Assessing the Contribution of the Environmental Parameters to Eutrophication with the Use of the “PaD” and “PaD2” Methods in a Hypereutrophic Lake
Abstract
:1. Introduction
2. Materials and Methods
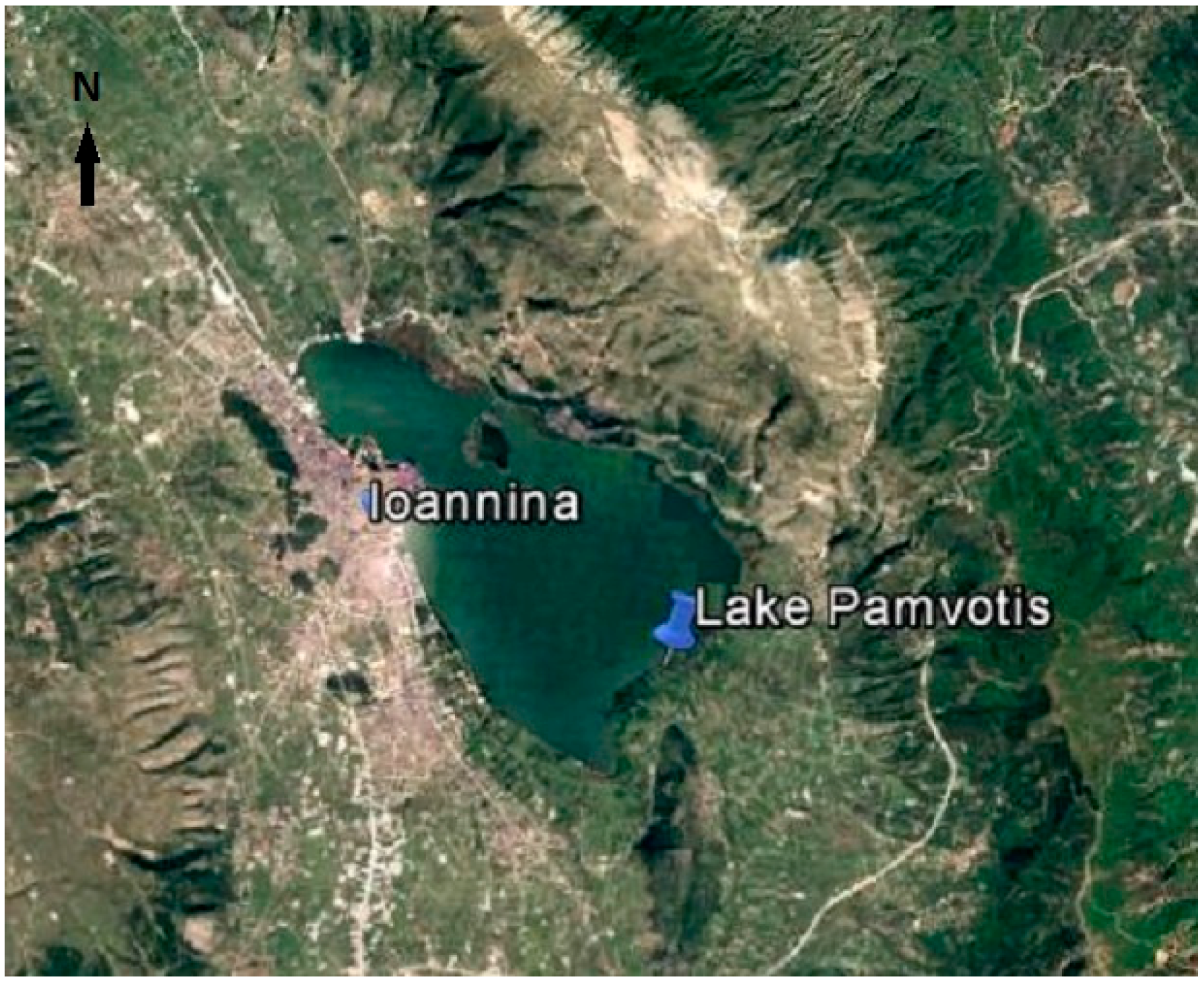
2.1. Study Area and Data Collection
2.2. ANNs Methodologies
2.3. ANN Model Development
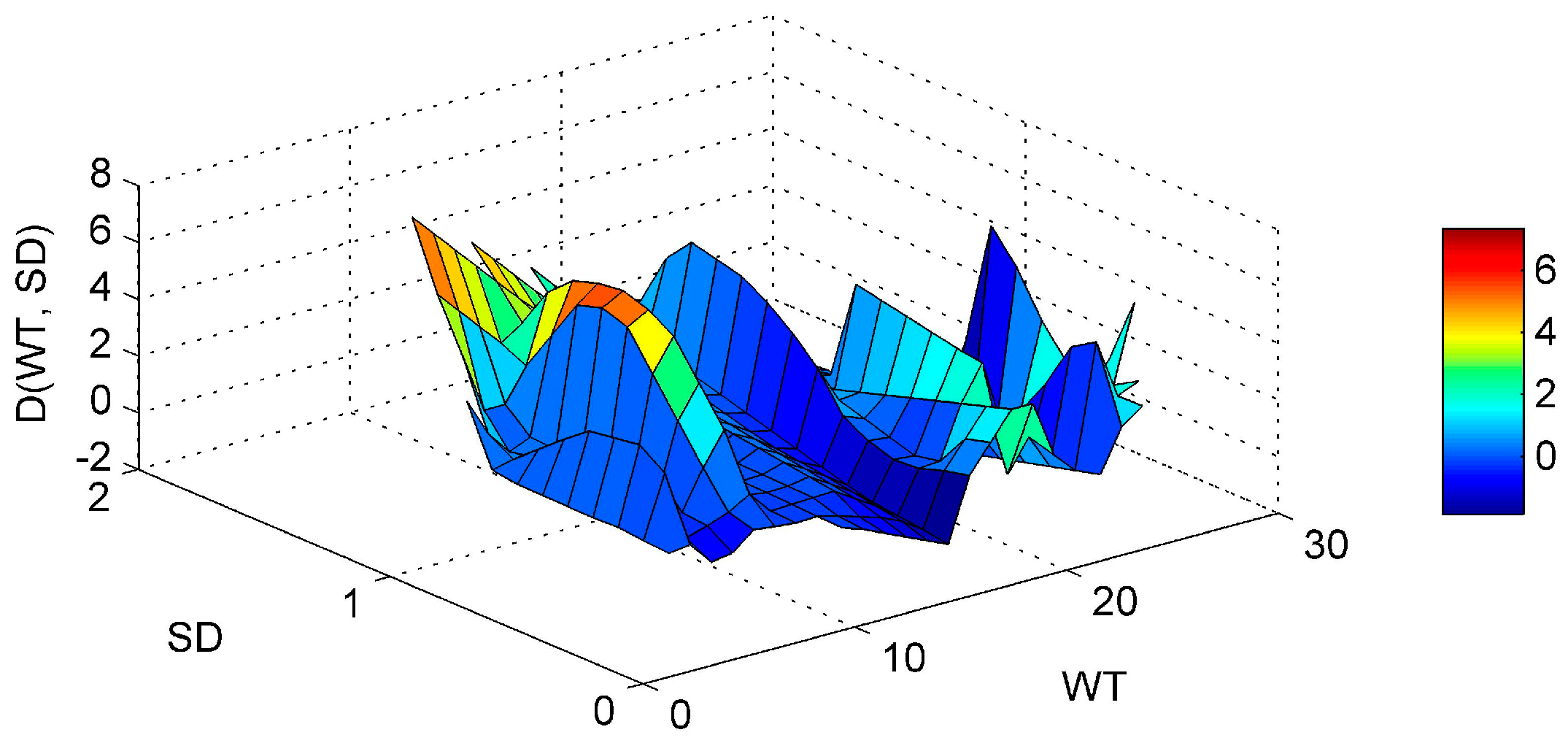
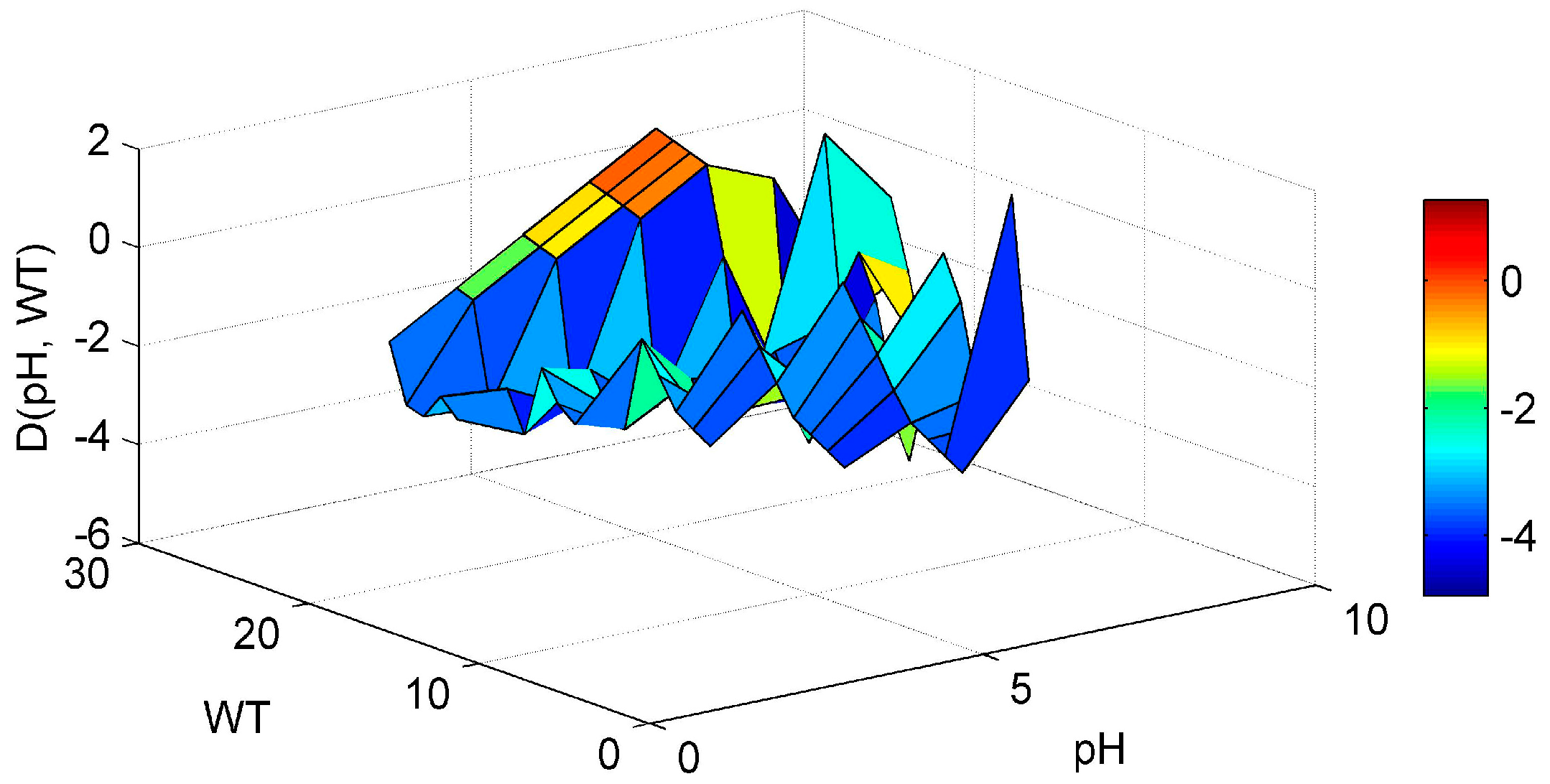
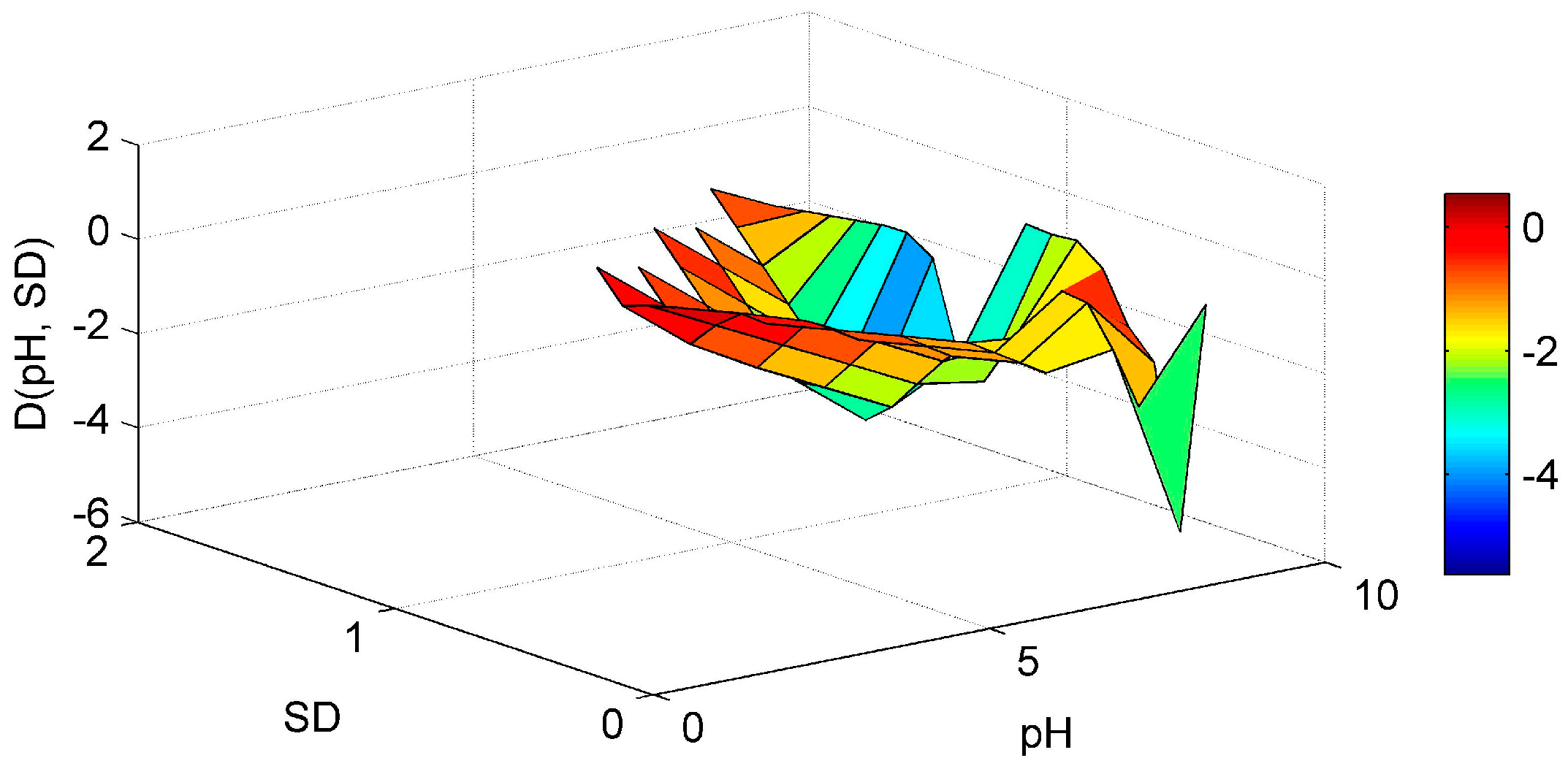
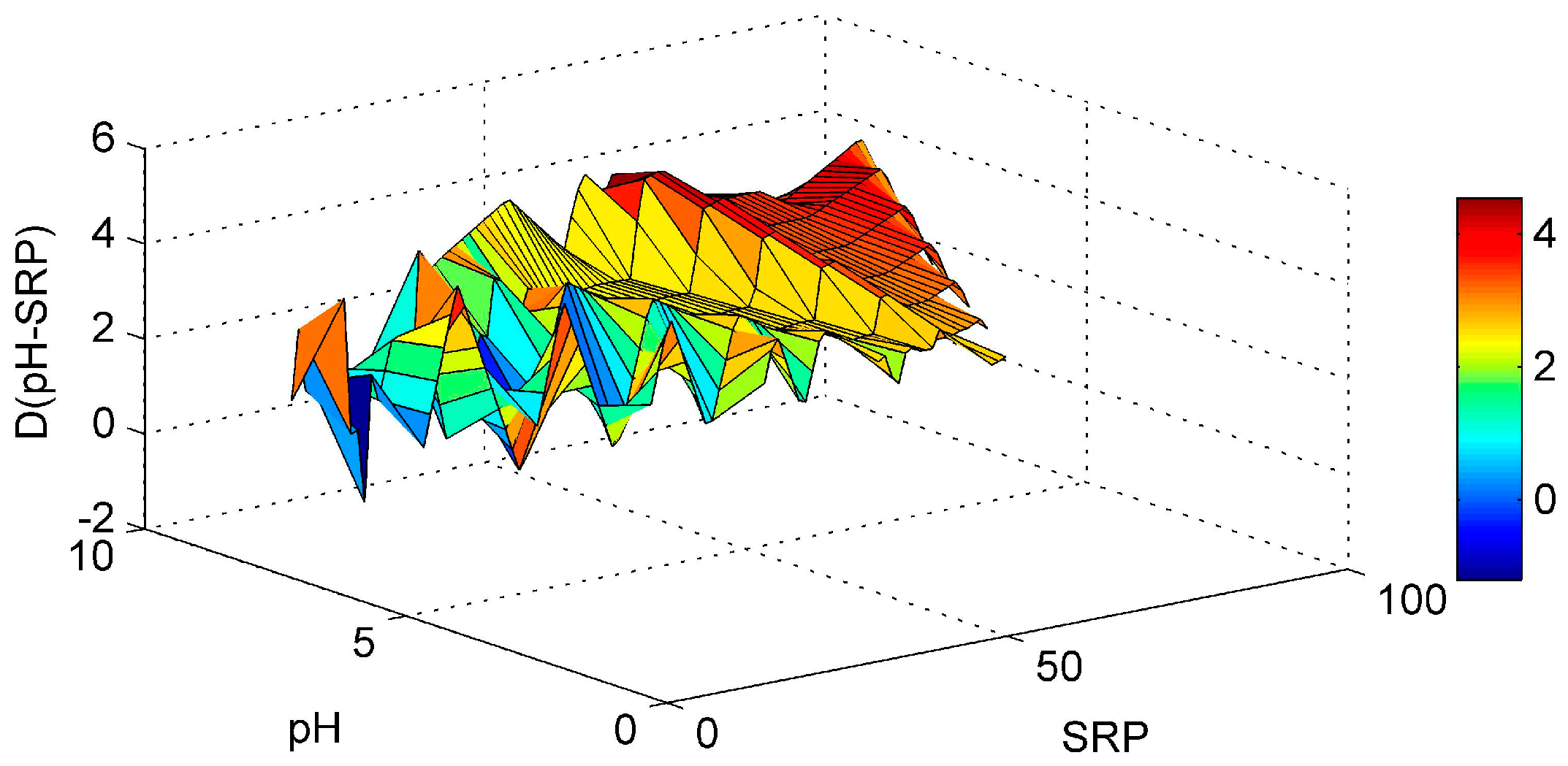
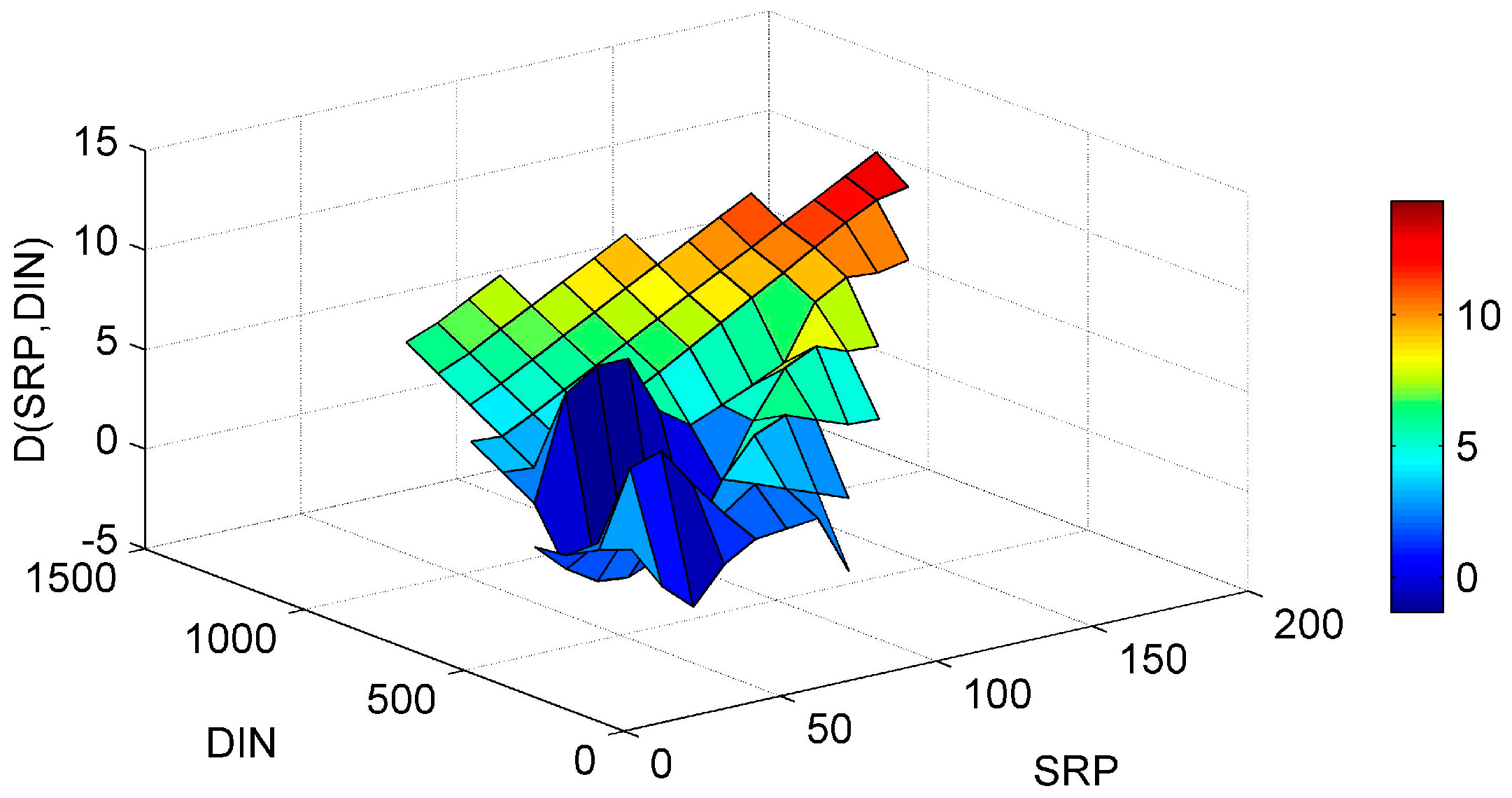
3. Results and Discussion
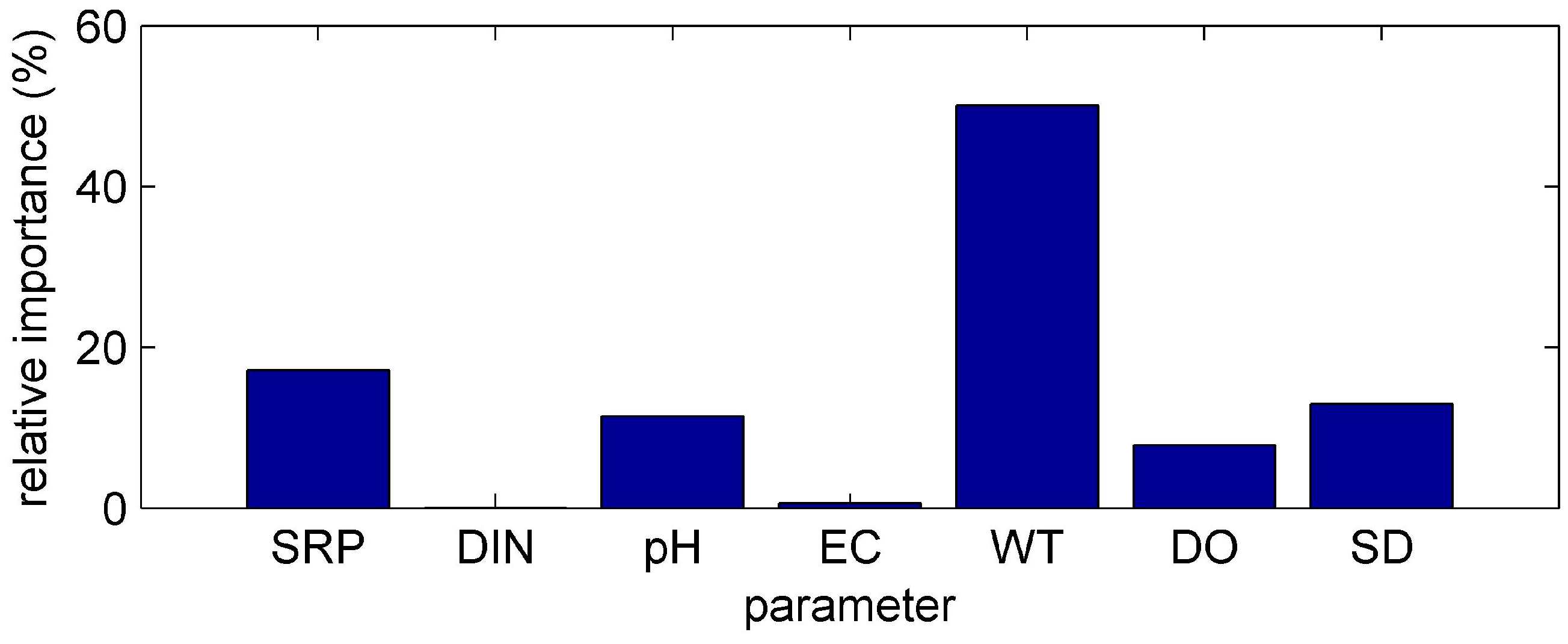
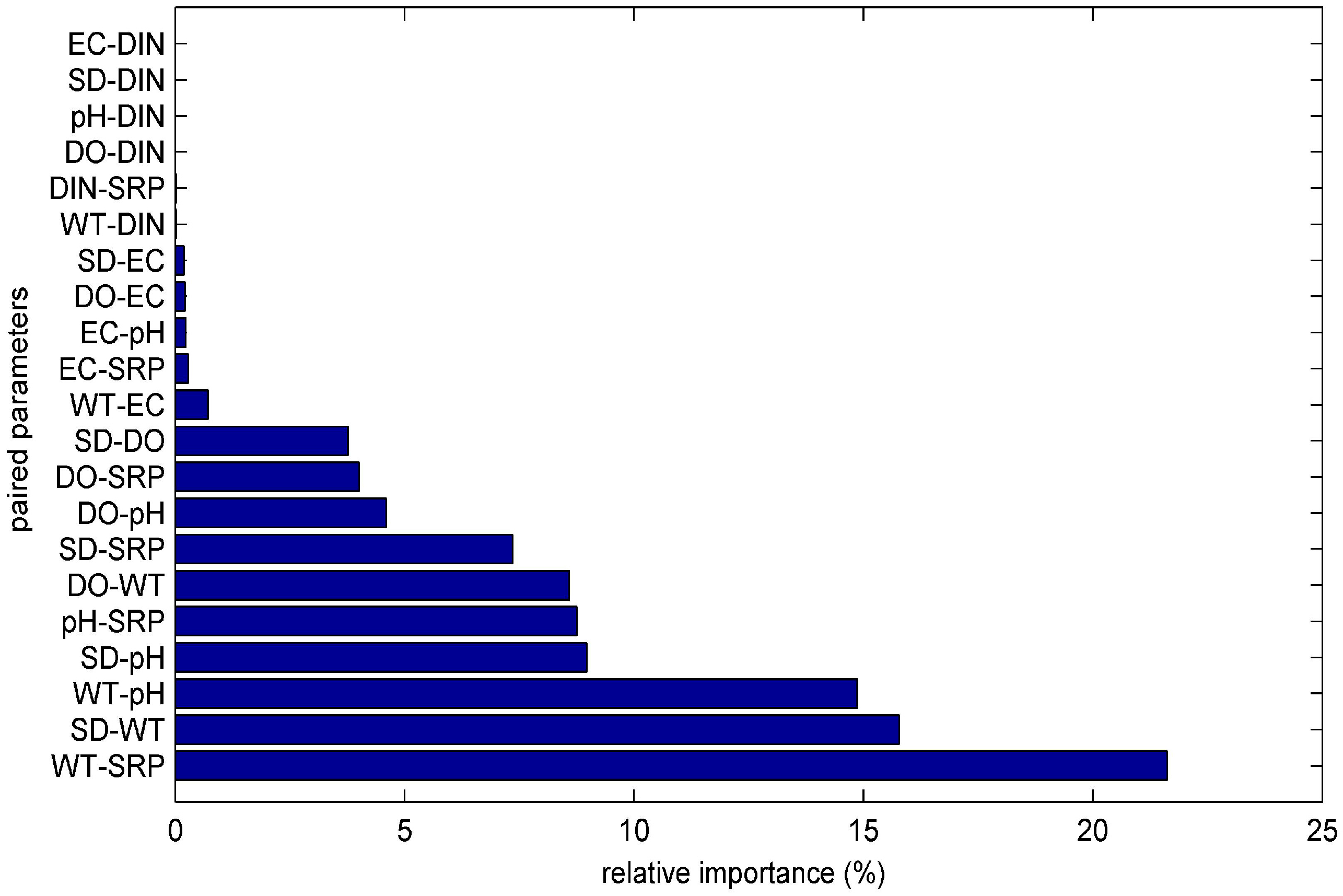
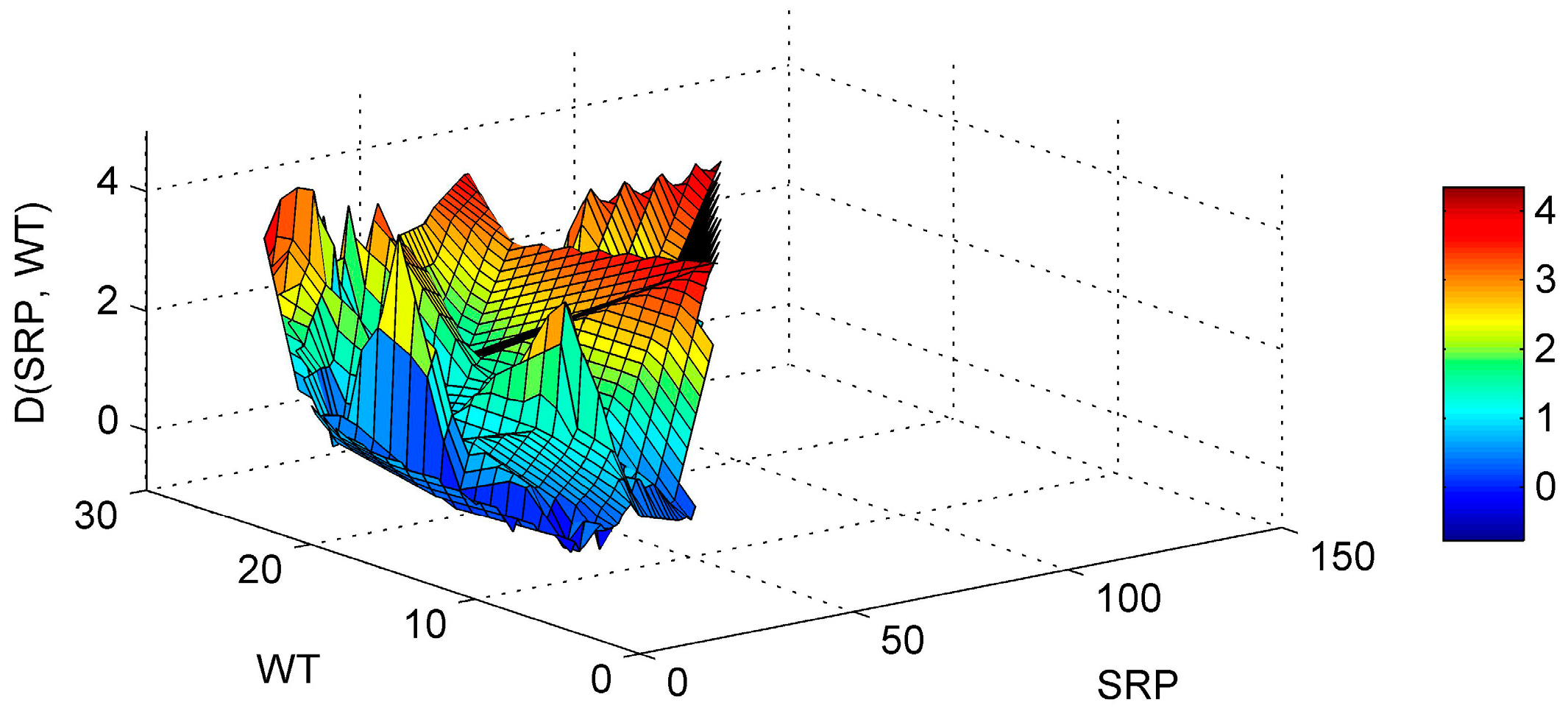
3.1. ANN’s Simulation Results
3.2. Implications for Management and Restoration
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| SRP | Soluble Reactive Phosphorus |
| DIN | Dissolved Inorganic Nitrogen |
| EC | Electrical Conductivity |
| WT | Water Temperature |
| DO | Dissolved Oxygen |
| SD | Secchi Disk |
| RE | Absolute Relative Error |
| R2 | Coefficient of Determination |
| HAB | Harmful Algal Bloom |
References
- Ferreira, J.G.; Bricker, S.B.; Simas, T.C. Application and sensitivity testing of a eutrophication assessment method on coastal systems in the United States and European Union. J. Environ. Manag. 2007, 82, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T. Molecular approaches to diagnosing nutritional physiology in harmful algae: Implications for studying the effects of eutrophication. Harmful Algae 2008, 8, 167–174. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Andersen, J.H.; Borja, A.; Bricker, S.B.; Camp, J.; Cardoso da Silva, M.; Garcés, E.; Heiskanen, A.S.; Humborg, C.; Ignatiades, L.; et al. Overview of eutrophication indicators to assess environmental status within the European Marine Strategy Framework Directive. Estuar. Coast. Shelf Sci. 2011, 93, 117–131. [Google Scholar] [CrossRef]
- Ha, M.H.; Pflugmacher, S. Phytotoxic effects of the cyanobacterial neurotoxin anatoxin-a: Morphological, physiological and biochemical responses in aquatic macrophyte, Ceratophyllum demersum. Toxicon 2013, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, S.J.; Hitzfeld, B.C.; Dietrich, D.R. Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol. Appl. Pharmacol. 2005, 203, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, W.; Li, D.; Huang, Z.; Shen, Y.; Liu, Y. Cyanobacteria-/cyanotoxin-contaminations and eutrophication status before Wuxi Drinking Water Crisis in Lake Taihu, China. J. Environ. Sci. 2011, 23, 575–581. [Google Scholar] [CrossRef]
- Koreiviene, J.; Anne, O.; Kasperoviciene, J.; Burskyte, V. Cyanotoxin management and human health risk mitigation in recreational waters. Environ. Monit. Assess. 2014, 186, 4443–4459. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Oliveri Conti, G.; Fiore, M.; Rapisarda, V. Harmful algal blooms in the Mediterranean Sea: Effects on human health. Euromediterr. Biomed. J. 2013, 8, 25–34. [Google Scholar] [CrossRef]
- Gubelit, Y.I.; Berezina, N.A. The causes and consequences of algal blooms: The Cladophora glomerata bloom and the Neva estuary (Eastern Baltic Sea). Mar. Pollut. Bull. 2010, 61, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Atoui, A.; Hafez, H.; Slim, K. Occurrence of toxic cyanobacterial blooms for the first time in Lake Karaoun, Lebanon. Water Environ. 2013, 27, 42–49. [Google Scholar] [CrossRef]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L. Eutrophication of U.S. freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W. Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: Interactive effects of human and climatic perturbations. Ecol. Eng. 2006, 26, 40–54. [Google Scholar] [CrossRef]
- Xu, F.L.; Tao, S.; Dawson, R.W.; Li, B.G. A GIS-based method of lake eutrophication assessment. Ecol. Model. 2001, 144, 231–244. [Google Scholar] [CrossRef]
- Lek, S.; Guégan, J.F. Artificial Neural Networks as a tool in ecological modelling, an introduction. Ecol. Model. 1999, 120, 65–73. [Google Scholar] [CrossRef]
- Papatheodorou, G.; Demopoulou, G.; Lambrakis, N. A long-term study of temporal hydrochemical data in a shallow lake using multivariate statistical techniques. Ecol. Model. 2006, 193, 759–776. [Google Scholar] [CrossRef]
- Kagalou, I.; Papastergiadou, E.; Leonardos, I. Long term changes in the eutrophication process in a shallow Mediterranean Lake ecosystem of W. Greece: Response after the reduction of external load. J. Environ. Manag. 2008, 87, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Papastergiadou, E.; Kagalou, I.; Stefanidis, K.; Retalis, A.; Leonardos, I. Effects of anthropogenic Influences on the trophic state, land uses and aquatic vegetation in a shallow Mediterranean Lake: Implications for restoration. Water Resour. Manag. 2010, 24, 415–435. [Google Scholar] [CrossRef]
- Gkelis, S.; Papadimitriou, T.; Zaoutsos, N.; Leonardos, I. Anthropogenic and climatic-induced change favors toxic cyanobacterial blooms: Evidence from monitoring a highly eutrophic, urban Mediterranean Lake. Harmful Algae 2014, 39, 322–333. [Google Scholar] [CrossRef]
- Kagalou, I.; Papastergiadou, E.; Tsimarakis, G.; Petridis, D. Evaluation of the trophic state of Lake Pamvotis Greece, a shallow urban lake. Hydrobiologia 2003, 506, 745–752. [Google Scholar] [CrossRef]
- Stefanidis, K.; Papastergiadou, E.S. Aquatic vegetation and related abiotic environment in a shallow urban lake of Greece. Belg. J. Bot. 2007, 140, 25–38. [Google Scholar]
- Hu, Y.H.; Hwang, J.N. Handbook of Neural Network Signal Processing; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kuo, J.T.; Hsieh, M.H.; Lung, W.S.; She, N. Using Artificial Neural Network for reservoir eutrophication prediction. Ecol. Model. 2007, 200, 171–177. [Google Scholar] [CrossRef]
- Kazemi Yazdi, S.; Scholz, M. Assessing the storm water detention systems treating road runoff with an Artificial Neural Network predicting fecal indicator organisms. Water Air Soil Pollut. 2010, 206, 35–47. [Google Scholar] [CrossRef]
- Salami Shahid, E.; Ehteshami, M. Application of Artificial Neural Networks to estimating DO and salinity in San Joaquin River Basin. Desalination Water Treat. 2016, 57, 4888–4897. [Google Scholar] [CrossRef]
- Wang, L.; Kisi, O.; Zounemat-Kermani, M.; Hu, B.; Gong, W. Modeling and Comparison of hourly photosynthetically active radiation in different ecosystems. Renew. Sustain. Energy Rev. 2016, 56, 436–453. [Google Scholar] [CrossRef]
- Karul, C.; Soyupak, S.; Yurteri, C. Neural network models as a management tool in lakes. Hydrobiologia 1999, 408, 139–144. [Google Scholar] [CrossRef]
- Demuth, H.; Beale, M.; Hagan, M. Neural Network Toolbox User’s Guide; MathWorks, Inc.: Natick, MA, USA, 2007. [Google Scholar]
- Maier, H.R.; Dandy, G.C.; Burch, M.D. Use of Artificial Neural Networks for modelling cyanobacteria Anabaena spp. in the River Murray, South Australia. Ecol. Model. 1998, 105, 257–272. [Google Scholar] [CrossRef]
- Özesmi, S.L.; Tan, C.O.; Özesmi, U. Methodological issues in building, training, and testing artificial neural networks in ecological applications. Ecol. Model. 2006, 195, 83–93. [Google Scholar] [CrossRef]
- Karul, C.; Soyupak, S.; Çilesiz, A.F.; Akbay, N.; Germen, E. Case studies on the use of neural networks in eutrophication modeling. Ecol. Model. 2000, 134, 145–152. [Google Scholar] [CrossRef]
- Dimopoulos, I.; Bourret, P.; Lek, S. Use of some sensitivity criteria for choosing networks with good generalization ability. Neural Process. Lett. 1995, 2, 1–4. [Google Scholar] [CrossRef]
- Gevrey, M.; Dimopoulos, I.; Sovan, L. Review and comparison of methods to study the contribution of variables in Artificial Neural Networks models. Ecol. Model. 2003, 160, 249–264. [Google Scholar] [CrossRef]
- Gevrey, M.; Dimopoulos, I.; Lek, S. Two-way interaction of input variables in the sensitivity analysis of neural network models. Ecol. Model. 2006, 195, 43–50. [Google Scholar] [CrossRef]
- Van Maanen, B.; Coco, G.; Bryan, K.R.; Ruessink, B.G. The use of Artificial Neural Networks to analyze and predict alongshore sediment transport. Nonlinear Process. Geophys. 2010, 17, 395–404. [Google Scholar] [CrossRef]
- Zounemat-Kermani, M. Principal Component Analysis (PCA) for estimating chlorophyll concentration using forward and generalized regression neural networks. Appl. Artif. Intell. 2014, 28, 16–29. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, B.; He, L.; Cheng, F.; Zhao, P.; Wei, C.; Liu, Y. Temporal and spatial simulation of atmospheric pollutant PM2.5 changes and risk assessment on population exposure to pollution using optimization algorithms of the back propagation-Artificial Neural Network model and GIS. Int. J. Environ. Res. Public Health 2015, 12, 12171–12195. [Google Scholar] [CrossRef] [PubMed]
- Muttil, N.; Chau, K.W. Machine-learning paradigms for selecting ecologically significant input variables. Eng. Appl. Artif. Intell. 2007, 20, 735–744. [Google Scholar] [CrossRef]
- Scardi, M.; Harding, L.W., Jr. Developing an empirical model of phytoplankton primary production: A neural network case study. Ecol. Model. 1999, 120, 213–223. [Google Scholar] [CrossRef]
- Tota-Maharaj, K.; Scholz, M. Artificial Neural Network simulation of combined permeable pavement and earth energy systems treating storm water. J. Environ. Eng. 2012, 138, 499–509. [Google Scholar] [CrossRef]
- Sener, E.; Terzi, O.; Sener, S.; Kucukkara, R. Modeling of water temperature based on GIS and ANN techniques: Case study of Lake Egirdir (Turkey). Ekoloji 2012, 21, 44–52. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, G.; Li, H.; Luo, L.; Cheng, X.; Jin, Y.; Trolle, D. Modelling the response of phytoplankton to reduced external nutrient load in a subtropical Chinese reservoir using DYRESM-CAEDYM. Lake Reserv. Manag. 2016, 32, 146–157. [Google Scholar] [CrossRef]
- Dedecker, A.; Goethals, P.; D’Heygere, T.; Gevrey, M.; Lek, S.; De Pauw, N. Application of Artificial Neural Network models to analyse the relationships between Gammarus Pulex L. (Crustacea, Amphipoda) and river characteristics. Environ. Monit. Assess. 2005, 111, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Trolle, D.; Staehr, P.A.; Davidson, T.A.; Bjerring, R.; Lauridsen, T.L.; Søndergaard, M.; Jeppesen, E. Seasonal dynamics of CO2 flux across the surface of shallow temperate lakes. Ecosystems 2012, 15, 336–347. [Google Scholar] [CrossRef]
- LaBounty, J.F. Secchi transparency of Boulder Basin, Lake Mead, Arizona-Navada: 1990–2007. Lake Reserv. Manag. 2008, 24, 207–218. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Sagrario, M.A.G.; Jeppesen, E.; Gomà, J.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.; Landkildehus, F. Does high nitrogen loading prevent clear-water conditions in shallow lakes at moderately high phosphorus concentrations? Freshw. Biol. 2005, 50, 27–41. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Olesen, J.E.; Audet, J.; Sondergaard, M.; Hoffmann, C.C.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Larsen, S.E.; et al. Climatic change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 2011, 663, 1–21. [Google Scholar] [CrossRef]
- Mateus, M.; Almeida, C.; Brito, D.; Neves, R. From eutrophic to mesotrophic: Modelling watershed management scenarios to change the trophic status of a reservoir. Int. J. Environ. Res. Public Health 2014, 11, 3015–3031. [Google Scholar] [CrossRef] [PubMed]
- Feuchtmayr, H.; Moran, R.; Hatton, K.; Connor, L.; Heyes, T.; Moss, B.; Harvey, I.; Atkinson, D. Global warming and eutrophication: Effects on water chemistry and autotrophic communities in experimental hypertrophic shallow lake mesocosms. J. Appl. Ecol. 2009, 46, 713–723. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Ye, C.; Shen, Z.; Zhang, T.; Fan, M.; Lei, Y.; Zhang, J. Long term effect of nutrients and temperature increase on algal growth in Lake Taihu, China. J. Environ. Sci. 2011, 23, 222–227. [Google Scholar] [CrossRef]
- Mooij, W.M.; De Senerpont Domis, L.N.; Janse, J.H. Linking species- and ecosystem-level impacts of climate change in lakes with a complex and a minimal model. Ecol. Model. 2009, 220, 3011–3020. [Google Scholar] [CrossRef]
- Guneralp, B.; Barlas, Y. Dynamic modelling of a shallow freshwater lake for ecological and economic sustainability. Ecol. Model. 2003, 167, 115–138. [Google Scholar] [CrossRef]
- Sagehashi, M.; Sakoda, A.; Suzuki, M. A mathematical model of a shallow and eutrophic lake (the Keszthely Basin, Lake Balaton) and simulation of restorative manipulations. Water Res. 2001, 35, 1675–1686. [Google Scholar] [CrossRef]
| Model Performance | R2 | RE |
|---|---|---|
| Training set | 0.76 | 0.57 |
| Test set | 0.82 | 0.64 |
| Whole set | 0.77 | 0.59 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjisolomou, E.; Stefanidis, K.; Papatheodorou, G.; Papastergiadou, E. Assessing the Contribution of the Environmental Parameters to Eutrophication with the Use of the “PaD” and “PaD2” Methods in a Hypereutrophic Lake. Int. J. Environ. Res. Public Health 2016, 13, 764. https://doi.org/10.3390/ijerph13080764
Hadjisolomou E, Stefanidis K, Papatheodorou G, Papastergiadou E. Assessing the Contribution of the Environmental Parameters to Eutrophication with the Use of the “PaD” and “PaD2” Methods in a Hypereutrophic Lake. International Journal of Environmental Research and Public Health. 2016; 13(8):764. https://doi.org/10.3390/ijerph13080764
Chicago/Turabian StyleHadjisolomou, Ekaterini, Konstantinos Stefanidis, George Papatheodorou, and Evanthia Papastergiadou. 2016. "Assessing the Contribution of the Environmental Parameters to Eutrophication with the Use of the “PaD” and “PaD2” Methods in a Hypereutrophic Lake" International Journal of Environmental Research and Public Health 13, no. 8: 764. https://doi.org/10.3390/ijerph13080764
APA StyleHadjisolomou, E., Stefanidis, K., Papatheodorou, G., & Papastergiadou, E. (2016). Assessing the Contribution of the Environmental Parameters to Eutrophication with the Use of the “PaD” and “PaD2” Methods in a Hypereutrophic Lake. International Journal of Environmental Research and Public Health, 13(8), 764. https://doi.org/10.3390/ijerph13080764









