Visualizing the Heterogeneity of Effects in the Analysis of Associations of Multiple Myeloma with Glyphosate Use. Comments on Sorahan, T. Multiple Myeloma and Glyphosate Use: A Re-Analysis of US Agricultural Health Study (AHS) Data. Int. J. Environ. Res. Public Health 2015, 12, 1548–1559
Abstract
:1. Introduction
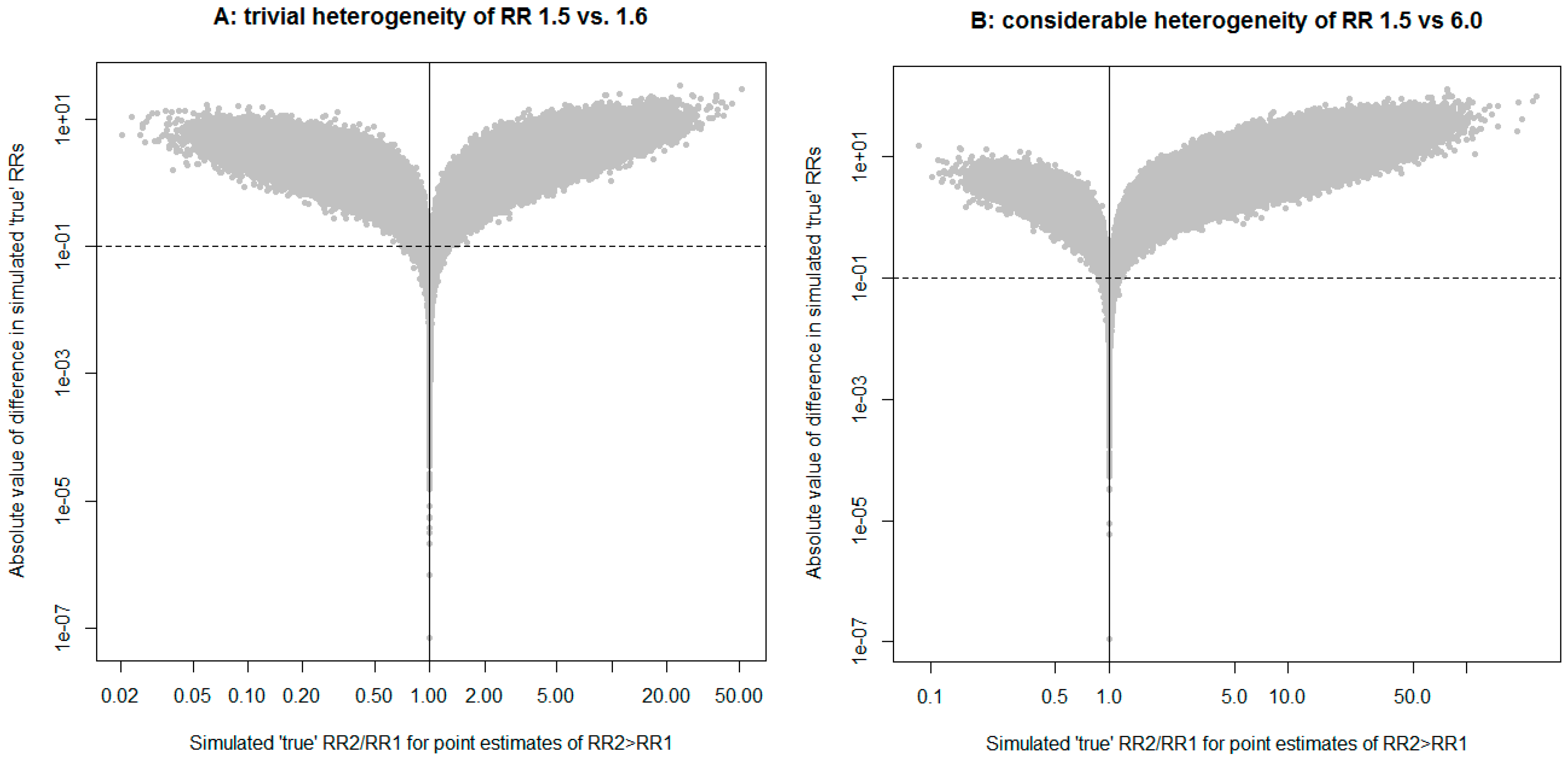
2. Description of a New Method
3. Application to a Case Study of the Risk of Multiple Myeloma in Relation to Glyphosate Use
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
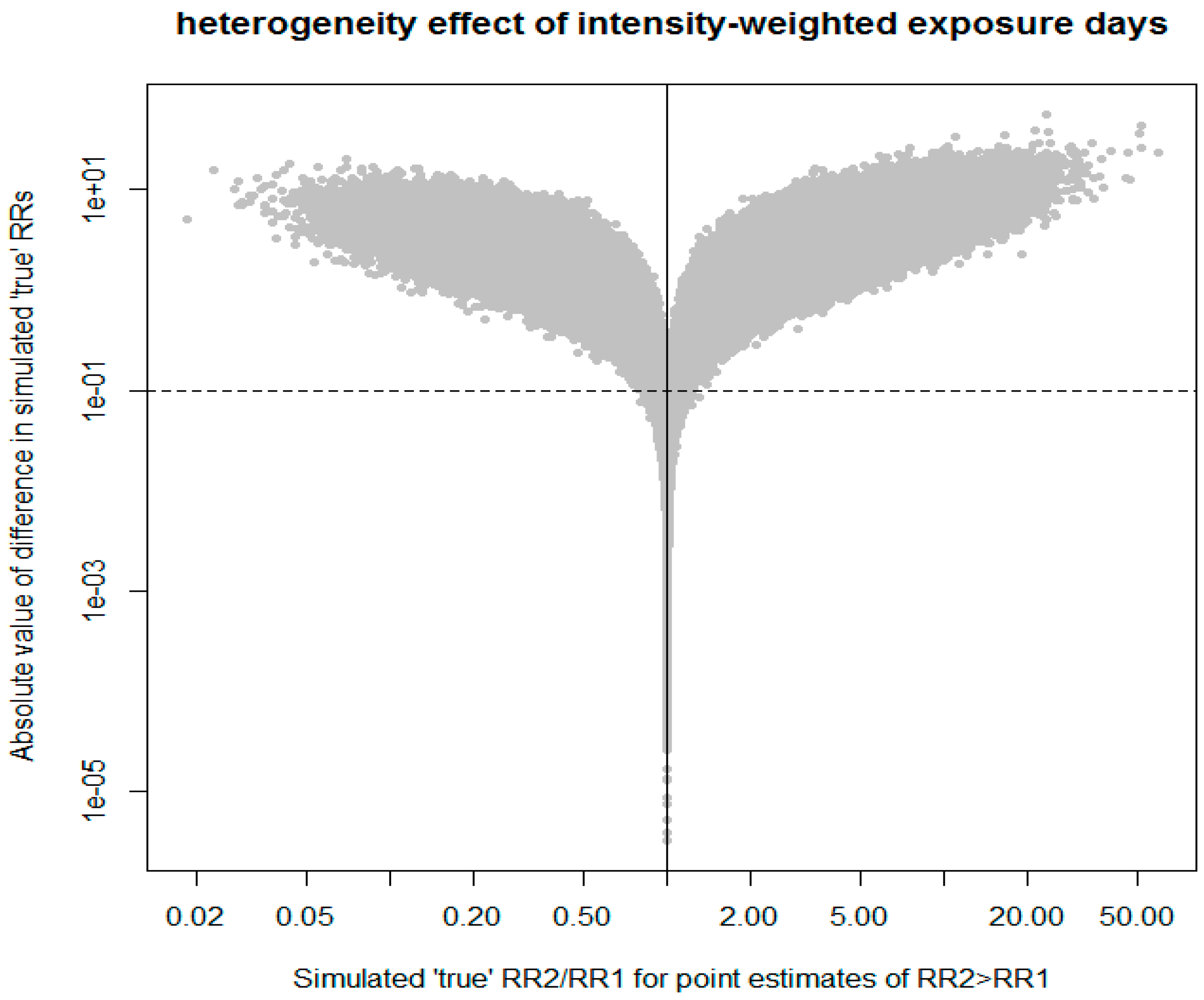
#INPUTS INTO SIMULATION set.seed(585310) #Sorahan T “the highest category ofintensity-weighted exposure days in the fully adjusted model” #RR 1.87, 95% CI 0.67 to 5.27 logrr1=log(1.87) #log-RR1 se11=(log(5.27)-log(0.67))/3.92 #log-SE(RR1) estimated from 95% CI #De Roos highest “Intensity-weighted exposure days” (Table 3 of De Roos et al., 2005) #RR 2.1, 95% CI 0.6 to 7.0 logrr2=log(2.1) se22=(log(7)-log(0.6))/3.92 #SIMULATION #draw samples from each estimate to simulate fix true value of RR #compare if two estimates are consistent with different true values #if we take point estimates to be true values than RR1<RR2 n=1000000 true1=rnorm(n,logrr1,se11) true2=rnorm(n,logrr2,se22) #chance that RR1 is actually bigger than RR2 SmallBigger <- ifelse(true1>true2,1,0) 100*sum(SmallBigger)/n #count virtually identical estimates: differ in 2nd digit of RR. trivial=0.1 rr1<-exp(true1) rr2<-exp(true2) ratio<-rr2/rr1 diff2<-abs(rr1-rr2) same<-ifelse(diff2<trivial, 1,0) 100*sum(same)/n #plots #FIGURE2A plot(ratio, diff2, log=“xy”, xlab=“Simulated ‘true’ RR2/RR1 for point estimates of RR2>RR1”, ylab=“Absolute value of difference in simulated ‘true’ RRs”, cex=1, pch=20, col=“#C0C0C0”) abline(v=1, lty=1) abline(h =trivial, lty=2) title(main=“heterogeneity effect of intensity-weighted exposure days”) #order correct and difference >=trivial right<-ifelse(SmallBigger==0 & same==0,1,0) 100*sum(right)/n
References
- Sorahan, T. Multiple myeloma and glyphosate use: A re-analysis of us agricultural health study (AHS) data. Int. J. Environ. Res. Public Health 2015, 12, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- De Roos, A.J.; Blair, A.; Rusiecki, J.A.; Hoppin, J.A.; Svec, M.; Dosemeci, M.; Sandler, D.P.; Alavanja, M.C. Cancer incidence among glyphosate-exposed pesticide applicators in the agricultural health study. Environ. Health Perspect. 2005, 113, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Acquavella, J.; Garabrant, D.; Marsh, G.; Sorahan, T.; Weed, D.L. Glyphosate epidemiology expert panel review: A weight of evidence systematic review of the relationship between glyphosate exposure and non-Hodgkin’s lymphoma or multiple myeloma. Crit. Rev. Toxicol. 2016, 46, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.S.; MacLehose, R.F. Which of these things is not like the others? Cancer 2013, 119, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- The R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burstyn, I.; De Roos, A.J. Visualizing the Heterogeneity of Effects in the Analysis of Associations of Multiple Myeloma with Glyphosate Use. Comments on Sorahan, T. Multiple Myeloma and Glyphosate Use: A Re-Analysis of US Agricultural Health Study (AHS) Data. Int. J. Environ. Res. Public Health 2015, 12, 1548–1559. Int. J. Environ. Res. Public Health 2017, 14, 5. https://doi.org/10.3390/ijerph14010005
Burstyn I, De Roos AJ. Visualizing the Heterogeneity of Effects in the Analysis of Associations of Multiple Myeloma with Glyphosate Use. Comments on Sorahan, T. Multiple Myeloma and Glyphosate Use: A Re-Analysis of US Agricultural Health Study (AHS) Data. Int. J. Environ. Res. Public Health 2015, 12, 1548–1559. International Journal of Environmental Research and Public Health. 2017; 14(1):5. https://doi.org/10.3390/ijerph14010005
Chicago/Turabian StyleBurstyn, Igor, and Anneclaire J. De Roos. 2017. "Visualizing the Heterogeneity of Effects in the Analysis of Associations of Multiple Myeloma with Glyphosate Use. Comments on Sorahan, T. Multiple Myeloma and Glyphosate Use: A Re-Analysis of US Agricultural Health Study (AHS) Data. Int. J. Environ. Res. Public Health 2015, 12, 1548–1559" International Journal of Environmental Research and Public Health 14, no. 1: 5. https://doi.org/10.3390/ijerph14010005
APA StyleBurstyn, I., & De Roos, A. J. (2017). Visualizing the Heterogeneity of Effects in the Analysis of Associations of Multiple Myeloma with Glyphosate Use. Comments on Sorahan, T. Multiple Myeloma and Glyphosate Use: A Re-Analysis of US Agricultural Health Study (AHS) Data. Int. J. Environ. Res. Public Health 2015, 12, 1548–1559. International Journal of Environmental Research and Public Health, 14(1), 5. https://doi.org/10.3390/ijerph14010005






