How Does Ambient Air Temperature Affect Diabetes Mortality in Tropical Cities?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
2.2. Geographical Location
2.3. Statistical Specifications
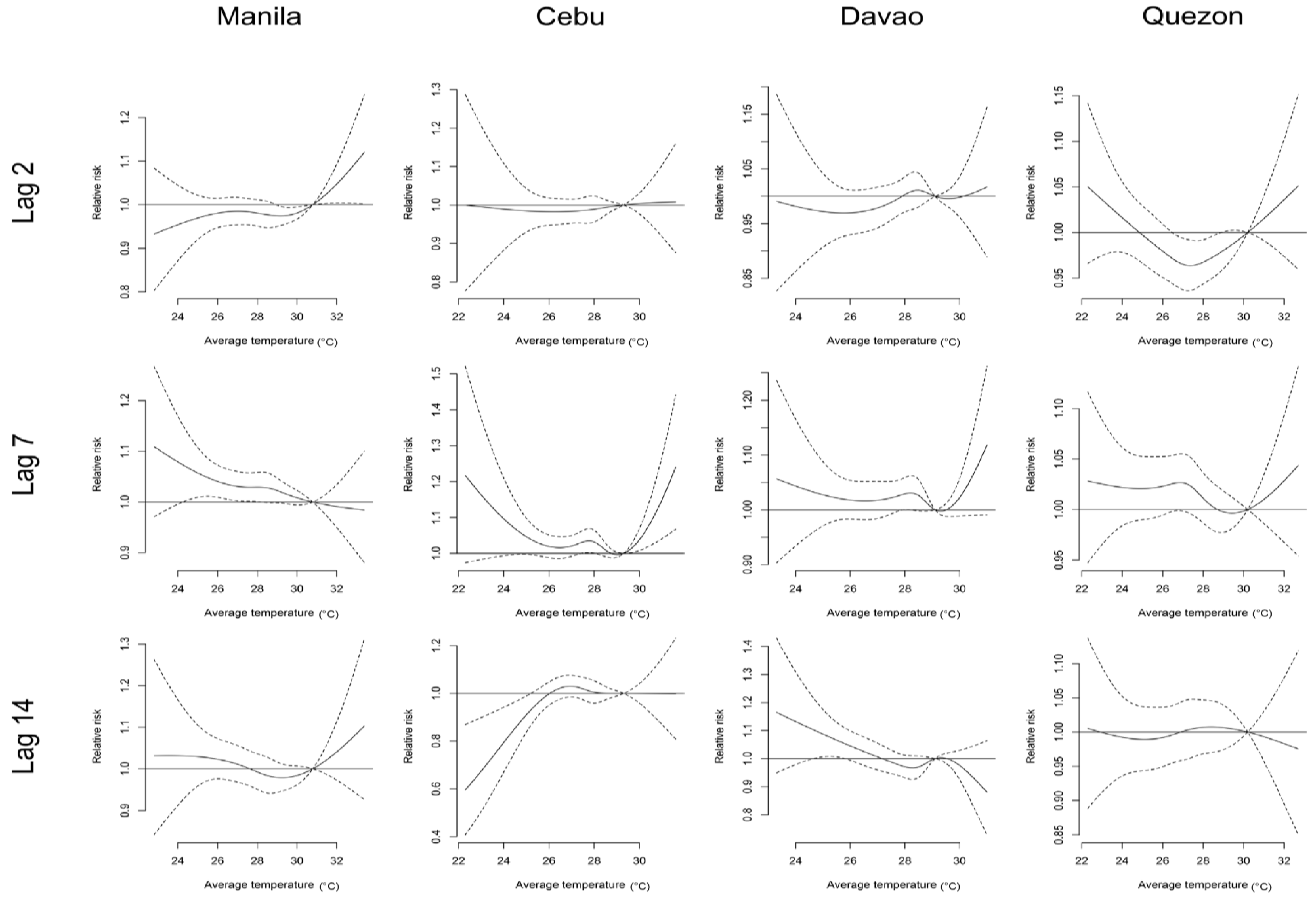
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Kitabchi, A.E.; Miles, J.M.; Umpierrez, G.E.; Fisher, J.N. Hyperglycemic Crises in Adult Patients with Diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Morales, D.D.; Punzalan, F.E.; Paz-Pacheco, E.; Sy, R.G.; Duante, C.A. Metabolic syndrome in the Philippine general population: Prevalence and risk for atherosclerotic cardiovascular disease and diabetes mellitus. Diabetes Vasc. Dis. Res. 2008, 5, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H. Diabetes Care in the Philippines. Ann. Glob. Health 2015, 81, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Soria, M.L.; Sy, R.G.; Vega, B.S.; Ty-Willing, T.; Abenir-Gallardo, A.; Velandria, F.; Punzalan, F.E. The incidence of type 2 diabetes mellitus in the Philippines: A 9-year cohort study. Diabetes Res. Clin. Pract. 2009, 86, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.B.; Wellik, K.E.; Fowke, M. Geoenvironmental diabetology. J. Diabetes Sci. Technol. 2011, 5, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Dain, K.; Hadley, L. Diabetes and climate change—Two interconnected global challenges. Diabetes Res. Clin. Pract. 2012, 97, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Armstrong, B. Time series analysis on the health effects of temperature: Advancements and limitations. Environ. Res. 2010, 110, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklov, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Guo, Y.; Gasparrini, A.; Armstrong, B.; Li, S.; Tawatsupa, B.; Tobias, A.; Lavigne, E.; de Sousa Zanotti Stagliorio Coelho, M.; Leone, M.; Pan, X.; et al. Global variation in the effects of ambient temperature on mortality: A systematic evaluation. Epidemiology 2014, 25, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gasparrini, A.; Armstrong, B.G.; Tawatsupa, B.; Tobias, A.; Lavigne, E.; Coelho, M.S.; Pan, X.; Kim, H.; Hashizume, M.; et al. Temperature Variability and Mortality: A Multi-Country Study. Environ. Health Perspect. 2016, 124, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Seposo, X.T.; Dang, T.N.; Honda, Y. Effect modification in the temperature extremes by mortality subgroups among the tropical cities of the Philippines. Glob. Health Action 2016, 9, 31500. [Google Scholar] [CrossRef] [PubMed]
- Seposo, X.T.; Dang, T.N.; Honda, Y. Evaluating the effects of temperature on mortality in Manila City (Philippines) from 2006 to 2010 using a distributed lag nonlinear model. Int. J. Environ. Res. Public Health 2015, 12, 6842–6857. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.R.; MacDonald, I.A.; Bennett, T.; Tattersall, R.B. Abnormal thermoregulation in diabetic autonomic neuropathy. Diabetes 1988, 37, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.A.; Childs, R.D.; Seifert, K.M.; Boyle, M.E.; Fowke, M.; Iniguez, P.; Cook, C.B. Managing diabetes in the heat: Potential issues and concerns. Endocr. Pract. 2010, 16, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, P.; Zhou, M.G.; Ou, C.Q.; Li, M.M.; Liu, Y.N.; Gao, J.H.; Chen, B.; Liu, J.M.; Bai, L.; et al. The effect of ambient temperature on diabetes mortality in China: A multi-city time series study. Sci. Total Environ. 2016, 543, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Z.; Tian, L.W.; Qiu, H.; Chan, K.P.; Tsang, H.; Tang, R.; Lee, R.S.Y.; Thach, T.Q.; Wong, C.M. The influence of pre-existing health conditions on short-term mortality risks of temperature: Evidence from a prospective Chinese elderly cohort in Hong Kong. Environ. Res. 2016, 148, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lan, L.; Wang, Y.; Yang, C.; Tang, W.; Cui, G.; Luo, S.; Cheng, Y.; Liu, Y.; Liu, J.; et al. Extremely cold and hot temperatures increase the risk of diabetes mortality in metropolitan areas of two Chinese cities. Environ. Res. 2014, 134, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Gasparrini, A.; Wang, X.; Chen, H.; Yagouti, A.; Fleury, M.D.; Cakmak, S. Extreme ambient temperatures and cardiorespiratory emergency room visits: Assessing risk by comorbid health conditions in a time series study. Environ. Health 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Pearson, D.; Malig, B.; Broadwin, R.; Green, R. The effect of high ambient temperature on emergency room visits. Epidemiology 2012, 23, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Green, R.S.; Basu, R.; Malig, B.; Broadwin, R.; Kim, J.J.; Ostro, B. The effect of temperature on hospital admissions in nine California counties. Int. J. Public Health 2010, 55, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Who is sensitive to extremes of temperature? A case-only analysis. Epidemiology 2005, 16, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, S.J.; Nagalingam, V.; Newnham, H.H. Impact of the 2009 Melbourne heatwave on a major public hospital. Int. Med. J. 2013, 43, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramon, M.; Zanobetti, A.; Cavanagh, D.P.; Schwartz, J. Extreme temperatures and mortality: Assessing effect modification by personal characteristics and specific cause of death in a multi-city case-only analysis. Environ. Health Perspect. 2006, 114, 1331–1336. [Google Scholar] [CrossRef]
- Vaneckova, P.; Bambrick, H. Cause-Specific Hospital Admissions on Hot Days in Sydney, Australia. PLoS ONE 2013, 8, e55459. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; O’Neill, M.S.; Gronlund, C.J.; Schwartz, J.D. Susceptibility to Mortality in Weather Extremes Effect Modification by Personal and Small-Area Characteristics. Epidemiology 2013, 24, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G. The relationship between relative humidity and the dewpoint temperature in moist air—A simple conversion and applications. Bull. Am. Meteorol. Soc. 2005, 86, 225. [Google Scholar] [CrossRef]
- Philippine Statistics Authority-National Statistics Office (PSA-NSO). 2015 Census of Population; PSA-NSO: Manila, Philippines, 2015.
- Cinco, T.A.; Hilario, F.D.; de Guzman, R.G.; Ares, E.D. Climate Trends and Projections in the Philippines. In Proceedings of the 12th National Convention on Statistics (NCS), Mandaluyong City, Philippines, 1–2 October 2013; p. 20. [Google Scholar]
- Yang, C.; Meng, X.; Chen, R.; Cai, J.; Zhao, Z.; Wan, Y.; Kan, H. Long-term variations in the association between ambient temperature and daily cardiovascular mortality in Shanghai, China. Sci. Total Environ. 2015, 538, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, G.; Cui, Y.; Jiang, G.; Pan, X. Estimating Temperature-Mortality Exposure-Response Relationships and Optimum Ambient Temperature at the Multi-City Level of China. Int. J. Environ. Res. Public Health 2016, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.N.; Seposo, X.T.; Duc, N.H.; Thang, T.B.; An, D.D.; Hang, L.T.; Long, T.T.; Loan, B.T.; Honda, Y. Characterizing the relationship between temperature and mortality in tropical and subtropical cities: A distributed lag non-linear model analysis in Hue, Vietnam, 2009–2013. Glob. Health Action 2016, 9, 28738. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Li, Y.; Cheng, Y.; Cui, G.; Peng, C.; Xu, Y.; Wang, Y.; Liu, Y.; Liu, J.; Li, C.; Wu, Z.; et al. Association between high temperature and mortality in metropolitan areas of four cities in various climatic zones in China: A time-series study. Environ. Health 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Rocklov, J.; Ebi, K.; Forsberg, B. Mortality related to temperature and persistent extreme temperatures: A study of cause-specific and age-stratified mortality. Occup. Environ. Med. 2011, 68, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Fealey, R.D.; Low, P.A.; Thomas, J.E. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin. Proc. 1989, 64, 617–628. [Google Scholar] [CrossRef]
- Reichard, P.; Jensen-Urstad, K.; Ericsson, M.; Jensen-Urstad, M.; Lindblad, L.E. Autonomic neuropathy—A complication less pronounced in patients with Type 1 diabetes mellitus who have lower blood glucose levels. Diabet. Med. 2000, 17, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, K.E. Silent coronary artery disease in diabetes—A feature of autonomic neuropathy or accelerated atherosclerosis? Diabetologia 2001, 44, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.; Costa, F.; Biaggioni, I. Spectrum of autonomic cardiovascular neuropathy in diabetes. Diabetes Care 2003, 26, 2174–2180. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Donaghue, K.C.; Cho, Y.H.; Craig, M.E. Autonomic neuropathy in young people with type 1 diabetes: A systematic review. Pediatr. Diabetes 2013, 14, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.P.; Sigal, R.J.; McGinn, R. Body temperature regulation in diabetes. Temperature 2016, 3, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Armstrong, B.G.; Gouveia, N.; Wilkinson, P. Mortality displacement of heat-related deaths: A comparison of Delhi, Sao Paulo, and London. Epidemiology 2005, 16, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, Q.; Wang, J.; Lavigne, E.; Gasparrini, A.; Copes, R.; Yagouti, A.; Burnett, R.T.; Goldberg, M.S.; Villeneuve, P.J.; et al. Hospitalizations from Hypertensive Diseases, Diabetes, and Arrhythmia in Relation to Low and High Temperatures: Population-Based Study. Sci. Rep. 2016, 6, 30283. [Google Scholar] [CrossRef] [PubMed]
- Tien, K.J.; Yang, C.Y.; Weng, S.F.; Liu, S.Y.; Hsieh, M.C.; Chou, C.W. The impact of ambient temperature on HbA1c in Taiwanese type 2 diabetic patients: The most vulnerable subgroup. J. Formos. Med. Assoc. 2016, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.L.; Brimacombe, M.; Xie, M.; Rajan, M.; Wang, H.; Kolassa, J.; Crystal, S.; Chen, T.C.; Pogach, L.; Safford, M. Seasonal patterns in monthly hemoglobin A1c values. Am. J. Epidemiol. 2005, 161, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, A.; Kiefer, F.W. Brown adipose tissue and thermogenesis. Horm. Mol. Biol. Clin. Investig. 2014, 19, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, P. Brown adipose tissue thermogenesis in humans. Diabetologia 2013, 56, 2110–2112. [Google Scholar] [CrossRef] [PubMed]
- Poher, A.L.; Altirriba, J.; Veyrat-Durebex, C.; Rohner-Jeanrenaud, F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front. Physiol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Tingelstad, H.C.; Mantha, O.L.; Gosselin, C.; Haman, F. Maintaining thermogenesis in cold exposed humans: Relying on multiple metabolic pathways. Compr. Physiol. 2014, 4, 1383–1402. [Google Scholar] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.G.; Bell, M.L. Weather-related mortality: How heat, cold, and heat waves affect mortality in the United States. Epidemiology 2009, 20, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ou, C.Q.; Ding, Y.; Zhou, Y.X.; Chen, P.Y. Daily temperature and mortality: A study of distributed lag non-linear effect and effect modification in Guangzhou. Environ. Health Glob. 2012, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Lim, Y.H.; Honda, Y.; Guo, Y.L.; Hashizume, M.; Bell, M.L.; Chen, B.Y.; Kim, H. Mortality related to extreme temperature for 15 cities in northeast Asia. Epidemiology 2015, 26, 255–262. [Google Scholar] [CrossRef] [PubMed]

| Variables | Manila | Quezon | ||||
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | |
| Diabetes mortality count | 2.14 ± 1.47 | 0 | 9 | 2.76 ± 1.68 | 0 | 9 |
| Maximum Temperature | 31.5 ± 1.94 | 23.1 | 39.3 | 32 ± 2.21 | 24.4 | 38.4 |
| Mean Temperature | 28.5 ± 1.45 | 22.8 | 33.4 | 27.3 ± 1.56 | 22.3 | 32.8 |
| Minimum Temperature | 25.6 ± 1.45 | 18 | 31.3 | 23.4 ± 1.62 | 12.4 | 28.2 |
| Relative Humidity | 75.4 ± 8.31 | 42.8 | 96.9 | 80.0 ± 8.94 | 45.8 | 99.4 |
| Variables | Cebu | Davao | ||||
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | |
| Diabetes mortality count | 1.46 ± 1.26 | 0 | 8 | 1.39 ± 1.20 | 0 | 7 |
| Maximum Temperature | 31.3 ± 1.63 | 24.8 | 40.5 | 31.9 ± 1.63 | 24 | 39.9 |
| Mean Temperature | 27.9 ± 1.24 | 22.3 | 31.6 | 28.3 ± 1.11 | 23.2 | 31 |
| Minimum Temperature | 24.8 ± 1.20 | 14.2 | 28 | 24.1 ± 0.78 | 16.2 | 27 |
| Relative Humidity | 85.1 ± 5.20 | 67.2 | 123.6 | 82.3 ± 4.57 | 66.7 | 97.5 |
| City | Lag (days) | Extreme Low | Moderate Low | Moderate High | Extreme High |
|---|---|---|---|---|---|
| Manila | |||||
| 0–2 | 0.75 (0.43–1.29) | 0.84 (0.60–1.16) | 1.36 (1.03–1.80) | 1.68 (1.03–2.74) | |
| 0–7 | 1.09 (0.51–2.37) | 1.06 (0.67–1.67) | 1.54 (1.09–2.17) | 2.11 (1.15–3.85) | |
| 0–15 | 1.11 (0.40–3.07) | 1.07 (0.58–1.97) | 1.59 (1.04–2.42) | 2.20 (1.04–4.65) | |
| 0–21 | 1.01 (0.30–3.37) | 1.02 (0.49–2.08) | 1.52 (0.93–2.47) | 2.04 (0.86–4.84) | |
| Cebu | |||||
| 0–2 | 1.13 (0.53–2.44) | 1.08 (0.68–1.71) | 1.21 (0.95–1.54) | 1.38 (0.87–2.19) | |
| 0–7 | 1.77 (0.58–5.41) | 1.40 (0.72–2.75) | 1.57 (1.20–2.04) | 2.32 (1.38–3.89) | |
| 0–15 | 0.60 (0.13–2.75) | 0.73 (0.29–1.83) | 1.55 (1.16–2.08) | 2.27 (1.27–4.04) | |
| 0–21 | 0.37 (0.06–2.15) | 0.54 (0.19–1.58) | 1.54 (1.12–2.12) | 2.20 (1.16–4.19) | |
| Davao | |||||
| 0–2 | 1.02 (0.58–1.80) | 1.01 (0.72–1.42) | 0.98 (0.78–1.22) | 1.02 (0.60–1.72) | |
| 0–7 | 1.30 (0.54–3.09) | 1.16 (0.69–1.96) | 1.10 (0.82–1.48) | 1.45 (0.71–2.98) | |
| 0–15 | 1.44 (0.44–4.72) | 1.25 (0.61–2.54) | 1.04 (0.72–1.49) | 1.14 (0.46–2.80) | |
| 0–21 | 3.87 (1.00–15.0) | 2.26 (1.01–5.08) | 1.20 (0.82–1.76) | 1.75 (0.67–4.57) | |
| Quezon | |||||
| 0–2 | 1.34 (0.97–1.87) | 1.20 (0.99–1.44) | 1.16 (0.94–1.43) | 1.29 (0.88–1.90) | |
| 0–7 | 1.33 (0.81–2.18) | 1.19 (0.90–1.57) | 1.29 (0.99–1.68) | 1.58 (0.98–2.57) | |
| 0–15 | 1.75 (0.89–3.42) | 1.38 (0.94–2.01) | 1.32 (0.96–1.80) | 1.65 (0.93–2.94) | |
| 0–21 | 1.28 (0.58–2.84) | 1.15 (0.73–1.81) | 1.30 (0.92–1.84) | 1.62 (0.85–3.07) | |
| Pooled | |||||
| 0–2 | 1.03 (0.75–1.41) | 1.01 (0.85–1.20) | 1.24 (1.02–1.52) | 1.35 (1.04–1.77) | |
| 0–7 | 1.36 (0.96–1.93) | 1.18 (0.98–1.41) | 1.33 (1.09–1.62) | 1.61 (1.21–2.15) | |
| 0–15 | 1.39 (0.87–2.24) | 1.19 (0.93–1.53) | 1.28 (1.00–1.64) | 1.55 (1.10–2.19) | |
| 0–21 | 1.20 (0.54–2.69) | 1.10 (0.71–1.71) | 1.27 (0.95–1.71) | 1.55 (1.06–2.29) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seposo, X.T.; Dang, T.N.; Honda, Y. How Does Ambient Air Temperature Affect Diabetes Mortality in Tropical Cities? Int. J. Environ. Res. Public Health 2017, 14, 385. https://doi.org/10.3390/ijerph14040385
Seposo XT, Dang TN, Honda Y. How Does Ambient Air Temperature Affect Diabetes Mortality in Tropical Cities? International Journal of Environmental Research and Public Health. 2017; 14(4):385. https://doi.org/10.3390/ijerph14040385
Chicago/Turabian StyleSeposo, Xerxes T., Tran Ngoc Dang, and Yasushi Honda. 2017. "How Does Ambient Air Temperature Affect Diabetes Mortality in Tropical Cities?" International Journal of Environmental Research and Public Health 14, no. 4: 385. https://doi.org/10.3390/ijerph14040385
APA StyleSeposo, X. T., Dang, T. N., & Honda, Y. (2017). How Does Ambient Air Temperature Affect Diabetes Mortality in Tropical Cities? International Journal of Environmental Research and Public Health, 14(4), 385. https://doi.org/10.3390/ijerph14040385







