Cruz [58] | 2009 | Ethn. Dis. | Review | Doxycycline | When high-risk and short-term exposure to leptospira is anticipated, chemoprophylaxis is effective. Doxycycline prophylaxis does not prevent leptospiral infection in endemic areas, but has a protective effect in reducing morbidity and mortality during outbreaks. |
| Pavli [59] | 2008 | J. Travel Med. | Review | Doxycycline | Pre-exposure doxycycline chemoprophylaxis should be considered for adventure travelers, athletes, and military recruits involved in high-risk activities in endemic areas. |
| Christopher [60] | 2005 | Milit. Med. | Review | Doxycycline | Dr. E.T. Takafuji and colleagues at the Walter Reed Army Institute of Research demonstrated that prophylaxis using doxycycline conferred a 95% risk reduction. |
| Edwards [61] | 2004 | Exp. Rev. Anti Infect. Ther. | Review | Doxycycline | Short-term chemoprophylaxis with doxycycline in healthy young adults is effective but larger studies are required to demonstrate effectiveness in other ages and resident populations. |
| Faucher [45] | 2004 | Exp. Opin. Pharmacother. | Review | Doxycycline | The efficacy of pre-exposure doxycycline has been established by two randomized studies performed in different epidemiological environments. However, the efficacy of doxycycline in post exposure prophylaxis is not firmly established. |
| Levett [62] | 2004 | Clin. Appl. Immunol. Rev. | Review | Doxycycline | Doxycycline may be considered for chemoprophylaxis if high-risk exposures are anticipated. |
| Bharti [44] | 2003 | Lancet Infect. Dis. | Review | Doxycycline | Chemoprophylaxis may be impractical to administer in highly endemic areas, but is likely to be useful for adventure travelers and military personnel who visit endemic areas, and also in accidental laboratory infection. |
| Lo Re III [63] | 2003 | Am. Fam. Physic. | Clinical recommendation | Doxycycline | Doxycycline is an effective prophylaxis for travelers to endemic areas who have a high risk of exposure. |
| Haake [36] | 2002 | Clin. Infect. Dis. | Case report | Doxycycline | The benefits of doxycycline prophylaxis must be weighed against the potential adverse side effects of prophylaxis. Doxycycline may be used as a chemoprophylaxis strategy against both malaria and leptospirosis for patients who anticipate having a relatively high level of exposure. Clinical trials are needed to validate antibiotics with longer serum half-lives, such as azithromycin. |
| Gilks [64] | 1988 | Postgrad. Med. J. | Case report | Doxycycline and penicillin | Doxycycline, 100 mg twice weekly, over a period of 6 weeks provides a rational regimen for post-exposure prophylaxis, which takes into account the possibility of prolonged incubation. |
References
- Intergovernmental Panel on Climate Change. Climate Change 2014 Synthesis Report: Summary for Policymakers; IPCC: Geneva, Switzerland, 2014; p. 32. [Google Scholar]
- Guha-Sapir, D.; Below, R.; Hoyois, P. EM-DAT: The International Disaster Database. Available online: www.emdat.be (accessed on 15 February 2017).
- Coumou, D.; Rahmstorf, S. A decade of weather extremes. Nat. Clim. Chang. 2012, 2, 491–496. [Google Scholar] [CrossRef]
- National Centers for Environmental Information. Global Climate Report—Annual 2010. Available online: https://www.ncdc.noaa.gov/sotc/global/201013 (accessed on 15 February 2017).
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.; Zinsstag, J.; Hattendorf, J. Environmental and behavioural determinants of leptospirosis transmission: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, e0003843. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Dobson, A.J.; Smythe, L.D.; Fearnley, E.J.; Skelly, C.; Clements, A.C.; Craig, S.B.; Fuimaono, S.D.; Weinstein, P. Leptospirosis in American Samoa 2010: Epidemiology, environmental drivers, and the management of emergence. Am. J. Trop. Med. Hyg. 2012, 86, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Amilasan, A.S.; Ujiie, M.; Suzuki, M.; Salva, E.; Belo, M.C.; Koizumi, N.; Yoshimatsu, K.; Schmidt, W.P.; Marte, S.; Dimaano, E.M.; et al. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg. Infect. Dis. 2012, 18, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, C.; Sabroza, P.C. The place behind the case: Leptospirosis risks and associated environmental conditions in a flood-related outbreak in Rio de Janeiro. Cad. Saude Publica. 2001, 17, S59–S67. [Google Scholar] [CrossRef]
- Liverpool, J.; Francis, S.; Liverpool, C.E.; Dean, G.T.; Mendez, D.D. Leptospirosis: Case reports of an outbreak in Guyana. Ann. Trop. Med. Parasitol. 2008, 102, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Goarant, C.; Laumond-Barny, S.; Perez, J.; Vernel-Pauillac, F.; Chanteau, S.; Guigon, A. Outbreak of leptospirosis in new Caledonia: Diagnosis issues and burden of disease. Trop. Med. Int. Health 2009, 14, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Trevejo, R.T.; Rigau-Pérez, J.G.; Ashford, D.A.; McClure, E.M.; Jarquín-González, C.; Amador, J.J.; José, O.; Gonzalez, A.; Zaki, S.R.; Shieh, W.-J. Epidemic leptospirosis associated with pulmonary hemorrhage—Nicaragua, 1995. J. Infect. Dis. 1998, 178, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Pellizzer, P.; Todescato, A.; Benedetti, P.; Colussi, P.; Conz, P.; Cinco, M. Leptospirosis following a flood in the Veneto area, North-East Italy. Ann. Ig. 2006, 18, 453–456. [Google Scholar] [PubMed]
- Gaynor, K.; Katz, A.R.; Park, S.Y.; Nakata, M.; Clark, T.A.; Effler, P.V. Leptospirosis on Oahu: An outbreak associated with flooding of a university campus. Am. J. Trop. Med. Hyg. 2007, 76, 882–885. [Google Scholar] [PubMed]
- Intergovernmental Panel on Climate Change. Summary for Policymakers. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 7–22. [Google Scholar]
- Melillo, J.M.; Richmond, T.; Yohe, G.W. Climate Change Impacts in the United States: The Third National Climate Assessment; U.S. Global Change Research Program: Washington, DC, USA, 2014; p. 841.
- Watson, J.T.; Gayer, M.; Connolly, M.A. Epidemics after natural disasters. Emerg. Infect. Dis. 2007, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Tirado, M.C.; Rereddy, S.; Dugas, R.; Borda, M.I.; Peralta, E.A.; Aldighieri, S.; Cosivi, O. Natural disasters and communicable diseases in the Americas: Contribution of veterinary public health. Vet. Ital. 2012, 48, 193–218. [Google Scholar] [PubMed]
- Pan American Health Organization. Health in the Americas—Regional Outlook and Country Profiles, 2012 ed.; PAHO: Washington DC, USA, 2012. [Google Scholar]
- Schneider, M.C.; Jancloes, M.; Buss, D.F.; Aldighieri, S.; Bertherat, E.; Najera, P.; Galan, D.I.; Durski, K.; Espinal, M.A. Leptospirosis: A silent epidemic disease. Int. J. Environ. Res. Public Health 2013, 10, 7229–7234. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control; WHO: Geneva, Switzerland, 2003; pp. 1–109. [Google Scholar]
- Acha, P.; Szyfres, B. Zoonoses and Communicable Diseases Common to Man and Animals: Bacterioses and Mycoses, 3rd ed.; Pan American Health Organization: Washington, DC, USA, 2003; Volume 1. [Google Scholar]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl. Trop. Dis. 2015, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbial. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Transact. Royal Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Leonel, D.G.; Hamrick, P.N.; Caldas, E.; Velasquez, R.; Paez, F.A.M.; Arrebato, J.G.; Gerger, A.; Pereira, M.M.; Aldighieri, S. Leptospirosis in Latin America: Exploring the first set of regional data. Rev. Panam. Salud Publica 2017, 41, e81. [Google Scholar]
- Boza, R. Leptospirosis anictérica: Análisis de una epidemia en Costa Rica. Acta Med. Costarric 1990, 33, 74–80. (In Spainish) [Google Scholar]
- Pan American Health Organization. Impacto del huracán mitch en Centro América. Boletin. Epidemiologico. 1998, 19, 1–13. (In Spainish) [Google Scholar]
- Rodríguez, T.; Cruz, L.; Rangel, G.S.; Vides, R. Brote de leptospirosis en el caserío las guarumas, cantón cerco de piedra, municipio de chapeltique, san miguel, el salvador, 15 de febrero de 2002. Encuesta Transversal 2002, 1–9. (In Spainish) [Google Scholar]
- Naranjo, M.; Suárez, M.; Fernández, C.; Amador, N.; González, M.; Batista, N.; González, I.; Valdés, Y.; Infante, J.F.; Sierra, G. Estudio de un brote de leptospirosis en Honduras tras el paso del huracán Mitch y potencialidad profiláctica de vax-SPIRAL®. Vaccimonitor 2007, 16, 13–18. (In Spainish) [Google Scholar]
- Munoz, F.; Jarquin, C.; Gonzalez, A.; Amador, J.; de los Reyes, J.; Jimenez, R.; Lamy, F.; Jiron, N.; Pinheiro, F. Outbreak of acute febrile illness and pulmonary hemorrhage—Nicaragua, 1995. MMWR 1995, 44, 841–843. [Google Scholar]
- Pan American Health Organization. Regional Core Health Data Initiative. Available online: http://www.paho.org/hq/index.php?option=com_tabs&view=article&id=2151&Itemid=3632&lang=en (accessed on 16 August 2016).
- Bacallao, J.; Schneider, M.C.; Najera, P.; Aldighieri, S.; Soto, A.; Marquiño, W.; Sáenz, C.; Jiménez, E.; Moreno, G.; Chávez, O. Socioeconomic factors and vulnerability to outbreaks of leptospirosis in Nicaragua. Int. J. Environ. Res. Public Health 2014, 11, 8301–8318. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.C.; Nájera, P.; Aldighieri, S.; Bacallao, J.; Soto, A.; Marquiño, W.; Altamirano, L.; Saenz, C.; Marin, J.; Jimenez, E. Leptospirosis outbreaks in Nicaragua: Identifying critical areas and exploring drivers for evidence-based planning. Int. J. Environ. Res. Public Health 2012, 9, 3883–3910. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Disaster Risk Management for Health Overview Fact Sheet. Available online: http://www.who.int/hac/techguidance/preparedness/factsheets/en/ (accessed on 16 August 2016).
- Dechet, A.M.; Parsons, M.; Rambaran, M.; Mohamed-Rambaran, P.; Florendo-Cumbermack, A.; Persaud, S.; Baboolal, S.; Ari, M.D.; Shadomy, S.V.; Zaki, S.R.; et al. Leptospirosis outbreak following severe flooding: A rapid assessment and mass prophylaxis campaign; Guyana, January–February 2005. PLoS ONE 2012, 7, e39672. [Google Scholar] [CrossRef] [PubMed]
- Haake, D.A.; Dundoo, M.; Cader, R.; Kubak, B.M.; Hartskeerl, R.A.; Sejvar, J.J.; Ashford, D.A. Leptospirosis, water sports, and chemoprophylaxis. Clin. Infect. Dis. 2002, 34, e40–e43. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.; Bancroft, E.; Winthrop, K.; Bettinger, J.; Bajani, M.; Bragg, S.; Shutt, K.; Kaiser, R.; Marano, N.; Popovic, T.; et al. Leptospirosis in “eco-challenge” athletes, Malaysian Borneo, 2000. Emerg. Infect. Dis. 2003, 9, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Chusri, S.; McNeil, E.B.; Hortiwakul, T.; Charernmak, B.; Sritrairatchai, S.; Santimaleeworagun, W.; Pattharachayakul, S.; Suksanan, P.; Thaisomboonsuk, B.; Jarman, R.G. Single dosage of doxycycline for prophylaxis against leptospiral infection and leptospirosis during urban flooding in southern Thailand: A non-randomized controlled trial. J. Infect. Chemother. 2014, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Brett-Major, D.M.; Lipnick, R.J. Antibiotic prophylaxis for leptospirosis. Cochrane Database Syst. Rev. 2009, 8, Cd007342. [Google Scholar]
- Wohlin, C. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering, New York, NY, USA, 13–14 May 2014; ACM: London, UK, 2014; pp. 1–10. [Google Scholar]
- Breban, R. Role of environmental persistence in pathogen transmission: A mathematical modeling approach. J. Mathem. Boil. 2013, 66, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.; Davis, S.; Leirs, H. A model of leptospirosis infection in an African rodent to determine risk to humans: Seasonal fluctuations and the impact of rodent control. Acta. Trop. 2006, 99, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.L. Control of Communicable Diseases Manual; American Public Health Association: Washington, DC, USA, 2008. [Google Scholar]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Faucher, J.F.; Hoen, B.; Estavoyer, J.M. The management of leptospirosis. Expert Opin. Pharmacother. 2004, 5, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Belmaker, I.; Alkan, M.; Barnea, A.; Dukhan, L.; Yitzhaki, S.; Gross, E. Risk of transmission of leptospirosis from infected cattle to dairy workers in southern Israel. Isr. Med. Assoc. J. 2004, 6, 24–27. [Google Scholar] [PubMed]
- Sehgal, S.C.; Sugunan, A.P.; Murhekar, M.V.; Sharma, S.; Vijayachari, P. Randomized controlled trial of doxycycline prophylaxis against leptospirosis in an endemic area. Int. J. Antimicrob. Agents 2000, 13, 249–255. [Google Scholar] [CrossRef]
- Shivaraj, B.; Ts, R.; Anithraj, B.Y.; Bayari, R. A study on prophylactic doxycycline to reduce the incidence of leptospirosis among paddy field farmers in a coastal district of India. Int. J. Infect. Dis. 2012, 16, e462. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kosambiya, J.K.; Vikas, K.D.; Karan, J. Chemoprophylaxis with doxycycline in suspected epidemic of leptospirosis during floods: Does this really work? Afr. Health sci. 2010, 10, 199–200. [Google Scholar] [PubMed]
- Galloway, R.L.; Levett, P.N.; Tumeh, J.W.; Flowers, C.R. Assessing cost effectiveness of empirical and prophylactic therapy for managing leptospirosis outbreaks. Epidemiol. Infect. 2009, 137, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Illangasekera, V.L.; Kularatne, S.A.; Kumarasiri, P.V.; Pussepitiya, D.; Premaratne, M.D. Is oral penicillin an effective chemoprophylaxis against leptospirosis? A placebo controlled field study in the Kandy district, Sri Lanka. South. Asian J. Trop. Med. Public Health 2008, 39, 882–884. [Google Scholar]
- Gonsalez, C.R.; Casseb, J.; Monteiro, F.G.; Paula-Neto, J.B.; Fernandez, R.B.; Silva, M.V.; Camargo, E.D.; Mairinque, J.M.; Tavares, L.C. Use of doxycycline for leptospirosis after high-risk exposure in Sao Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 1998, 40, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, E.T.; Kirkpatrick, J.W.; Miller, R.N.; Karwacki, J.J.; Kelley, P.W.; Gray, M.R.; McNeill, K.M.; Timboe, H.L.; Kane, R.E.; Sanchez, J.L. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N Engl. J. Med. 1984, 310, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Guidugli, F.; Castro, A.A.; Atallah, A.N. Antibiotics for preventing leptospirosis. Cochrane Database Syst. Rev. 2000, Cd001305. [Google Scholar]
- Devishree, R.A. Management of leptospirosis: A short review. J. Pharmaceut. Sci. Res. 2015, 7, 759–761. [Google Scholar]
- Charan, J.; Saxena, D.; Mulla, S. Prophylaxis and treatment for leptospirosis: Where are the evidences? Nat. J. Physiol. Pharm. Pharmacol. 2012, 2, 78–83. [Google Scholar] [CrossRef]
- McBride, W.J.H. Chemoprophylaxis of tropical infectious diseases. Pharmaceuticals 2010, 3, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.S.; Vargas, R.; Lopes, A.A. Leptospirosis: A worldwide resurgent zoonosis and important cause of acute renal failure and death in developing nations. Ethn. Dis. 2009, 19, S137–S141. [Google Scholar]
- Pavli, A.; Maltezou, H.C. Travel-acquired leptospirosis. J. Travel Med. 2008, 15, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Christopher, G.W.; Agan, B.K.; Cieslak, T.J.; Olson, P.E. History of U.S. Military contributions to the study of bacterial zoonoses. Military Med. 2005, 170, 39–48. [Google Scholar] [CrossRef]
- Edwards, C.N.; Levett, P.N. Prevention and treatment of leptospirosis. Expert Rev. Anti. Infect. Ther. 2004, 2, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis: A forgotten zoonosis? Clin. Appl. Immunol. Rev. 2004, 4, 435–448. [Google Scholar] [CrossRef]
- Re, V.L., III; Gluckman, S.J. Fever in the returned traveler. Am. Fam. Physic. 2003, 68, 1343–1350. [Google Scholar]
- Gilks, C.F.; Lambert, H.P.; Broughton, E.S.; Baker, C.C. Failure of penicillin prophylaxis in laboratory acquired leptospirosis. Postgr. Med. J. 1988, 64, 236–238. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kosambiya, J.K.; Desai, V.K. A case control study to explore the risk factors for acquisition of leptospirosis in Surat city, after flood. Indian J. Med. Sci. 2008, 62, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Allan, K.J.; Biggs, H.M.; Halliday, J.E.B.; Kazwala, R.R.; Maro, V.P.; Cleaveland, S.; Crump, J.A. Epidemiology of leptospirosis in Africa: A systematic review of a neglected zoonosis and a paradigm for “one health” in Africa. PLoS Negl. Trop. Dis. 2015, 9, e0003899. [Google Scholar] [CrossRef] [PubMed]
- De Vries, S.G.; Visser, B.J.; Nagel, I.M.; Goris, M.G.; Hartskeerl, R.A.; Grobusch, M.P. Leptospirosis in Sub-Saharan Africa: A systematic review. Int. J. Infect. Dis. 2014, 28, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Zanzi, C.; Mason, M.; Encina, C.; Astroza, A.; Romero, A. Leptospira contamination in household and environmental water in rural communities in Southern Chile. Int. J. Environ. Res. Public Health 2014, 11, 6666–6680. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, J.; Portanova, A.; Zuckerman, I.; Loftis, A.; Ceccato, P.; Willingham, A.; Verma, A. Molecular detection of leptospiral DNA in environmental water on St. Kitts. Int. J. Environ. Res. Public Health 2014, 11, 7953–7960. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-P.; Chan, T.-C.; Chang, C.-C. Typhoon-related leptospirosis and melioidosis, Taiwan, 2009. Emerg. Infect. Dis. J. 2011, 17, 1322–1324. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G. Experience in Controlling Outbreaks of Leptospirosis in Leon; National Forum of Leptospirosis of Nicaragua: Managua, Nicaragua, 2012.
- Chavez, O. Experience in Controlling Outbreaks of Leptospirosis in Chinandega; National Forum of Leptospirosis of Nicaragua: Managua, Nicaragua, 2012.
- Pena, E. Experience in Human Leptospirosis in the Dominican Republic; National Forum of Leptospirosis of Nicaragua: Managua, Nicaragua, 2012.
- Hatch, M.; Thomas, D. Measurement issues in environmental epidemiology. Environ. Health Perspect. 1993, 101, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, R.C.R.M.; Daher, E.F.; Camargo, E.D.; Spinosa, C.; de Silva, M.V. Leptospirosis severity may be associated with the intensity of humoral immune response. Rev. Inst. Med. Trop. Sao Paulo 2002, 44, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, G.; Hie, P.; Gourinat, A.-C.; Huguon, E.; Polfrit, Y.; Goarant, C.; D’Ortenzio, E.; Missotte, I. Association between age and severity to leptospirosis in children. PLoS Negl. Trop. Dis. 2013, 7, e2436. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Danon, L. Mathematical modelling of infectious diseases. Br. Med. Bull. 2009, 92, 33–42. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Mathematical and Biological Synthesis. Nimbios Working Group: Leptospirosis Modeling. Available online: http://www.nimbios.org/workinggroups/WG_leptospira (accessed on 29 March 2017).
- Ministério da Saúde do Brasil. Plano de Contingência de Vigilância em Saúde Frente a Inundações; Secretaria de Vigilância em Saúde: Brasília, Brazil, 2005. (In Portuguese)
- Durski, K.N.; Jancloes, M.; Chowdhary, T.; Bertherat, E. A global, multi-disciplinary, multi-sectorial initiative to combat leptospirosis: Global leptospirosis environmental action network (GLEAN). Int. J. Environ. Res. Public Health 2014, 11, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Global Leptospirosis Environmental Action Network. 3rd Glean Meeting Report. Annex 3: Recommendations for Outbreak Control. Available online: https://docs.google.com/viewer?a=v&pid=sites&srcid=ZGVmYXVsdGRvbWFpbnxnbGVhbmxlcHRvfGd4OjY0ZTQxZWIzMTc3NGIyNmE (accessed on 29 March 2017).
- Jancloes, M.; Bertherat, E.; Schneider, C.; Belmain, S.; Munoz-Zanzi, C.; Hartskeerl, R.; Costa, F.; Denis, J.; Benschop, J. Towards a “one health” strategy against leptospirosis. Planet. Risk 2014, 2, 204–206. [Google Scholar]
- Pereira, M.M.; Schneider, M.C.; Munoz-Zanzi, C.; Costa, F.; Benshop, J.; Hartskeerl, R.; Martinez, J.; Jancloes, M.; Bertherat, E. Rio report—A roadmap for leptospirosis research and health policies based on country needs. Rev. Panam. Salud. Publica. 2017, in press. [Google Scholar]
| Parameter | Description | Baseline | References |
|---|---|---|---|
| β | Per capita contact rate (1/day) | 1.42 | Set parameter (default) |
| k | Half-saturation density | 0.5 | Set parameter (default) |
| µ | Mortality rate by other causes | 0 | Set parameter (default) |
| h | Leptospire recruitment rate into environment (1/day) | 0.002 | Set parameter (default) |
| d | Leptospire clearance rate in environment (1/day) | 0.2 | [42] |
| γ | Incubation period (days) | 10 | [20] |
| η | Infectious period (days) | 7 | [43] |
| p | Probability of severe leptospirosis given infection | 0.2 | Based on Bharti et al. for icteric and other severe forms [44] |
| q | Probability of mild leptospirosis given infection | 0.3 | Based on Bharti et al. and Faucher et al. 80% of infections are mild or asymptomatic [44,45] |
| θ | Chemoprophylaxis administration rate (1/day) | 0.2 | Set parameter (default) |
| φ | Chemoprophylaxis duration (days) | 14 | [46] |
| p1 | Probability of developing severe leptospirosis after chemoprophylaxis | 0.1 | Set parameter. 50% lower than before chemoprophylaxis |
| p2 | Probability of developing mild leptospirosis after chemoprophylaxis | 0.15 | Set parameter. 50% lower than before chemoprophylaxis |
| q1 | Probability of not being infected after chemoprophylaxis | 0.3 | Based on Seghal et al. but with a lower protective effect [47] |
| Author [Ref] | Year | Country | Antibiotics | Target Population | Situation | Administration | Effectiveness of Treatment |
|---|---|---|---|---|---|---|---|
| Chusri [38] | 2014 | Thailand | Doxycycline (200 mg single dose) | Local residents | Flooding | Post exposure | Protective efficacy for leptospiral infection: 92.0% (CI = 81.2%–96.6%) and for leptospirosis: 95.6% (CI = 78.2%–99.3%), among participants with laceration wound. Protective efficacy for leptospiral infection: 89.2% (CI = 63.6%–96.67%), among participants exposed to flood water ≤3 h/day. |
| Shivaraj [48] | 2012 | India | Doxycycline (200 mg/week) | Paddy field farmers | Farming | Pre-exposure | Incidence of leptospirosis: nil in the test group and 7.29% in the control group (p = 0.017) |
| Bhardwaj [49] | 2010 | India | Doxycycline (200 mg/week) | Local residents | Flooding | Post exposure | Univariate analysis: OR = 0.43 (CI = 0.23–0.78). Multivariate analysis: Adj OR = 0.77 (C.I = 0.35–1.69) |
| Galloway [50] | 2009 | N/A * | Doxycycline and azithromycin | N/A | N/A | N/A | Prophylaxis with doxycycline compared to no-prophylaxis strategy. Prophylaxis provided cost savings, decreased severity of illness and mortality, and improved health outcomes. |
| Illangasekera [51] | 2008 | Sri Lanka | Penicillin (500 mg/day for a month) | Farmers | Farming | Pre-exposure | Of 5 patients hospitalized with fever, 3 tested positive for leptospirosis, all from the placebo group. |
| Belmaker [46] | 2004 | Israel | Doxycycline (200 mg/week) | Dairy workers | Animal husbandry | Post exposure | Either with or without chemoprophylaxis, no dairy workers exposed to herds infected with Leptospira serovar Hardjo showed evidence of seroconversion or disease. |
| Sejvar [37] | 2003 | Malaysia | Doxycycline (200 mg/week) | Athletes | Race in risk area | Pre-exposure | Taking doxycycline before or during the race was protective (RR = 0.4, 95% CI = 0.2–1.2). |
| Sehgal [47] | 2000 | India | Doxycycline (200 mg/week) | Local residents | Endemic area | Pre-exposure | No statistically difference was observed in the infection rates among the doxycycline and the placebo group. A statistically significant difference was observed in the clinical disease attack rates (3.11 vs. 6.82%) between the two groups. |
| Gonsalez [52] | 1998 | Brazil | Doxycycline (200 mg single dose) | Local residents | Flooding | Post exposure | A protective association of doxycycline for confirmed leptospirosis cases (RR = 2.3) and seroconversion only (RR = 2.0) was observed, but it was not statistically significant. |
| Takafuji [53] | 1984 | Panama | Doxycycline (200 mg/week) | US Army during deployment | Training | Pre-exposure | 95% efficacy. Attack rate of 4.2% in the placebo group compared to an attack rate of 0.2% in the doxycycline group (p < 0.001). |
| Brett-Major [39] | 2009 | N/A* | Doxycycline | Varied (Meta-analysis) | Varied (Meta-analysis) | Varied (Meta-analysis) | Three randomized clinical trials met the inclusion criteria. Pre-exposure antibiotic prophylaxis with doxycycline may decrease laboratory identified Leptospira infection. |
| Guidugli [54] | 2000 | N/A* | Doxycycline | Varied (Meta-analysis) | Varied (Meta-analysis) | Varied (Meta-analysis) | Two randomized clinical trials met the inclusion criteria. Doxycycline seems to be an efficient intervention when used in a specific clinical situation, i.e., soldiers who train in endemic areas with high risk of exposure. |
| Time of Administration (t0, day) | Chemoprophylaxis Rate (θ, 1/day) | Proportion of Cases Prevented |
|---|---|---|
| 5 | 0.01 | 0.12 |
| 5 | 0.05 | 0.47 |
| 5 | 0.1 | 0.67 |
| 5 | 0.2 | 0.84 |
| 5 | 0.5 | 0.93 |
| 10 | 0.01 | 0.12 |
| 10 | 0.05 | 0.43 |
| 10 | 0.1 | 0.62 |
| 10 | 0.2 | 0.77 |
| 10 | 0.5 | 0.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, M.C.; Velasco-Hernandez, J.; Min, K.-d.; Leonel, D.G.; Baca-Carrasco, D.; Gompper, M.E.; Hartskeerl, R.; Munoz-Zanzi, C. The Use of Chemoprophylaxis after Floods to Reduce the Occurrence and Impact of Leptospirosis Outbreaks. Int. J. Environ. Res. Public Health 2017, 14, 594. https://doi.org/10.3390/ijerph14060594
Schneider MC, Velasco-Hernandez J, Min K-d, Leonel DG, Baca-Carrasco D, Gompper ME, Hartskeerl R, Munoz-Zanzi C. The Use of Chemoprophylaxis after Floods to Reduce the Occurrence and Impact of Leptospirosis Outbreaks. International Journal of Environmental Research and Public Health. 2017; 14(6):594. https://doi.org/10.3390/ijerph14060594
Chicago/Turabian StyleSchneider, Maria Cristina, Jorge Velasco-Hernandez, Kyung-duk Min, Deise Galan Leonel, David Baca-Carrasco, Matthew E. Gompper, Rudy Hartskeerl, and Claudia Munoz-Zanzi. 2017. "The Use of Chemoprophylaxis after Floods to Reduce the Occurrence and Impact of Leptospirosis Outbreaks" International Journal of Environmental Research and Public Health 14, no. 6: 594. https://doi.org/10.3390/ijerph14060594
APA StyleSchneider, M. C., Velasco-Hernandez, J., Min, K.-d., Leonel, D. G., Baca-Carrasco, D., Gompper, M. E., Hartskeerl, R., & Munoz-Zanzi, C. (2017). The Use of Chemoprophylaxis after Floods to Reduce the Occurrence and Impact of Leptospirosis Outbreaks. International Journal of Environmental Research and Public Health, 14(6), 594. https://doi.org/10.3390/ijerph14060594






