The Trend of Voluntary Warnings in Electronic Nicotine Delivery System Magazine Advertisements
Abstract
:1. Introduction
2. Data and Methods
2.1. Data
2.1.1. Magazine Ads from Kantar Media
2.1.2. Contents of Magazine Ads
2.2. Methods
2.2.1. Categorization of Voluntary Warnings
2.2.2. Measures
2.2.3. Analyses
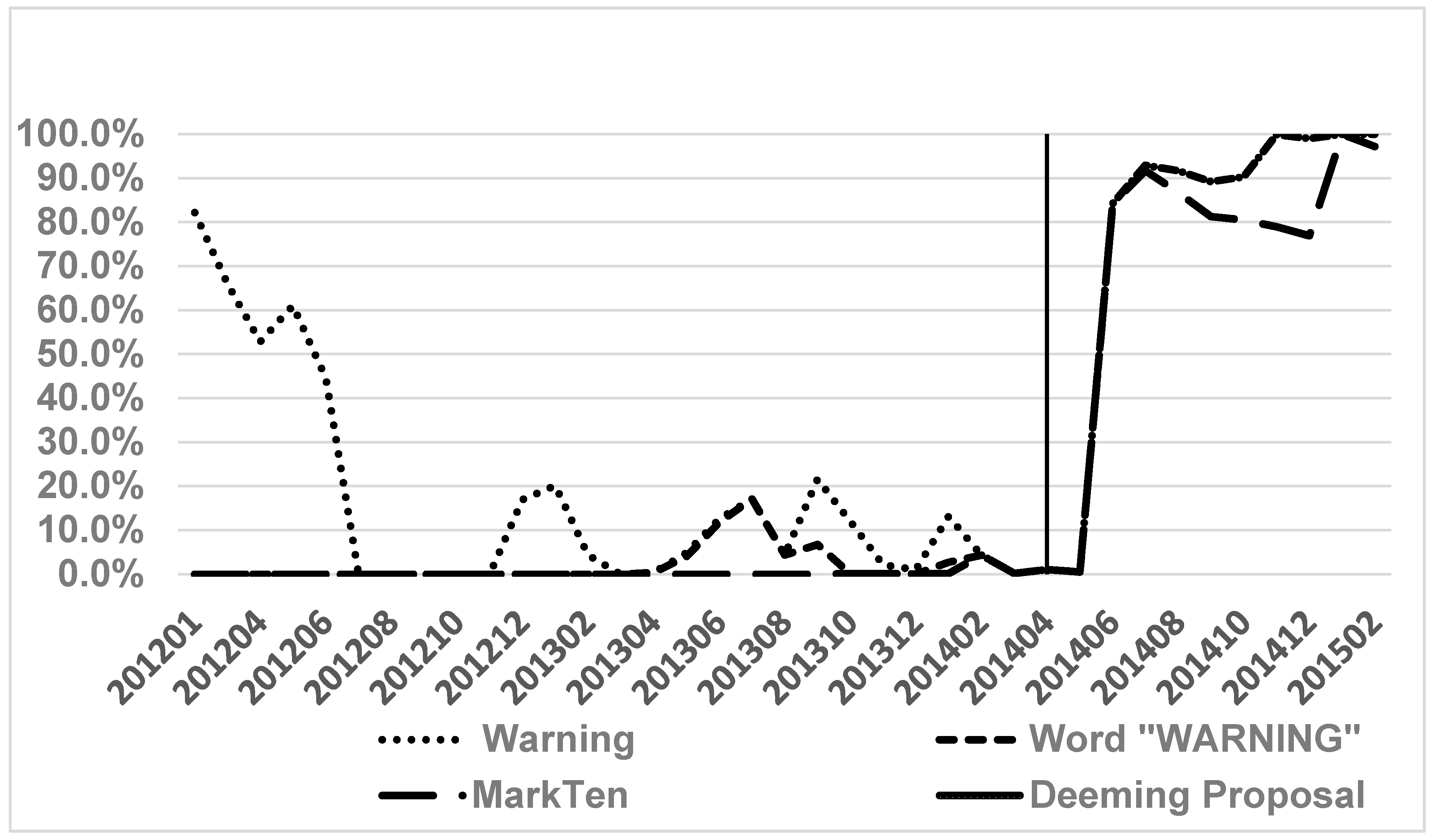
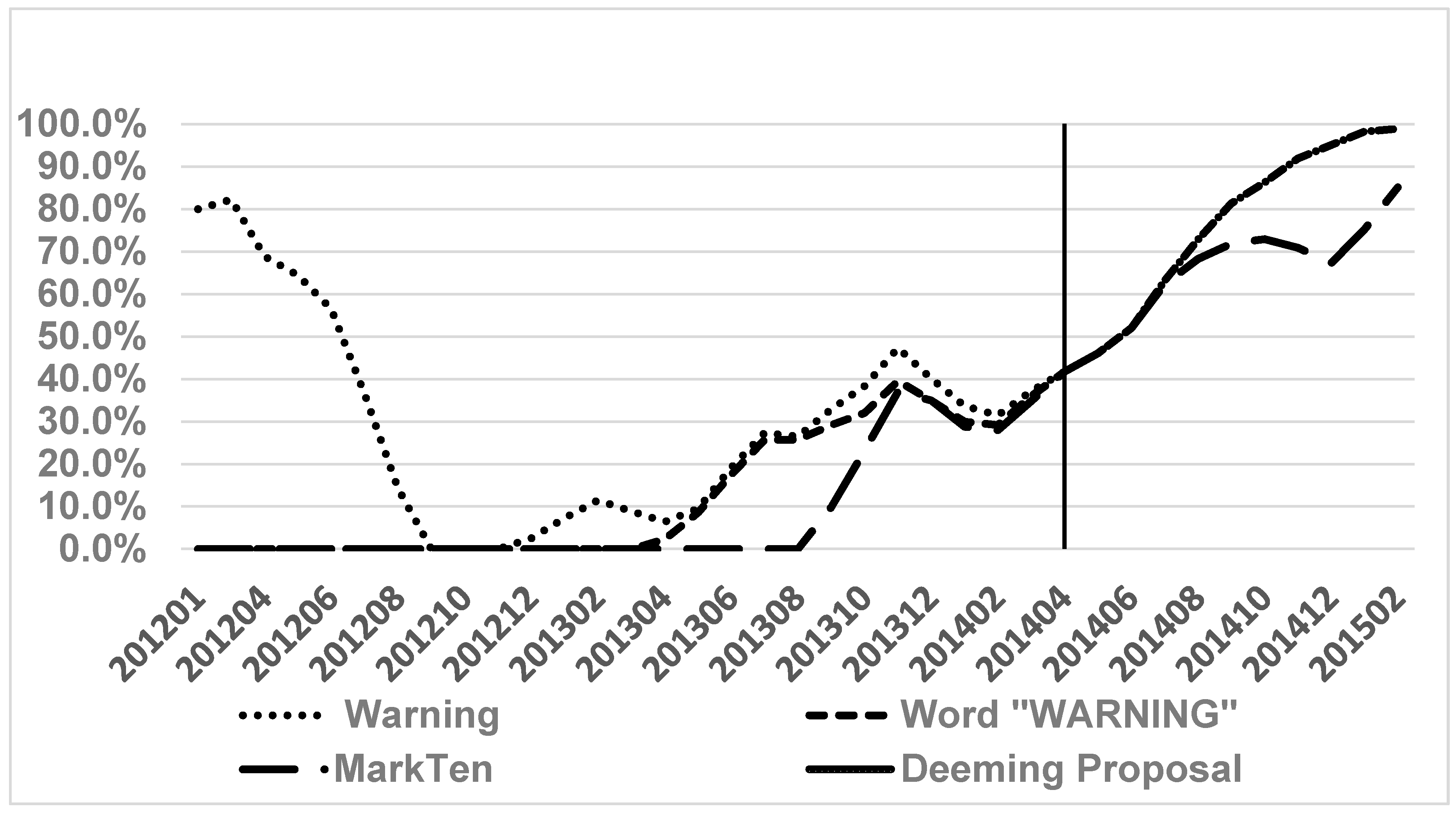
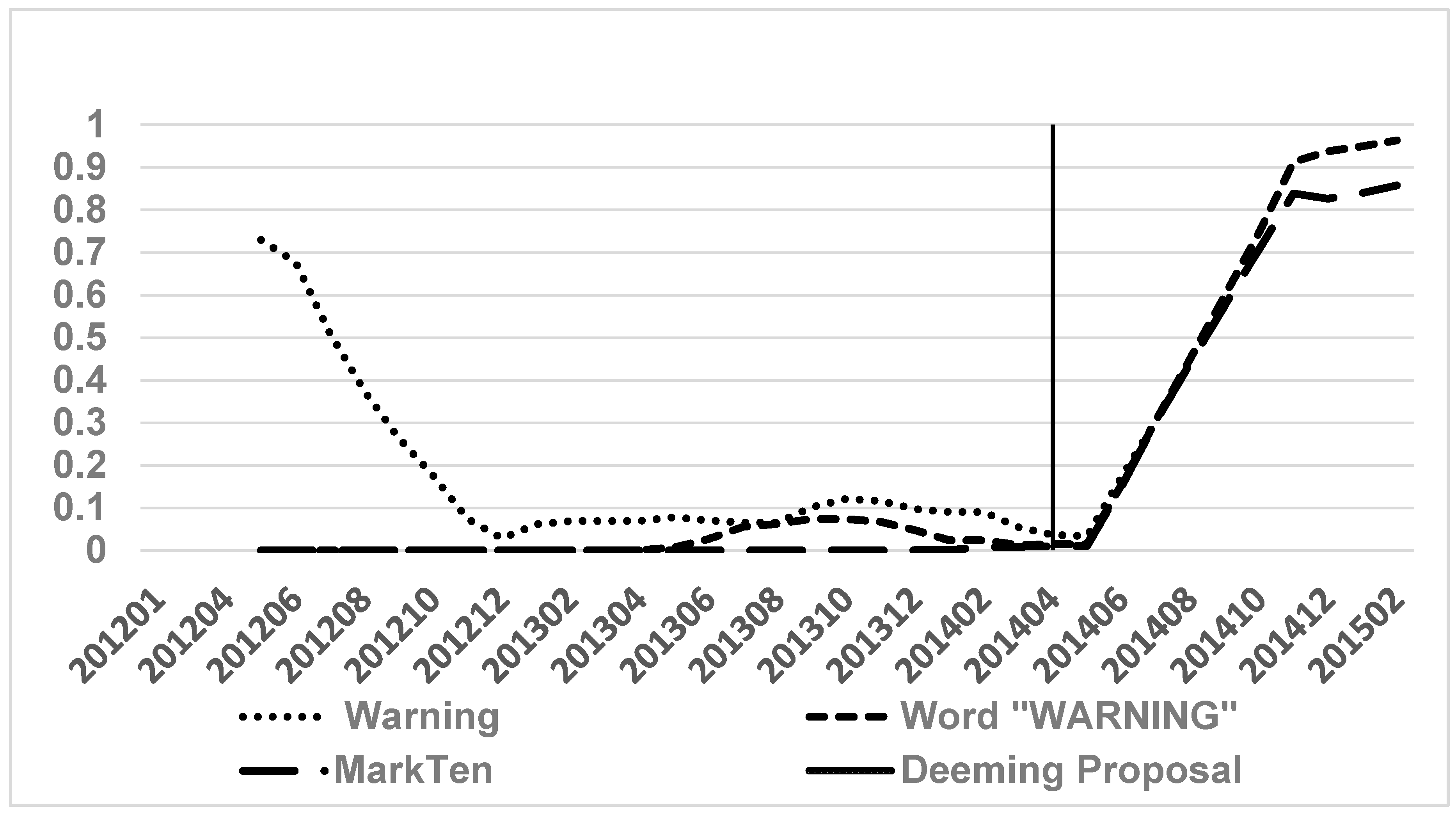
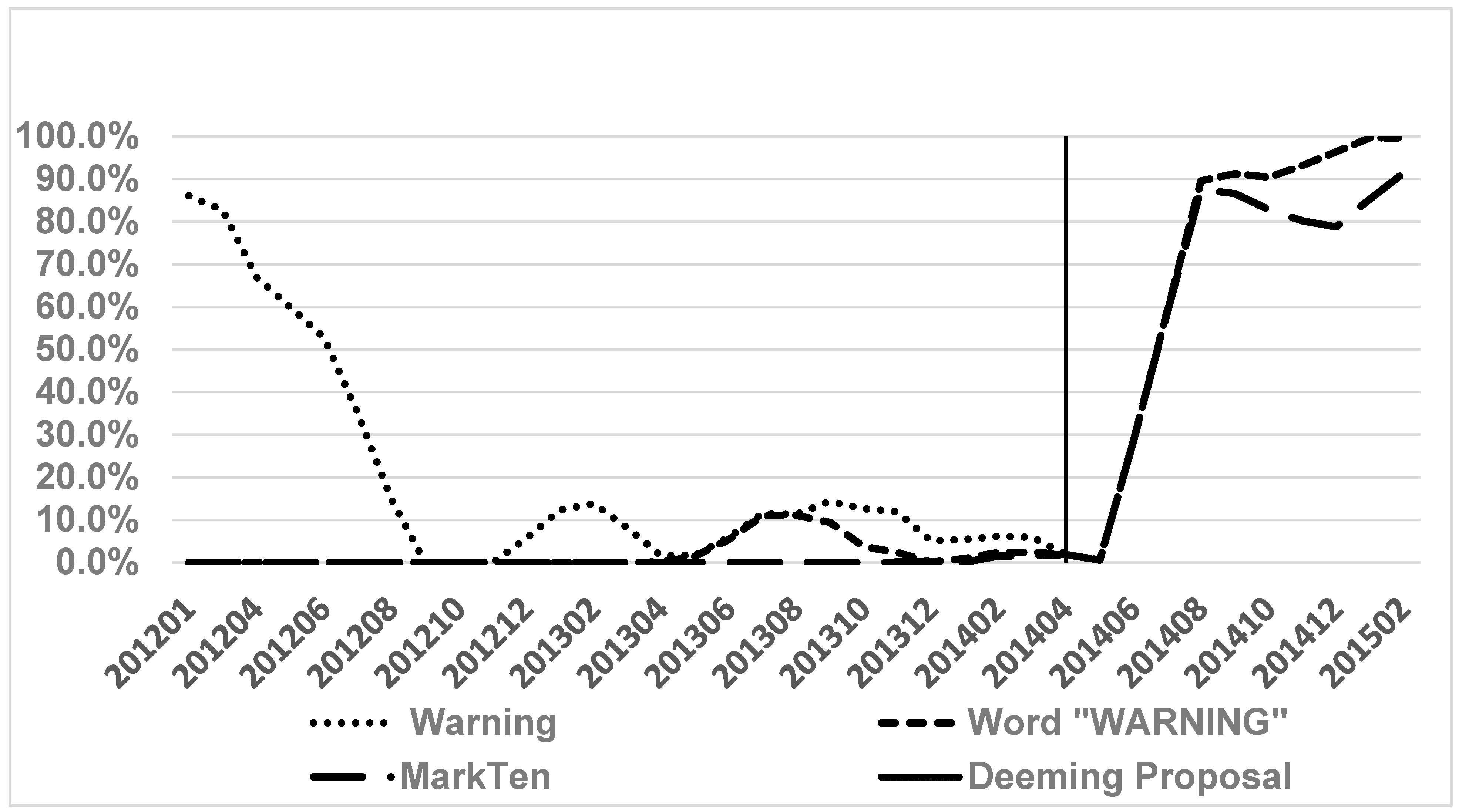
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Centers for Disease Control and Prevention. Tobacco use among middle and high school students—United States, 2011–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 361–367. [Google Scholar]
- U.S. Department of Health and Human Services. E-Cigarette Use among Youth and Young Adults. A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2016.
- Hammond, D. Health warning messages on tobacco products: A review. Tob. Control 2011, 20, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Sanders-Jackson, A.; Schleicher, N.C.; Fortmann, S.P.; Henriksen, L. Effect of warning statements in e-cigarette advertisements: An experiment with young adults in the United States. Addiction 2015, 110, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Popova, L.; Ling, P.M. Nonsmokers’ responses to new warning labels on smokeless tobacco and electronic cigarettes: An experimental study. BMC Public Health 2014, 14, 997. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.O.; Shafer, P.R.; Eggers, M.E.; Kim, A.E.; Parvanta, S.A.; Nonnemaker, J.M. Effect of a voluntary e-cigarette warning label on risk perceptions. Tob. Regulat. Sci. 2016, 2, 82–93. [Google Scholar] [CrossRef]
- Wackowski, O.A.; Manderski, M.T.; Delnevo, C.D. Smokers’ sources of e-cigarette awareness and risk information. Prev. Med. Rep. 2015, 2, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Czoli, C.D.; Goniewicz, M.; Islam, T.; Kotnowski, K.; Hammond, D. Consumer preferences for electronic cigarettes: Results from a discrete choice experiment. Tob. Control 2015. [Google Scholar] [CrossRef] [PubMed]
- Mays, D.; Smith, C.; Johnson, A.C.; Tercyak, K.P.; Niaura, R.S. An experimental study of the effects of electronic cigarette warnings on young adult nonsmokers’ perceptions and behavioral intentions. Tob. Induc. Dis. 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Wackowski, O.A.; O’Connor, R.J.; Strasser, A.A.; Hammond, D.; Villanti, A.C.; Delnevo, C.D. Smokers’ and e-cigarette users’ perceptions of modified risk warnings for e-cigarettes. Prev. Med. Rep. 2016, 4, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Wackowski, O.A.; Hammond, D.; O’Connor, R.J.; Strasser, A.A.; Delnevo, C.D. Smokers’ and e-cigarette users’ perceptions about e-cigarette warning statements. Int. J. Environ. Res. Public Health 2016, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services, Food and Drug Administration, 21 CFR Parts 1100, 1140, and 1143 (Docket No. FDA-2014-N-0189), RIN 0910-AG38. “Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products” ACTION: Final Rule. Available online: http://www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm394909.htm (accessed on 7 June 2016).
- Department of Health and Human Services, Food and Drug Administration, Docket No. FDA-2014-N-0189, RIN 0910-AG38, “Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Regulations Restricting the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Product Packages and Advertisements”. Available online: http://federalregister.gov/a/2014-09491 (accessed on 3 June 2014).
- Gateway to Addiction? A Survey of Popular Electronic Cigarette Manufacturers and Targeted Marketing to Youth. Available online: http://www.merkley.senate.gov/imo/media/doc/DurbineCigarettepercent20Survey.pdf (accessed on 20 August 2016).
- Ohlheiser, A. Big Tobacco Companies Are Putting Big Warning Labels on Their Ecigarettes. The Washington Post. 29 September 2014. Available online: http://www.washingtonpost.com/news/to-yourhealth/wp/2014/09/29/big-tobacco-companies-are-putting-big-warning-labels-on-their-e-cigarettes/ (accessed on 20 February 2015).
- Dire Warnings by Big Tobacco on E-Smoking. Available online: http://mobile.nytimes.com/2014/09/29/business/dire-warnings-by-big-tobacco-on-e-smoking-.html?partner=rss&emc=rss&smid=tw-nytimes&_r=0&referrer= (accessed on 20 August 2016).
- American Legacy Foundation. Vaporized: E-Cigarettes, Advertising, and Youth. May 2014. Available online: http://legacyforhealth.org/content/download/4542/63436/version/1/file/LEG-Vaporized-E-cig_ReportMay2014.pdf (accessed on 24 June 2015).
- McCarthy, M. Youth exposure to e-cigarette advertising on U.S. television soars. BMJ 2014, 348. [Google Scholar] [CrossRef]
- Duke, J.C.; Lee, Y.O.; Kim, A.E.; Watson, K.A.; Arnold, K.Y.; Nonnemaker, J.M.; Porter, L. Exposure to electronic cigarette television advertisements among youth and young adults. Pediatrics 2014, 134. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kornfield, R.; Szczypka, G.; Emery, S.L. A cross-sectional examination of marketing of electronic cigarettes on Twitter. Tob. Control 2014, 23. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.L.; Vera, L.; Huang, J.; Szczypka, G. Wanna know about vaping? Patterns of message exposure, seeking and sharing information about e-cigarettes across media platforms. Tob. Control 2014, 23. [Google Scholar] [CrossRef] [PubMed]
- Kornfield, R.; Huang, J.; Vera, L.; Emery, S.L. Rapidly increasing promotional expenditures for e-cigarettes. Tob. Control 2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.E.; Arnold, K.Y.; Makarenko, O. E-cigarette advertising expenditures in the U.S., 2011–2012. Am. J. Prev. Med. 2014, 46, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Shuk, E.; Greene, K.; Ostroff, J. Content analysis of trends in print magazine tobacco advertisements. Tob. Regul. Sci. 2015, 1, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Prior, M. Weighted content analysis of political advertisements. Polit. Commun. 2001, 18, 335–345. [Google Scholar] [CrossRef]
- Anderson, S.P.; Ciliberto, F.; Liaukonyte, J. Information content of advertising: Empirical evidence from the OTC analgesic industry. Int. J. Ind. Org. 2013, 31, 355–367. [Google Scholar] [CrossRef]
- Altria to Launch MarkTen E-Cigarette Nationally. Available online: http://www.wsj.com/articles/SB10001424052702304914204579393083711733854 (accessed on 20 August 2016).
| Section A, by Magazine Shares (43 Magazines) | |||||
| Magazines | Freq. (Percent) | Magazines | Freq. (Percent) | Magazines | Freq. (Percent) |
| Allure | 8 (0.88) | Hot Rod | 5 (0.55) | Popular Mechanics | 29 (3.2) |
| Automobile Magazine | 8 (0.88) | In Style | 12 (1.32) | Popular Science | 17 (1.87) |
| Car and Driver | 22 (2.43) | In Touch Weekly | 33 (3.64) | Rolling Stone | 79 (8.71) |
| Cosmopolitan | 19 (2.09) | Life & Style Weekly | 32 (3.53) | Soap Opera Digest | 6 (0.66) |
| Country Weekly | 11 (1.21) | Marie Claire | 12 (1.32) | Southern Living | 3 (0.33) |
| Cycle World | 8 (0.88) | Maxim | 35 (3.86) | Spin | 4 (0.44) |
| Ebony | 7 (0.77) | Men’s Journal | 44 (4.85) | Sports Illustrated | 40 (4.41) |
| Elle | 10 (1.1) | Motor Trend | 10 (1.1) | Star | 112 (12.35) |
| Entertainment Weekly | 61 (6.73) | National Enquirer | 2 (0.22) | Time | 12 (1.32) |
| ESPN Magazine | 18 (1.98) | New York Magazine | 3 (0.33) | TV Guide | 37 (4.08) |
| Esquire | 22 (2.43) | OK! Weekly | 27 (2.98) | US Weekly | 67 (7.39) |
| Essence | 1 (0.11) | Out | 6 (0.66) | Vanity Fair | 9 (0.99) |
| Field & Stream | 7 (0.77) | People | 10 (1.1) | Vogue | 8 (0.88) |
| Glamour | 9 (0.99) | Playboy | 33 (3.64) | Wired | 1 (0.11) |
| GQ | 8 (0.88) | ||||
| Section B, by Brand Shares (18 Brands) | |||||
| Brands | Freq. (Percent) | Brands | Freq. (Percent) | Brands | Freq. (Percent) |
| American Blue Tip | 4 (0.44) | MarkTen E-Vapor | 239 (26.35) | Swisher | 1 (0.11) |
| Apollo | 1 (0.11) | Markten | 108 (11.91) | TRYST | 5 (0.55) |
| Blu Cigs | 406 (44.76) | Mistic | 40 (4.41) | Vapir Rise | 2 (0.22) |
| Blu Plus Cigs | 26 (2.87) | Njoy King | 15 (1.65) | Vapor Nation | 1 (0.11) |
| Cigirex | 2 (0.22) | Playboy Premium | 1 (0.11) | VaporGenie | 14 (1.54) |
| Fin | 40 (4.41) | Starfire | 1 (0.11) | Vuse | 1 (0.11) |
| Section C, Variables | |||||
| Variable | Percent | Variable | Mean | ||
| Any Warning 1 | 53.36 | Ad national equivalence | 0.95 | ||
| “WARNING” 1 | 49.39 | Expenditure | 165,938 | ||
| MarkTen 1 | 38.29 | Units | 1.01 | ||
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, C.; Chaloupka, F.J. The Trend of Voluntary Warnings in Electronic Nicotine Delivery System Magazine Advertisements. Int. J. Environ. Res. Public Health 2017, 14, 62. https://doi.org/10.3390/ijerph14010062
Shang C, Chaloupka FJ. The Trend of Voluntary Warnings in Electronic Nicotine Delivery System Magazine Advertisements. International Journal of Environmental Research and Public Health. 2017; 14(1):62. https://doi.org/10.3390/ijerph14010062
Chicago/Turabian StyleShang, Ce, and Frank J. Chaloupka. 2017. "The Trend of Voluntary Warnings in Electronic Nicotine Delivery System Magazine Advertisements" International Journal of Environmental Research and Public Health 14, no. 1: 62. https://doi.org/10.3390/ijerph14010062
APA StyleShang, C., & Chaloupka, F. J. (2017). The Trend of Voluntary Warnings in Electronic Nicotine Delivery System Magazine Advertisements. International Journal of Environmental Research and Public Health, 14(1), 62. https://doi.org/10.3390/ijerph14010062







