Water Quality, Sanitation, and Hygiene Conditions in Schools and Households in Dolakha and Ramechhap Districts, Nepal: Results from A Cross-Sectional Survey
Abstract
:1. Introduction
2. Materials and Methods
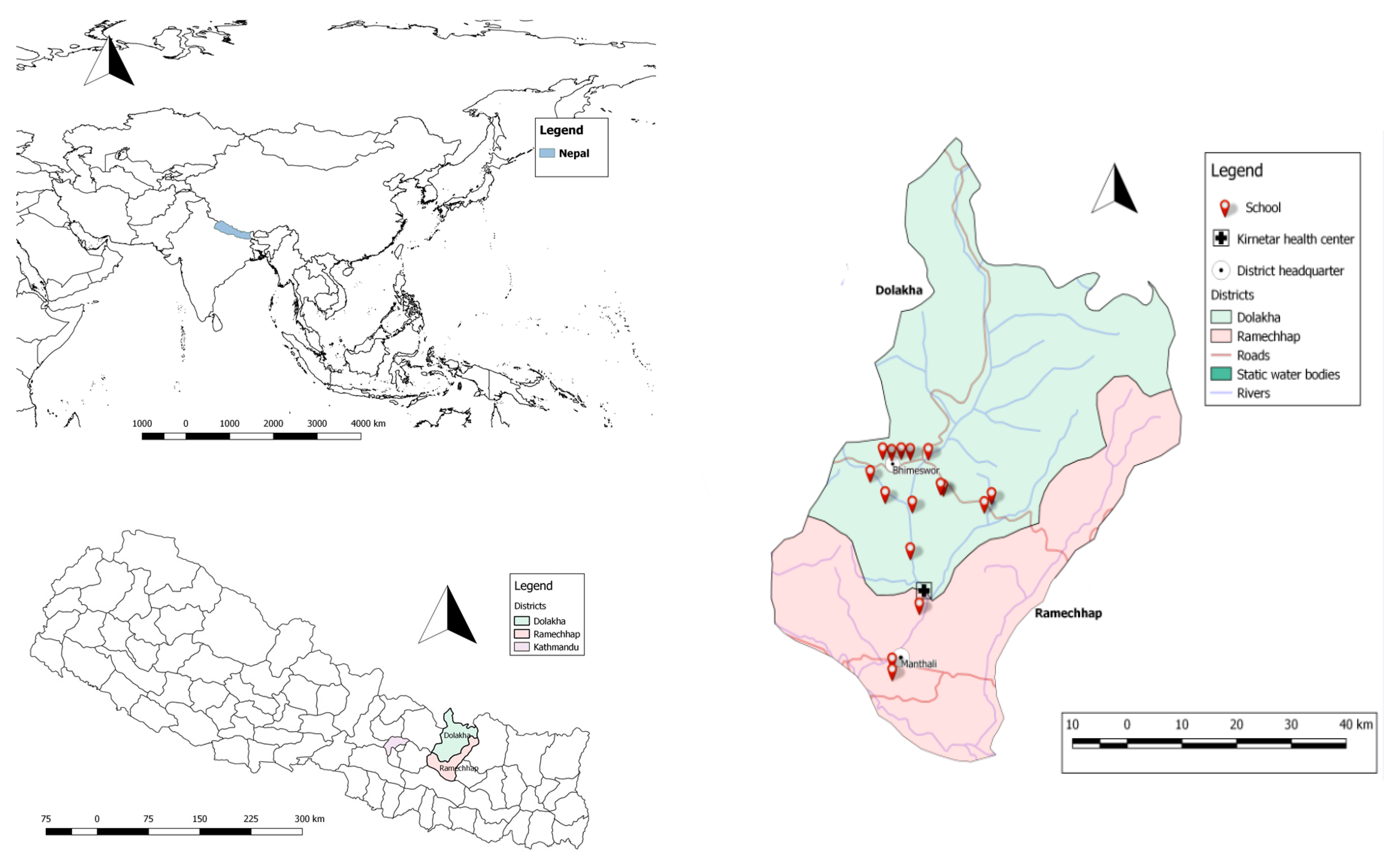
2.1. Study Sites
2.2. School Selection
- schools benefiting from a school garden intervention only;
- schools allocated a nutrition and health education programme, including school garden implementation;
- schools benefiting from nutrition and WASH intervention; and
- schools without any interventions (considered as control schools).
2.3. Study Population and Sample Size
- the prevalence of intestinal protozoan and helminth infections is about 30% [36] and remains constant in the absence of any intervention;
- the probability of new intestinal protozoa and helminth infections at the end of follow-up is 15%;
- the same effect odds ratios (ORs) apply to incidence and persistence of intestinal protozoa and helminth infection; and
- each of the two interventions reduces the odds of infection by 50%, and their effects are additive on the logit-scale.
2.4. Sample Collection and Treatment
- schools, between 30 May and 6 June 2015, from one main functioning drinking water point in each of 16 schools and 13 point-of-use (water container, cups);
- households, between 6 and 30 June 2015, at point-of-use in every 562 surveyed schoolchildren’s households; and
- communities, from 1 to 10 June 2015, in approximately 10% of the water sources in the community. Of note, 43 drinking water sources were collected from 40 communities selected at random (at least one sample per community). Water was collected from stand pipes (n = 37), protected springs (n = 3), protected wells (n = 2), and ponds (n = 1).
2.5. Physical, Chemical, and Microbiological Parameters
2.6. Drinking Water Quality Analysis
2.7. Heavy Metal Analysis
2.8. Questionnaire Survey
2.9. Statistical Analysis
2.10. Ethics Statement
3. Results
3.1. Study Compliance and Population Characteristics
3.2. School and Community WASH Characteristics
3.3. KAP of Schoolchildren on WASH at Schools and Households
3.4. WASH Characteristics of Households
3.5. Association of TTC with Household WASH Predictors
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- The World Health Organization /United Nations Children’s Fund. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Cairncross, S.; Bartram, J.; Cumming, O.; Brocklehurst, C. Hygiene, sanitation, and water: What needs to be done? PLoS Med. 2010, 7, e1000365. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Üstün, A.; Bos, R.; Gore, F.; Bartram, J. Safe Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Alexander, K.T.; Mwaki, A.; Adhiambo, D.; Cheney-Coker, M.; Muga, R.; Freeman, M.C. The life-cycle costs of school water, sanitation and hygiene access in Kenyan primary schools. Int. J. Environ. Res. Public Health 2016, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Jasper, C.; Le, T.-T.; Bartram, J. Water and sanitation in schools: A systematic review of the health and educational outcomes. Int. J. Environ. Res. Public Health 2012, 9, 2772–2787. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Raising Even More Clean Hands; UNICEF: New York, NY, USA, 2012. [Google Scholar]
- Bowen, A.; Ma, H.; Ou, J.; Billhimer, W.; Long, T.; Mintz, E.; Hoekstra, R.M.; Luby, S. A cluster-randomized controlled trial evaluating the effect of a handwashing-promotion program in Chinese primary schools. Am. J. Trop. Med. Hyg. 2007, 76, 1166–1173. [Google Scholar] [PubMed]
- Freeman, M.C.; Greene, L.E.; Dreibelbis, R.; Saboori, S.; Muga, R.; Brumback, B.; Rheingans, R. Assessing the impact of a school-based water treatment, hygiene and sanitation programme on pupil absence in Nyanza province, Kenya: a cluster-randomized trial. Trop. Med. Int. Health 2012, 17, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Garn, J.V.; Greene, L.E.; Dreibelbis, R.; Saboori, S.; Rheingans, R.D.; Freeman, M.C. A cluster-randomized trial assessing the impact of school water, sanitation, and hygiene improvements on pupil enrollment and gender parity in enrollment. J. Water Sanit. Hyg. Dev. 2013, 4, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Prüss-Ustün, A.; Bonjour, S.; Corvalán, C. The impact of the environment on health by country: Ameta-synthesis. Environ. Health 2008, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Prüss-Ustün, A.; Cumming, O.; Bartram, J.; Bonjour, S.; Cairncross, S.; Clasen, T.; Colford, J.M.; Curtis, V.; de France, J.; et al. Assessing the impact of drinking water and sanitation on diarrhoeal disease in low- and middle-income settings: Systematic review and meta-regression. Trop. Med. Int. Health 2014, 19, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Tarrass, F.; Benjelloun, M. The effects of water shortages on health and human development. Perspect. Public Health 2012, 132, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.C.; Clasen, T.; Dreibelbis, R.; Saboori, S.; Greene, L.E.; Brumback, B.; Muga, R.; Rheingans, R. The impact of a school-based water supply and treatment, hygiene, and sanitation programme on pupil diarrhoea: a cluster-randomized trial. Epidemiol. Infect. 2014, 142, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Bartram, J.; Lewis, K.; Lenton, R.; Wright, A. Focusing on improved water and sanitation for health. Lancet 2005, 365, 810–812. [Google Scholar] [CrossRef]
- Dangour, A.D.; Watson, L.; Cumming, O.; Boisson, S.; Che, Y.; Velleman, Y.; Cavill, S.; Allen, E.; Uauy, R. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Hall, A.; Hewitt, G.; Tuffrey, V.; de Silva, N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern. Child Nutr. 2008, 4, 118–236. [Google Scholar] [CrossRef] [PubMed]
- Aryal, J.; Gautam, B.; Sapkota, N. Drinking water quality assessment. J. Nepal Health Res. Counc. 2012, 10, 192–196. [Google Scholar] [PubMed]
- Lalor, G.C.; Vutchkov, M.K.; Bryan, S.T.; Christie, C.D.C.; Donaldson, D.; Young, J.; Chambers, S. Acute lead poisoning associated with backyard lead smelting in Jamaica. West Indian Med. J. 2006, 55, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Mandour, R.A.; Ghanem, A.-A.; El-Azab, S.M. Correlation between lead levels in drinking water and mothers’ breast milk: Dakahlia, Egypt. Environ. Geochem. Health 2013, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.C.; Murphy, L.; Ekvall, S.; Gagnon, M. In-home toxic chemical exposures and children with intellectual and developmental disabilities. Pediatr. Nurs. 2006, 32, 596–603. [Google Scholar] [PubMed]
- Prüss-Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M.; Cumming, O.; Curtis, V.; Bonjour, S.; Dangour, A.D.; de France, J.; Fewtrell, L.; et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Warner, N.R.; Levy, J.; Harpp, K.; Farruggia, F. Drinking water quality in Nepal’s Kathmandu Valley: A survey and assessment of selected controlling site characteristics. Hydrogeol. J. 2008, 16, 321–334. [Google Scholar] [CrossRef]
- Government of Nepal. Sanitation and Hygiene Master Plan: Steering Committee for National Sanitation Action; Government of Nepal: Kathmandu, Nepal, 2011.
- Government of Nepal, Ministry of Education, Department of Education (GON/MOE/DOE) and WaterAid. A Study Report: WASH Financing in Community Schools of Nepal; GON/MOE/DOE and WaterAid: Kathmandu, Nepal, 2014.
- Aryal, K.K.; Joshi, H.D.; Dhimal, M.; Singh, S.P.; Dhakal, P.; Dhimal, B.; Bhusal, C.L. Environmental burden of diarrhoeal diseases due to unsafe water supply and poor sanitation coverage in Nepal. J. Nepal Health Res. Counc. 2012, 10, 125–129. [Google Scholar] [PubMed]
- Sah, R.B.; Yadav, S.; Jha, P.K.; Yadav, B.K.; Pokharel, P.K. Worm infestation among the school children of Dhankuta district. Nepal Med. Coll. J. 2013, 15, 8–13. [Google Scholar] [PubMed]
- Tandukar, S.; Ansari, S.; Adhikari, N.; Shrestha, A.; Gautam, J.; Sharma, B.; Rajbhandari, D.; Gautam, S.; Nepal, H.P.; Sherchand, J.B. Intestinal parasitosis in school children of Lalitpur district of Nepal. BMC Res. Notes 2013, 6, 449. [Google Scholar] [CrossRef] [PubMed]
- Grimes, J.E.T.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The relationship between water, sanitation and schistosomiasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e3296. [Google Scholar] [CrossRef] [PubMed]
- Ziegelbauer, K.; Speich, B.; Mäusezahl, D.; Bos, R.; Keiser, J.; Utzinger, J. Effect of sanitation on soil-transmitted helminth infection: Systematic review and meta-analysis. PLoS Med. 2012, 9, e1001162. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.A.; Kafle, B.K. The study of lead pollution in street dust and drinking water along Arniko Rajmarg of Nepal. J. Nepal Chem. Soc. 2009, 23, 106–108. [Google Scholar]
- Pokhrel, D.; Bhandari, B.S.; Viraraghavan, T. Arsenic contamination of groundwater in the Terai region of Nepal: An overview of health concerns and treatment options. Environ. Int. 2009, 35, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Mahat, R.K.; Shrestha, R. Metal contamination in ground water of Dang district. Nepal J. Sci. Technol. 2008, 9, 143–148. [Google Scholar] [CrossRef]
- Yadav, I.C.; Singh, S.; Devi, N.L.; Mohan, D.; Pahari, M.; Tater, P.S.; Shakya, B.M. Spatial distribution of arsenic in groundwater of southern Nepal. Rev. Environ. Contam. Toxicol. 2012, 218, 125–140. [Google Scholar] [PubMed]
- Erismann, S.; Shrestha, A.; Diagbouga, S.; Knoblauch, A.; Gerold, J.; Herz, R.; Sharma, S.; Schindler, C.; Odermatt, P.; Drescher, A.; et al. Complementary school garden, nutrition, water, sanitation and hygiene interventions to improve children’s nutrition and health status in Burkina Faso and Nepal: A study protocol. BMC Public Health 2016, 16, 244. [Google Scholar]
- Barnekow Lillesø, J.P.; Shrestha, T.B.; Dhakal, L.P.; Nayaju, R.P.; Shrestha, R. The Map of Potential Vegetation of Nepal: A Forestry/Agro-Ecological/Biodiversity Classification System Center for Skov, Landskab og Planlægning/Københavns Universitet; Forest and Landscape Denmark: Copenhagen, Denmark, 2005. [Google Scholar]
- Sah, R.B.; Bhattarai, S.; Yadav, S.; Baral, R.; Jha, N.; Pokharel, P.K. A study of prevalence of intestinal parasites and associated risk factors among the school children of Itahari, eastern region of Nepal. Trop. Parasitol. 2013, 3, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Delagua. Delagua Portable Water Testing Kit, User Manual: Version 5; Delagua Water Testing Limited: Marlborough, UK, 2015. [Google Scholar]
- Ministry of Physical Planning and Works and World Health Organization (MOPPW and WHO). National Drinking Water Quality Standards, Nepal; MOPPW and WHO: Kathmandu, Nepal, 2005. [Google Scholar]
- Jordanova, T.; Cronk, R.; Obando, W.; Medina, O.Z.; Kinoshita, R.; Bartram, J. Water, sanitation, and hygiene in schools in low socio-economic regions in Nicaragua: A cross-sectional survey. Int. J. Environ. Res. Public Health 2015, 12, 6197–6217. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.F.; Bain, R.E.S.; Cronk, R.; Wright, J.A.; Bartram, J. Association of supply type with fecal contamination of source water and household stored drinking water in developing countries: A bivariate meta-analysis. Environ. Health Perspect. 2015, 123, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, J.E.; Gumbo, J.R. Knowledge, attitude and practices (KAP) survey on water, sanitation and hygiene in selected schools in Vhembe district, Limpopo, South Africa. Int. J. Environ. Res. Public Health 2013, 10, 2282–2295. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.R. Ground water quality in the Kathmandu valley of Nepal. Environ. Monit. Assess. 2011, 178, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.D.; Poudyal, N.; Bhattacharya, S.K. Bacteriological quality of bottled drinking water versus municipal tap water in Dharan municipality, Nepal. J. Health Popul. Nutr. 2016, 35, 17. [Google Scholar] [CrossRef] [PubMed]
- Tabor, M.; Kibret, M.; Abera, B. Bacteriological and physicochemical quality of drinking water and hygiene-sanitation practices of the consumers in Bahir Dar city, Ethiopia. Ethiop. J. Health Sci. 2011, 21, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.T.G.; Sthiannopkao, S.; Kim, K.-W. Arsenic and other trace elements contamination in groundwater and a risk assessment study for the residents in the Kandal province of Cambodia. Environ. Int. 2009, 35, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Shaheed, A.; Orgill, J.; Ratana, C.; Montgomery, M.A.; Jeuland, M.A.; Brown, J. Water quality risks of “improved” water sources: Evidence from Cambodia. Trop. Med. Int. Health 2014, 19, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sustainable Development Goals (SDG). Clean Water and Sanitation: Why It Matters; SDG: Kathmandu, Nepal, 2015. [Google Scholar]
- Bartran, J.; Brocklehurst, C.; Fisher, M.B.; Luyendijk, R.; Hossain, R.; Wardlaw, T.; Gordon, B. Global monitoring of water supply and sanitation: history, methods and future challenges. Int. J. Environ. Res. Public Health 2014, 11, 8137–8165. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Ono, K.; Yanagida, J.I.; Kurokawa, M.; Rai, C.K. Status of drinking water contamination in Mountain Region, Nepal. Nepal Med. Coll. J. 2009, 11, 281–283. [Google Scholar] [PubMed]
- McMichael, C.; Robinson, P. Drivers of sustained hygiene behaviour change: A case study from mid-western Nepal. Soc. Sci. Med. 1982 2016, 163, 28–36. [Google Scholar] [CrossRef] [PubMed]
- United States Agency International Development (USAID) and Promoting Child Rights to End Child Poverty (Plan International). Assistance to Self-Help Shelter and Sanitation Solutions in Dolakha, Nepal; UNAIDS and Plan International: Washington DC, USA, 2016. [Google Scholar]
- Kovalsky, A.N.; Lacey, S.E.; Kaphle, U.R.; Vaughn, J.M. Risk perception and water purification practices for water-borne parasitic infections in remote Nepal. Trop. Doct. 2008, 38, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Aihara, Y.; Yoden, K.; Yamagata, Z.; Nishida, K.; Kondo, N. Access to improved water and its relationship with diarrhoea in Kathmandu Valley, Nepal: A cross-sectional study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Saboori, S.; Greene, L.E.; Moe, C.L.; Freeman, M.C.; Caruso, B.A.; Akoko, D.; Rheingans, R.D. Impact of regular soap provision to primary schools on handwashing and E. coli hand contamination among pupils in Nyanza province, Kenya: A cluster-randomized trial. Am. J. Trop. Med. Hyg. 2013, 89, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, S.; Shordt, K. It does last! Some findings from a multi-country study of hygiene sustainability. Waterlines 2004, 22, 4–7. [Google Scholar]
- Luby, S.P.; Halder, A.K.; Huda, T.M.N.; Unicomb, L.; Johnston, R.B. Using child health outcomes to identify effective measures of handwashing. Am. J. Trop. Med. Hyg. 2011, 85, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013, 380, 2224–2260. [Google Scholar] [CrossRef]
- Blanton, E.; Ombeki, S.; Oluoch, G.O.; Mwaki, A.; Wannemuehler, K.; Quick, R. Evaluation of the role of school children in the promotion of point-of-use water treatment and handwashing in schools and households—Nyanza province, Western Kenya, 2007. Am. J. Trop. Med. Hyg. 2010, 82, 664–671. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- The World Health Organization /United Nations Children’s Fund. Progress on Sanitation and Drinking-Water; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Erismann, S.; Diagbouga, S.; Odermatt, P.; Knoblauch, A.M.; Gerold, J.; Shrestha, A.; Grissoum, T.; Kaboré, A.; Schindler, C.; Utzinger, J.; et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasites Vectors 2016, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.A.; Nagel, C.L.; Rosa, G.; Iyakaremye, L.; Zambrano, L.D.; Clasen, T.F. Faecal contamination of household drinking water in Rwanda: A national cross-sectional study. Sci. Total Environ. 2016, 571, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Uwimpuhwe, M.; Reddy, P.; Barratt, G.; Bux, F. The impact of hygiene and localised treatment on the quality of drinking water in Masaka, Rwanda. J. Environ. Sci. 2014, 49, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Tubatsi, G.; Bonyongo, M.C.; Gondwe, M. Water use practices, water quality, and households’ diarrheal encounters in communities along the Boro-Thamalakane-Boteti river system, Northern Botswana. J. Health Popul. Nutr. 2015, 33, 21. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall N (%) | Dolakha n (%) | Ramechhap n (%) | |
|---|---|---|---|---|
| Sex of the schoolchildren (n = 708) | ||||
| Girls | 339 (47.9) | 261 (47.0) | 78 (51.0) | |
| Boys | 369 (52.1) | 294 (53.0) | 75 (49.0) | |
| Age of children (n = 708) | ||||
| Age group 1 (8–12 years) | 108 (15.2) | 86 (15.5) | 22 (14.4) | |
| Age group 2 (13–16 years) | 600 (84.8) | 469 (84.5) | 131 (85.6) | |
| Education level of children (n = 708) | ||||
| Grade 6 | 333 (47.0) | 258 (46.5) | 75 (49.0) | |
| Grade 7 | 375 (53.0) | 297 (53.5) | 78 (51.0) | |
| Educational attainment of caregivers (n = 562) | ||||
| No formal schooling | 210 (37.4) | 174 (39.2) | 36 (30.5) | |
| Primary education | 144 (25.6) | 130 (29.3) | 14 (11.9) | |
| Secondary education | 143 (25.4) | 82 (18.5) | 61 (51.7) | |
| Higher education | 65 (11.6) | 58 (13.0) | 7 (5.9) | |
| Age of caregivers (n = 562) | ||||
| 18–24 years | 2 (0.4) | 1 (0.2) | 1 (0.9) | |
| 24–40 years | 239 (42.5) | 184 (41.4) | 55 (46.6) | |
| >40 years | 321 (57.1) | 259 (58.3) | 62 (52.5) | |
| Ethnicity of caregivers (n = 562) | ||||
| Brahmin | 101 (17.9) | 97 (21.9) | 4 (3.4) | |
| Chhetri | 210 (37.4) | 154 (34.7) | 56 (47.5) | |
| Newar | 33 (5.9) | 22 (4.9) | 11 (9.3) | |
| Tamang | 213 (37.9) | 166 (37.4) | 47 (39.8) | |
| Janajati | 5 (0.9) | 5 (1.1) | 0 (0.0) | |
| Socioeconomic status of caregivers (n = 562) | ||||
| Roof material | Corrugated iron roof | 415 (73.8) | 325 (73.2) | 90 (76.3) |
| Wood and tiles | 147 (26.2) | 119 (26.8) | 28 (23.7) | |
| Wall material | Wood | 66 (11.7) | 61 (13.7) | 5 (4.2) |
| Corrugated iron | 407 (72.4) | 331 (74.6) | 76 (64.4) | |
| Bricks | 89 (15.9) | 52 (11.7) | 37 (31.4) | |
| Floor material | Mud | 524 (93.2) | 430 (96.9) | 94 (79.7) |
| Cement | 38 (6.8) | 14 (3.1) | 24 (20.3) | |
| Socioeconomic status * (n = 562) | High | 49 (8.7) | 39 (8.8) | 10 (8.5) |
| Middle | 215 (38.3) | 163 (36.7) | 52 (44.1) | |
| Poor | 298 (53.0) | 242 (54.5) | 56 (47.5) | |
| Owning agricultural land (n = 562) | 511 (90.9) | 412 (92.8) | 99 (83.9) | |
| Possession of domestic animals (n = 562) | 507 (90.2) | 401 (90.3) | 106 (89.8) | |
| Variables | Overall N (%) | Dolakha n (%) | Ramechhap n (%) | |
|---|---|---|---|---|
| School level | ||||
| Secondary | 10 (62.5) | 9 (69.2) | 1 (33.3) | |
| Above secondary | 6 (37.5) | 4 (30.8) | 2 (66.7) | |
| School building age | ||||
| 0–10 years | 7 (43.7) | 6 (46.2) | 1 (33.3) | |
| 11–20 years | 4 (25.0) | 3 (23.1) | 1 (33.3) | |
| >20 years | 5 (31.2) | 4 (30.8) | 1 (33.4) | |
| School size | ||||
| <500 students | 4 (25.0) | 3 (23.1) | 1 (33.3) | |
| >500 students | 12 (75.0) | 10 (76.9) | 2 (66.7) | |
| School conditions | ||||
| Electricity at the school | 11 (68.7) | 9 (69.2) | 2 (18.2) | |
| Roof material | Iron sheet | 15 (93.7) | 13 (100.0) | 2 (66.7) |
| Bamboo | 1 (6.3) | 0 (0.0) | 1 (33.3) | |
| Floor material | Cement | 2 (12.5) | 0 (0.0) | 1 (33.3) |
| Soil | 14 (87.5) | 12 (92.3) | 2 (66.7) | |
| Wall material | Brick | 2 (12.5) | 1 (7.7) | 1 (33.3) |
| Bamboo | 2 (12.5) | 0 (0.0) | 2 (66.7) | |
| Iron sheet | 12 (75.0) | 12 (92.3) | 0 (0.0) | |
| Water source * | ||||
| Surface water | 2 (12.5) | 0 (0.0) | 2 (66.7) | |
| Standpipe | 6 (37.5) | 5 (38.5) | 1 (33.3) | |
| Piped water | 8 (50.0) | 8 (61.5) | 0 (0.0) | |
| Drinking water * | ||||
| Drinking water available throughout year | 7 (43.8) | 7 (100.0) | 0 (0.0) | |
| Treatment of drinking water by school | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Water in school # | Inadequate | 14 (87.5) | 13 (100.0) | 1 (33.3) |
| Adequate | 2 (12.5) | 0 (0.0) | 2 (66.7) | |
| Hand washing facility in any area of school | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Latrine | ||||
| Flush toilet | 3 (18.8) | 2 (15.4) | 1 (33.3) | |
| Pit latrine with cement floor and composting latrine | 4 (25.0) | 4 (30.8) | 0 (0.0) | |
| Pit latrine without cement floors, hanging latrine | 9 (56.2) | 7 (53.9) | 2 (66.8) | |
| Latrine condition | ||||
| Presence of door | 16 (100.0) | 13 (100.0) | 3 (100.0) | |
| Sharing with opposite gender | 14 (87.5) | 12 (92.3) | 2 (66.7) | |
| Damaged floor (i) | 9 (56.3) | 8 (61.5) | 1 (33.3) | |
| No privacy (ii) | 8 (50.0) | 7 (53.9) | 1 (33.3) | |
| Clean floor (iii) | 5 (31.3) | 4 (30.8) | 1 (33.3) | |
| Clean wall (iv) | 7 (43.8) | 6 (46.2) | 1 (33.3) | |
| Flies present | 8 (50.0) | 5 (38.5) | 3 (100.0) | |
| Odour present | 15 (93.8) | 12 (92.3) | 3 (100.0) | |
| Regular water for anal cleansing | 2 (12.5) | 2 (15.4) | 0 (0.0) | |
| Washbasin for handwashing | 0 (16.0) | 0 (0.0) | 0 (0.0) | |
| Water for hand washing | 6 (37.5) | 4 (30.8) | 2 (66.4) | |
| Soap for hand washing | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Sanitation in school (v) | Inadequate | 8 (50.0) | 7 (53.9) | 1 (33.3) |
| Adequate | 8 (50.0) | 6 (46.1) | 2 (66.7) | |
| Hygiene in school (vi) | Inadequate | 8 (50.0) | 6 (46.2) | 2 (66.7) |
| Adequate | 8 (50.0) | 7 (53.9) | 1 (33.3) | |
| Safe solid waste disposal (vii) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Category | Parameter | Unit | Range | School | Community | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Dolakha | Ramechhap | Overall | Dolakha | Ramechhap | |||||||
| Main Source * (N = 16) | Point-of-Use ** (N = 13) | Main Source * (n = 13) | Point-of-Use (n = 10) | Main Source * (n = 3) | Point-of-Use (n = 3) | (n= 43) | (n = 33) | (n = 10) | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Physical characteristics | Turbidity | NTU | >5 | 0 (0.0) | 1 (7.7) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 4 (9.3) | 3 (9.1) | 1 (10.0) |
| 2–5 | 16 (100.0) | 12 (92.3) | 13 (100.0) | 9 (90.0) | 3 (100.0) | 3 (100.0) | 39 (90.7) | 30 (90.9) | 9 (90.9) | |||
| pH | 6.5–8.5 | 0 (0.0) | 13 (100.0) | 13 (100.0) | 10 (100.0) | 3 (100.0) | 3 (100.0) | 43 (100.0) | 33 (100.0) | 10 (100.0) | ||
| Chemical characteristics | Free residual chlorine *** | 0.1–0.2 | 16 (100.0) | 13 (100.0) | 13 (100.0) | 10 (100.0) | 3 (100.0) | 3 (100.0) | 43 (100.0) | 33 (100.0) | 10 (100.0) | |
| Total residual chlorine | mg/L | 0.2–0.5 | 16 (100.0) | 13 (100.0) | 13 (100.0) | 10 (100.0) | 3 (100.0) | 3 (100.0) | 41 (95.3) | 31 (93.9) | 10 (100.0) | |
| 0–0.199 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.7) | 2 (15.1) | 0 (0.0) | |||
| Lead | mg/L | <0.01 | 16 (100.0) | 0 (0.0) | 13 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | ||||
| Arsenic | mg/L | <0.05 | 16 (100.0) | 0 (0.0) | 13 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | ||||
| Microbiological characteristics | Thermo-tolerant coliforms (TTC) *** | CFU/100 mL | <1 | 4 (25.0) | 3 (23.1) | 3 (23.1) | 3 (30.0) | 1 (33.3) | 0 (0.0) | 26 (60.5) | 21 (63.6) | 5 (50.0) |
| 1–10 | 5 (31.3) | 4 (30.8) | 5 (38.5) | 2 (20.0) | 0 (0.0) | 2 (66.7) | 3 (7.0) | 2 (6.0) | 1 (10.0) | |||
| 11–100 | 6 (37.5) | 2 (15.4) | 4 (30.8) | 2 (20.0) | 2 (66.7) | 0 (0.0) | 8 (18.6) | 6 (18.2) | 2 (20.0) | |||
| >100 | 1 (6.2) | 4 (30.7) | 1 (7.6) | 3 (30.0) | 0 (0.0) | 1 (33.3) | 6 (13.9) | 4 (12.1) | 2 (20.0) | |||
| Variables (n = 708) | Overall N (%) | Dolakha n (%) | Ramechhap n (%) |
|---|---|---|---|
| KAP indicators: schoolchildren | |||
| Hand washing | |||
| Before eating | 525 (74.2) | 427 (76.9) | 98 (64.1) |
| After eating | 434 (61.3) | 357 (64.3) | 77 (50.3) |
| After playing | 422 (59.6) | 345 (62.2) | 77 (50.3) |
| After defecation | 534 (75.4) | 427 (76.9) | 107 (69.9) |
| Do not wash hands | 66 (11.7) | 45 (10.1) | 21 (17.8) |
| With water only | 687 (97.0) | 540 (97.3) | 147 (96.1) |
| With ash | 17 (2.4) | 12 (2.2) | 5 (3.3) |
| With mud/soil | 4 (0.6) | 4 (0.7) | 0 (0.0) |
| With water and soap | 689 (97.3) | 539 (97.1) | 150 (98.0) |
| Hygiene (ii) | |||
| Higher category | 261 (36.9) | 225 (40.5) | 36 (23.5) |
| Middle category | 211 (29.8) | 165 (29.7) | 46 (30.1) |
| Lower category | 236 (33.3) | 165 (29.7) | 71 (46.4) |
| Sanitary practices at school | |||
| Using latrine at school | 679 (95.9) | 543 (97.8) | 136 (88.9) |
| No latrine use | 29 (4.1) | 12 (2.2) | 17 (11.1) |
| Drinking water of children at school * | |||
| Drinking water from school | 637 (90.0) | 535 (96.4) | 102 (66.7) |
| Bringing water from home | 102 (14.4) | 67 (12.1) | 35 (22.9) |
| Households (n = 562) | |||
| Use of toilet at home | |||
| Latrine in the household | 394 (70.1) | 320 (72.1) | 74 (62.7) |
| Shared latrine | 68 (12.1) | 57 (12.8) | 11 (9.3) |
| Bush | 73 (13.0) | 57 (12.8) | 16 (13.5) |
| River, swamp, lake | 27 (4.8) | 10 (2.2) | 17 (14.4) |
| Type of latrine at home | |||
| Water seal latrine | 283 (50.4) | 233 (52.5) | 50 (42.4) |
| Open pit latrine with slab | 97 (17.3) | 77 (17.3) | 20 (16.9) |
| Open pit latrine without slab | 14 (2.5) | 12 (2.7) | 2 (1.7) |
| Soap in household for hand-washing | 417 (74.2) | 319 (71.9) | 98 (83.0) |
| Hygiene of caregivers (iii) (n = 252) | |||
| Lower category | 72 (28.7) | 60 (27.0) | 12 (41.4) |
| Middle category | 26 (10.4) | 23 (10.4) | 3 (10.3) |
| Better category | 153 (60.9) | 139 (62.6) | 14 (48.3) |
| Drinking water at home * | |||
| Drinking water source during dry season | |||
| Private tap | 287 (51.1) | 257 (57.9) | 30 (25.4) |
| Spring | 13 (2.3) | 3 (0.7) | 10 (8.5) |
| Public tap | 36 (6.4) | 36 (8.1) | 0 (0.0) |
| Other (iv) | 226 (40.2) | 148 (33.3) | 78 (66.1) |
| Drinking water source during rainy season | |||
| Private tap | 285 (50.7) | 258 (58.1) | 27 (22.9) |
| Spring | 1 (0.18) | 1 (0.2) | 0 (0.0) |
| Public tap | 44 (7.8) | 40 (9.0) | 4 (3.4) |
| Other (v) | 232 (41.3) | 145 (32.7) | 87 (73.7) |
| Container to fetch water at the principle source | |||
| Clay | 40 (7.1) | 16 (3.6) | 24 (20.3) |
| Plastic | 258 (45.9) | 205 (46.2) | 53 (44.9) |
| Metal | 264 (47.0) | 223 (50.2) | 41 (34.8) |
| Frequency of washing drinking water storage container with soap | |||
| Never | 40 (7.1) | 20 (4.5) | 20 (17.0) |
| Daily | 347 (61.8) | 277 (62.4) | 70 (59.3) |
| Weekly | 175 (31.1) | 147 (33.1) | 28 (23.7) |
| Status of drinking water container | |||
| Covered | 417 (74.2) | 322 (72.5) | 95 (80.5) |
| Uncovered | 145 (25.8) | 122 (27.5) | 23 (19.5) |
| Drinking water container used for other activities | 112 (19.9) | 89 (20.5) | 23 (19.5) |
| Regular water treatment | 76 (13.5) | 50 (11.3) | 26 (22.0) |
| Aware of boiling | 203 (36.1) | 181 (40.8) | 22 (18.6) |
| Aware of chlorination | 32 (5.7) | 28 (6.3) | 4 (3.4) |
| Aware of filtration | 70 (12.5) | 28 (6.3) | 42 (35.6) |
| Water sufficiency | 439 (78.1) | 333 (75.0) | 106 (89.8) |
| Safe solid waste disposal * | 273 (48.6) | 237 (53.4) | 36 (30.5) |
| Category | Parameter | Unit | Range | Overall N (%) | Dolakha n (%) | Ramechhap n (%) |
|---|---|---|---|---|---|---|
| Physical characteristics | Turbidity | NTU * | >5 | 131 (23.3) | 115 (25.9) | 16 (13.6) |
| 2–5 | 431 (76.7) | 329 (74.1) | 102 (86.4) | |||
| pH | 6.5–8.5 | 562 (100.0) | 444 (100.0) | 118 (100.0) | ||
| Chemical characteristics | Free residual chlorine | mg/L | 0.3–0.5 | 121 (21.5) | 105 (23.6) | 16 (13.6) |
| 0.1–0.2 | 441 (78.5) | 339 (76.4) | 102 (86.4) | |||
| Total residual chlorine | mg/L | >0.5 | 2 (0.4) | 1 (0.2) | 1 (0.8) | |
| 0.2–0.5 | 548 (97.5) | 439 (98.9) | 109 (92.4) | |||
| 0–0.199 | 12 (2.1) | 4 (0.9) | 8 (6.8) | |||
| Microbiological characteristics | Thermo-tolerant coliforms (TTC) | CFU/100 mL ** | <1 | 408 (72.6) | 333 (75.0) | 75 (63.6) |
| 1–10 | 42 (7.5) | 24 (5.4) | 18 (15.3) | |||
| 11–100 | 42 (7.5) | 36 (81.1) | 6 (5.1) | |||
| >100 | 70 (12.5) | 51 (11.5) | 19 (16.1) |
| Risk Factor | N (%) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | aOR | 95% CI | p-Value | ||
| District | |||||||
| Dolakha | 444 (79.0) | 1.00 | 1.00 | ||||
| Ramechhap | 118 (21.0) | 1.79 | 1.02–3.13 | 0.04 | 2.25 | 1.16–4.34 | 0.02 |
| Education of the respondent | |||||||
| No formal education | 210 (37.4) | 1.00 | |||||
| Primary education | 144 (25.6) | 1.21 | 0.74–1.99 | 0.44 | 1.26 | 0.76–2.07 | 0.37 |
| Secondary education | 143 (25.4) | 0.75 | 0.43–1.32 | 0.34 | 0.73 | 0.41–1.30 | 0.29 |
| Superior | 65 (11.6) | 0.76 | 0.36–1.61 | 0.47 | 0.92 | 0.43–1.95 | 0.82 |
| Socioeconomic status | |||||||
| Low | 298 (53.0) | 1.00 | |||||
| Medium | 215 (38.3) | 1.10 | 0.73–1.64 | 0.65 | 1.07 | 0.71–1.61 | 0.75 |
| High | 49 (8.7) | 1.06 | 0.53–2.11 | 0.87 | 1.02 | 0.50–2.06 | 0.97 |
| Household drinking water during the dry season | |||||||
| Private tap | 287 (51.1) | 1.00 | 1.00 | ||||
| Spring | 13 (2.3) | 3.98 | 1.14–13.97 | 0.03 | 2.48 | 0.64–9.66 | 0.19 |
| Public tap | 36 (6.4) | 1.67 | 0.70–3.95 | 0.24 | 1.68 | 0.71–3.96 | 0.23 |
| Other | 226 (40.2) | 0.93 | 0.59–1.44 | 0.73 | 0.87 | 0.55–1.37 | 0.55 |
| Container for fetching water | |||||||
| Metal container | 264 (47.0) | 1.00 | |||||
| Plastic container | 258 (45.9) | 1.11 | 0.73–1.69 | 0.62 | 0.96 | 0.60–1.52 | 0.85 |
| Clay pot | 40 (7.1) | 1.86 | 0.86–4.02 | 0.12 | 0.82 | 0.34–1.99 | 0.67 |
| Status of container during storage | |||||||
| Covered | 417 (74.2) | 1.00 | |||||
| Uncovered | 127 (22.6) | 0.97 | 0.60–1.57 | 0.89 | |||
| Not seen | 18 (3.2) | 0.90 | 0.30–2.70 | 0.86 | |||
| Container washing frequency | |||||||
| Daily | 347 (61.7) | 1.00 | |||||
| Never | 40 (7.1) | 0.82 | 0.35–1.93 | 0.65 | |||
| Weekly | 175 (31.1) | 0.95 | 0.60–1.51 | 0.82 | |||
| Drinking water container used for other activities | |||||||
| Yes | 451 (80.3) | 1.00 | |||||
| No | 111 (19.7) | 0.76 | 0.46–1.27 | 0.30 | |||
| Water treatment * | |||||||
| No | 486 (86.5) | 1.00 | |||||
| Yes | 76 (13.5) | 0.74 | 0.42–1.31 | 0.30 | |||
| Latrine in household | |||||||
| Yes | 395 (70.3) | 1.00 | |||||
| No | 167 (29.7) | 1.20 | 0.76–1.87 | 0.43 | |||
| Latrine type | |||||||
| Water seal latrine | 283 (50.4) | 1.00 | |||||
| No latrine | 168 (29.9) | 1.30 | 0.81–2.11 | 0.28 | |||
| Open pit latrine with slab | 97 (17.3) | 1.21 | 0.69–2.11 | 0.51 | |||
| Flushed toilet | 14 (2.5) | 0.91 | 0.24–3.49 | 0.89 | |||
| Solid waste disposal | |||||||
| Yes | 273 (48.6) | 1.00 | |||||
| No | 289 (51.4) | 0.99 | 0.67–1.48 | 0.99 | |||
| Possession of livestock | |||||||
| Yes | 507 (90.2) | 1.00 | |||||
| No | 55 (9.8) | 0.92 | 0.47–1.80 | 0.80 | |||
| Livestock kept inside household | |||||||
| No | 307 (54.6) | 1.00 | |||||
| Yes | 255 (45.4) | 0.63 | 0.41–0.95 | 0.03 | 1.64 | 1.08–2.50 | 0.02 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, A.; Sharma, S.; Gerold, J.; Erismann, S.; Sagar, S.; Koju, R.; Schindler, C.; Odermatt, P.; Utzinger, J.; Cissé, G. Water Quality, Sanitation, and Hygiene Conditions in Schools and Households in Dolakha and Ramechhap Districts, Nepal: Results from A Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2017, 14, 89. https://doi.org/10.3390/ijerph14010089
Shrestha A, Sharma S, Gerold J, Erismann S, Sagar S, Koju R, Schindler C, Odermatt P, Utzinger J, Cissé G. Water Quality, Sanitation, and Hygiene Conditions in Schools and Households in Dolakha and Ramechhap Districts, Nepal: Results from A Cross-Sectional Survey. International Journal of Environmental Research and Public Health. 2017; 14(1):89. https://doi.org/10.3390/ijerph14010089
Chicago/Turabian StyleShrestha, Akina, Subodh Sharma, Jana Gerold, Séverine Erismann, Sanjay Sagar, Rajendra Koju, Christian Schindler, Peter Odermatt, Jürg Utzinger, and Guéladio Cissé. 2017. "Water Quality, Sanitation, and Hygiene Conditions in Schools and Households in Dolakha and Ramechhap Districts, Nepal: Results from A Cross-Sectional Survey" International Journal of Environmental Research and Public Health 14, no. 1: 89. https://doi.org/10.3390/ijerph14010089
APA StyleShrestha, A., Sharma, S., Gerold, J., Erismann, S., Sagar, S., Koju, R., Schindler, C., Odermatt, P., Utzinger, J., & Cissé, G. (2017). Water Quality, Sanitation, and Hygiene Conditions in Schools and Households in Dolakha and Ramechhap Districts, Nepal: Results from A Cross-Sectional Survey. International Journal of Environmental Research and Public Health, 14(1), 89. https://doi.org/10.3390/ijerph14010089






