Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Urine Sample Collection and Urinary BPA Concentrations Measure
2.3. Statistical Analysis
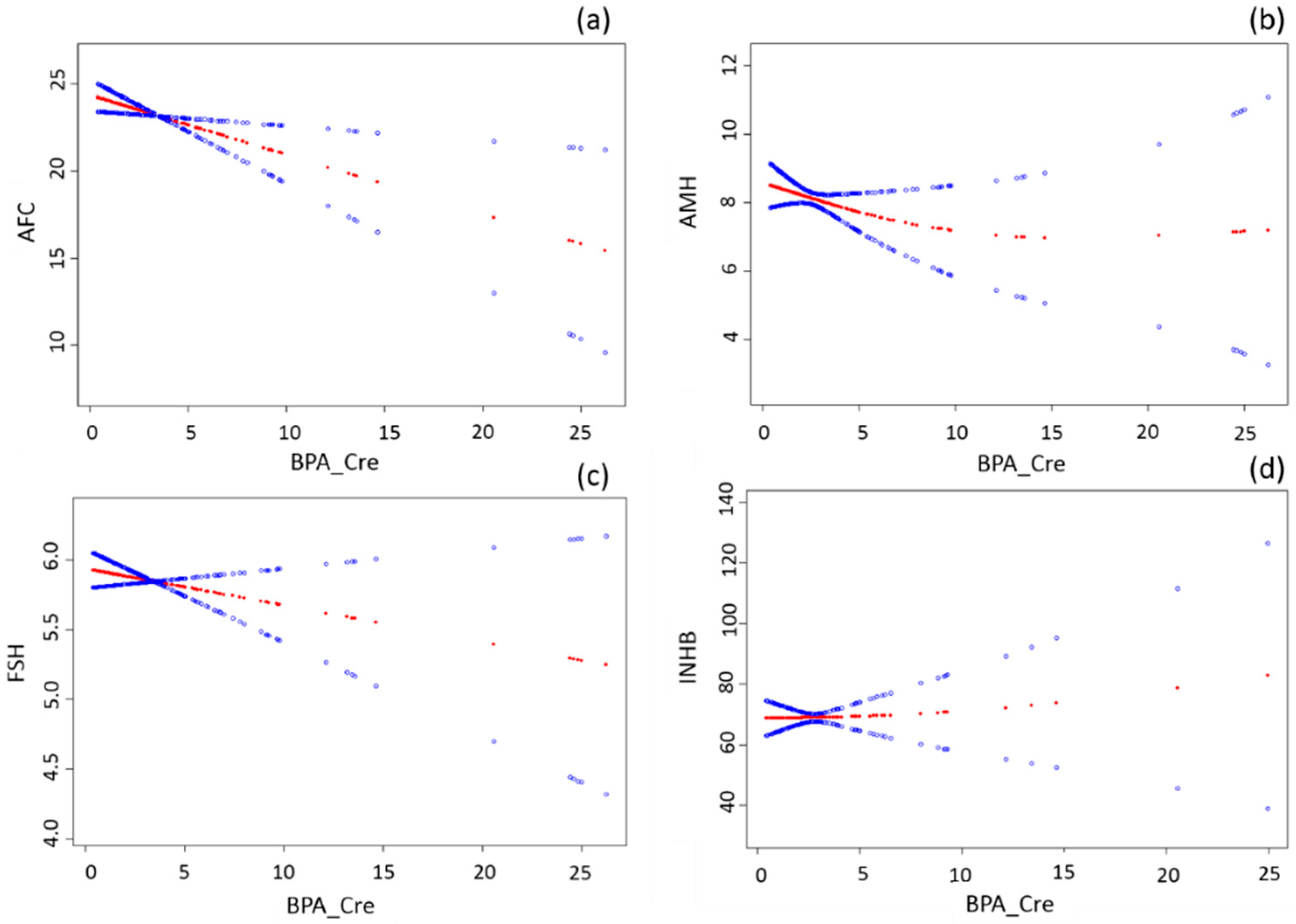
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho, S.M.; Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J.; et al. Female reproductive disorders: The roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Chen, D.; He, Y.; Zhu, W.; Zhou, W.; Zhang, J. Bisphenol A and female infertility: A possible role of gene-environment interactions. Int. J. Environ. Res. Public Health 2015, 12, 11101–11116. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Di Segni, N.; Mallozzi, M.; Giovanale, V.; Mantovani, A.; Marci, R.; Moscarini, M. Bisphenol A and the female reproductive tract: An overview of recent laboratory evidence and epidemiological studies. Reprod. Biol. Endocrinol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cienc. Saude Colet. 2012, 17, 407–434. [Google Scholar] [CrossRef]
- Souter, I.; Smith, K.W.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. The association of bisphenol A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 2013, 42, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, V.Y.; Kim, D.; vom Saal, F.S.; Lamb, J.D.; Taylor, J.A.; Bloom, M.S. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil. Steril. 2011, 95, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Nah, W.H.; Park, M.J.; Gye, M.C. Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin. Exp. Reprod. Med. 2011, 38, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Semiz, O.; Cinar, O. Bisphenol A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol. Hum. Reprod. 2005, 11, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mok-Lin, E.; Ehrlich, S.; Williams, P.L.; Petrozza, J.; Wright, D.L.; Calafat, A.M.; Ye, X.; Hauser, R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 2010, 33, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J. Pay more attention to ethnic differences in polycystic ovary syndrome phenotypic expression. Chin. Med. J. 2013, 126, 2003–2006. [Google Scholar] [PubMed]
- Tarantino, G.; Valentino, R.; Di Somma, C.; D’Esposito, V.; Passaretti, F.; Pizza, G.; Brancato, V.; Orio, F.; Formisano, P.; Colao, A.; et al. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clin. Endocrinol. 2013, 78, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fert. Steril. 2004, 81, 19–25. [Google Scholar]
- Zhao, S.-S.Z.; Tian, Y. Establishment and application of determination method for BPA, TCS and 4-N-NP in urine by HPLC-MS/MS. J. Environ. Occup. Med. 2015, 32, 846–851. [Google Scholar]
- Chao, H.H.; Zhang, X.F.; Chen, B.; Pan, B.; Zhang, L.J.; Li, L.; Sun, X.F.; Shi, Q.H.; Shen, W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem. Cell Biol. 2012, 137, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.A.; Santambrosio, N.; Santamaria, C.G.; Munoz-de-Toro, M.; Luque, E.H. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 2010, 30, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Rivera, O.E.; Varayoud, J.; Rodriguez, H.A.; Munoz-de-Toro, M.; Luque, E.H. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod. Toxicol. 2011, 32, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Couse, J.F.; Padilla-Banks, E.; Korach, K.S.; Newbold, R.R. Neonatal exposure to genistein induces estrogen receptor (ER) α expression and multioocyte follicles in the maturing mouse ovary: Evidence for ERB-mediated and nonestrogenic actions. Biol. Reprod. 2002, 67, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Liu, J.; Wang, W.; Li, H.; Zhu, J.; Weng, S.; Xiao, S.; Wu, T. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod. Toxicol. 2014, 44, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Sugiura, K.; Eppig, J.J. Mouse oocyte control of granulosa cell development and function: Paracrine regulation of cumulus cell metabolism. Semin. Reprod. Med. 2009, 27, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Osuga, Y.; Yano, T.; Morita, Y.; Tang, X.; Fujiwara, T.; Takai, Y.; Matsumi, H.; Koga, K.; Taketani, Y.; et al. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem. Biophys. Res. Commun. 2002, 292, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Ye, X.; Calafat, A.M.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum. Reprod. 2012, 27, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, J.; Liao, L.; Han, S.; Liu, J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008, 283, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cain, L.; Chatterjee, S.; Collins, T.J. In vitro folliculogenesis of rat preantral follicles. Endocrinology 1995, 136, 3369–3377. [Google Scholar] [PubMed]
| Characteristic | AFC (n = 215) | AMH (n = 257) | FSH (n = 250) | INHB (n = 131) |
|---|---|---|---|---|
| Age (years) | 27 (25, 31) | 27 (25, 30) | 27 (25, 30) | 28 (26, 32) |
| BMI (kg/m2) | 25.4 (22.5, 27.7) | 25.4 (22.1, 28.2) | 25.4 (22.1, 28.3) | 25.4 (22.0, 28.3) |
| Income (103 RMB/person/year) (n, (%)) | ||||
| <10 | 43 (20) | 53 (21) | 52 (21) | 22 (17) |
| 10–30 | 112 (52) | 138 (54) | 134 (54) | 66 (50) |
| 30–50 | 32 (15) | 37 (14) | 35 (14) | 25 (19) |
| 50–100 | 14 (6) | 13 (6) | 14 (6) | 9 (7) |
| >100 | 6 (3) | 6 (2) | 6 (2) | 5 (4) |
| Refuse to answer | 8 (4) | 9 (3) | 9 (3) | 4 (3) |
| BPA (ng/mL) | 2.3 (1.1, 3.8) | 2.1 (1.1, 3.8) | 2.1 (1.1, 3.8) | 2.3 (1.1, 3.7) |
| BPA_Cre (ng/g) | 2.2 (1.4, 3.9) | 2.2 (1.4, 3.7) | 2.2 (1.4, 3.9) | 2.2 (1.3, 3.5) |
| AFC | 23 (18, 28) | |||
| AMH (ng/mL) | 6.7 (4.1, 11.7) | |||
| FSH (IU/L) | 5.8 (5.1, 6.6) | |||
| INHB (pg/mL) | 66.2 (42.7, 87.8) | |||
| Indicators | n | Crude β (95% CI) | p-Value | Adjusted β (95% CI) b | p-Value |
|---|---|---|---|---|---|
| AFC | 215 | −0.32 (−0.57, −0.07) | 0.01 | −0.34 (−0.60, −0.08) | 0.01 |
| AMH | 257 | −0.06 (−0.21, 0.08) | 0.39 | −0.08 (−0.22, 0.07) | 0.30 |
| FSH | 250 | −0.02 (−0.06, 0.02) | 0.23 | −0.03 (−0.07, 0.01) | 0.20 |
| INHB | 131 | 0.48 (−1.30, 2.27) | 0.59 | 0.42 (−1.41, 2.25) | 0.65 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Fang, F.; Zhu, W.; Chen, Z.-J.; Du, Y.; Zhang, J. Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. Int. J. Environ. Res. Public Health 2017, 14, 18. https://doi.org/10.3390/ijerph14010018
Zhou W, Fang F, Zhu W, Chen Z-J, Du Y, Zhang J. Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. International Journal of Environmental Research and Public Health. 2017; 14(1):18. https://doi.org/10.3390/ijerph14010018
Chicago/Turabian StyleZhou, Wei, Fang Fang, Wenting Zhu, Zi-Jiang Chen, Yanzhi Du, and Jun Zhang. 2017. "Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome" International Journal of Environmental Research and Public Health 14, no. 1: 18. https://doi.org/10.3390/ijerph14010018
APA StyleZhou, W., Fang, F., Zhu, W., Chen, Z.-J., Du, Y., & Zhang, J. (2017). Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. International Journal of Environmental Research and Public Health, 14(1), 18. https://doi.org/10.3390/ijerph14010018






