Potential of Endophytic Bacterium Paenibacillus sp. PHE-3 Isolated from Plantago asiatica L. for Reduction of PAH Contamination in Plant Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of PAH-Degrading Endophytic Bacteria
2.2. Identification of PAH-Degrading Endophytic Bacteria
2.3. Biodegradation of PAHs by Endophytic Bacterium
2.3.1. Capacity of Degrading Phenanthrene
2.3.2. Activities of Catechol 2,3-dioxygenase
2.3.3. Capabilities of Degrading Other PAHs
Capabilities of Degrading Other PAHs Separately
Capacities of Degrading a Mixture of PAHs
2.4. Effects of Additional Nutrients on Phenanthrene Degradation
2.5. Detection of PAH Residues by HPLC
2.6. Statistical Analyses
3. Results and Discussion
3.1. Isolation and Identification of Strain PHE-3
3.2. Biodegradation of Phenanthrene by Strain PHE-3
3.3. Catechol 2,3-dioxygenase Activity
3.4. Biodegradation of Other PAHs
3.5. Optimal Environmental Conditions for Biodegradation of Phenanthrene
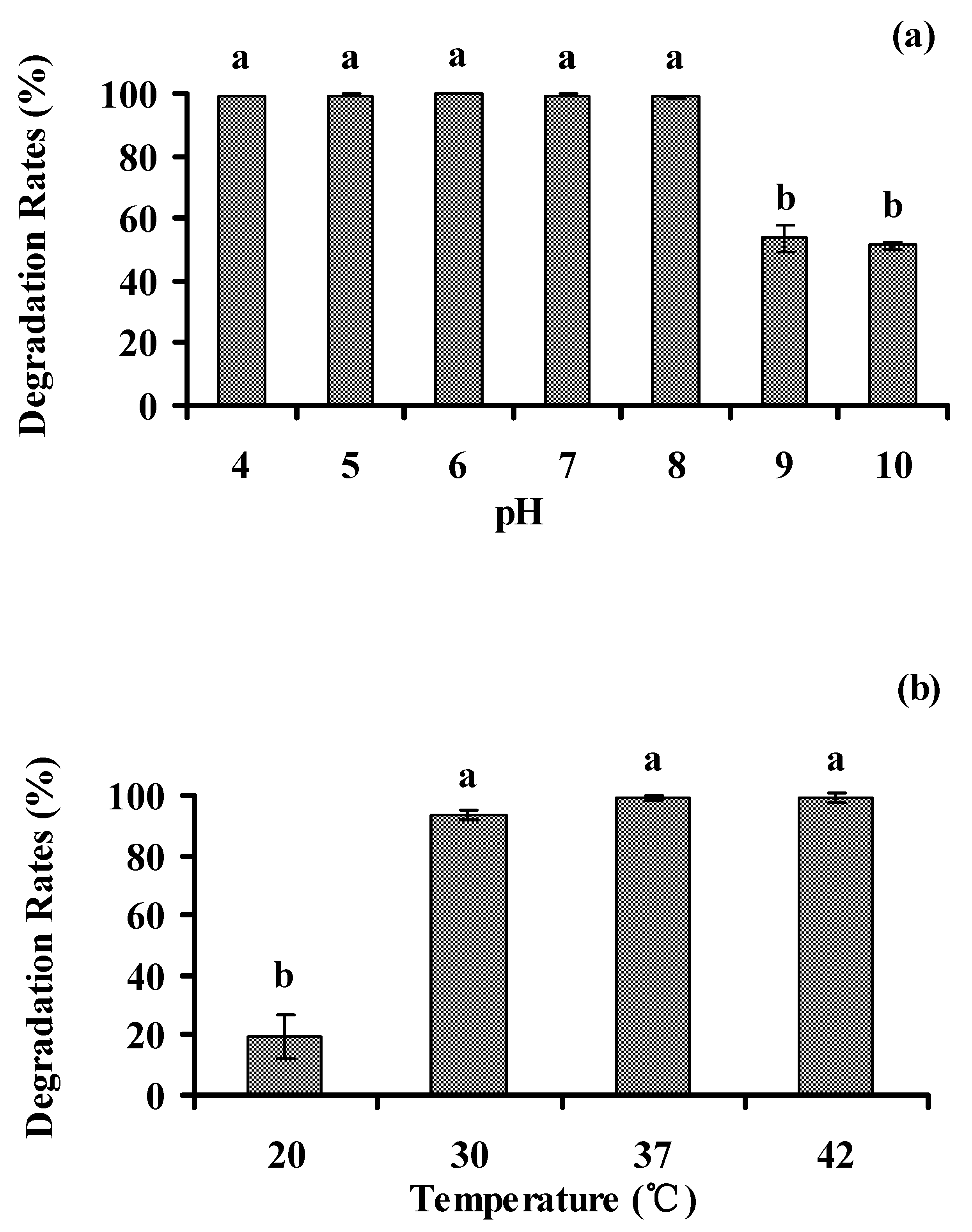
3.5.1. pH and Temperature
3.5.2. Additional Carbon and Nitrogen Nutrients
3.6. Possible Application for PAH-Degrading Endophytic Bacteria
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanaly, R.A.; Harayama, S. Advances in the field of high molecular-weight polycyclic aromatic hydrocarbon biodegradation by bacteria. Microb. Biotechnol. 2010, 3, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, G.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef]
- Su, D.; Li, P.J.; Frank, S.; Xiong, X.H. Biodegradation of benzo[a]pyrene in soil by Mucor sp. SF06 and Bacillus sp. SB02 co-immobilized on vermiculite. J. Environ. Sci. 2006, 18, 1204–1209. [Google Scholar] [CrossRef]
- Wu, Y.R.; Luo, Z.H.; Vrijmoed, L.L.P. Biodegradation of anthracene and benz[a]anthracene by two Fusarium solani strains isolated from mangrove sediments. Bioresour. Technol. 2010, 101, 9666–9672. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.W.; Gao, D.W.; Zhang, B.; Liang, H. Co-metabolic degradation of pyrene by indigenous white-rot fungus Pseudotrametes gibbosa from the northeast China. Int. Biodeterior. Biodegrad. 2011, 65, 600–604. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Krechel, A.; Ditz, M.; Sikora, R.A.; Ulrich, A.; Hallmann, J. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microb. Ecol. 2005, 51, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Liu, L.; Jin, L.; Gao, Y.Z. Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination. Plant Soil 2014, 374, 251–262. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Gao, Y.; Jin, L.; Gu, Y.; Wang, W. Isolation, plant colonization potential, and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6-gfp. Sci. Rep. 2014, 4, 5462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.Z.; Varma, A.; Qin, S.; Xiong, Z.; Huang, H.Y.; Zhu, W.Y.; Zhao, L.X.; Xu, L.H.; Zhang, S.; et al. An Endophytic Pseudonocardia Species Induces the Production of Artemisinin in Artemisia annua. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Afyuni, M.; Hajabbasi, M.A.; Nourbakhsh, F.; Sabzalian, M.R.; Christensen, J.H. Phytoremediation of an aged petroleum contaminated soil using endophyte infected and non-infected grasses. Chemosphere 2010, 81, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Parellada, E.A.; Ramos, A.N.; Ferrero, M.; Cartagena, E.; Bardon, A.; Neske, A. Effect of the annonaceous acetogenins, squamocin and laherradurin, on the degradation of naphthalene mediated by Pseudomonas plecoglossicida J26. Int. Biodeterior. Biodegrad. 2012, 72, 82–87. [Google Scholar] [CrossRef]
- Weyens, N.; Truyens, S.; Dupae, J.; Newman, L.; Taghavi, S.; van der Lelie, D.; Carleer, R.; Vangronsveld, J. Potential of the TCE-Degrading endophyte pseudomonas putida W619-TCE to improve plant growth and reduce TCE phytotoxicity and evapotranspiration in poplar cuttings. Environ. Pollut. 2010, 158, 2915–2919. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Z.; Cai, M.Y. Manual of Systematic Identification for Common Bacteria; Science Press: Beijing, China, 2001; pp. 80–110. (In Chinese) [Google Scholar]
- Byers, H.K.; Stackebrandt, E.; Hayward, C.; Blackall, L.L. Molecular investigation of a microbial mat associated with the Great Artesian Basin. FEMS Microbiol. Ecol. 1998, 25, 391–403. [Google Scholar] [CrossRef]
- GenBank Database. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 1 June 2016).
- Tian, L.; Ma, P.; Zhong, J.J. Kinetics and key enzyme activities of phenanthrene degradation by Pseudomonas mendocina. Process Biochem. 2002, 37, 1431–1437. [Google Scholar] [CrossRef]
- Herrera, S.D.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Raj, A.; Purohit, H.J.; Kapley, A. Characterisation and optimisation of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 2007, 67, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Konishi, J.; Okada, H.; Hirasawa, K.; Onaka, T.; Suzuki, M. Operon structure and functional analysis of the genes encoding thermophilic desulfurizing enzymes of Paenibacillus sp. A11–2. Biochem. Biophys. Res. Commun. 2000, 270, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Septiningrum, K.; Ohi, H.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Sermsathanaswadi, J.; Deng, L.; Prawitwong, P.; Kosugi, A. The GH67α-glucuronidase of Paenibacillus curdlanolyticus B-6removes hexenuronic acid groups and facilitates biodegradation of the model xylooligosaccharide hexenuronosyl xylotriose. Enzyme Microb. Tech. 2015, 71, 28–35. [Google Scholar] [CrossRef] [PubMed]
- AL-Saleh, E.; Obuekwe, C. Crude oil biodegradation activity in potable water. Int. Biodeterior. Biodegrad. 2014, 93, 18–24. [Google Scholar] [CrossRef]
- Daane, L.L.; Harjono, I.; Barns, S.M.; Launen, L.A.; Palleroni, N.J.; Haggblom, M.M. PAH-degradation by Paenibacillus spp. and description of Paenibacillus naphthalenovorans sp. nov., a naphthalene degrading bacterium from the rhizosphere of salt marsh plants. Int. J. Syst. Evol. Microbiol. 2002, 52, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Lobianco, A.; Renzi, M.; Perra, G.; Bernardini, E.; Marvasi, M.; Gasperini, S.; Volterrani, M.; Franchi, E.; Heipieper, H.J.; et al. Two naphthalene degrading bacteria belonging to the genera Paenibacillus and Pseudomonas isolated from a highly polluted lagoon perform different sensitivities to the organic and heavy metal contaminants. Extremophiles 2009, 13, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, N.; Dourado, A.C.; Alves, P.I.; Cortes-Pallero, A.M.; Delgado-Rodriguez, A.I.; Prazeres, A.; Borges, N.; Sanchez, C.; Crespo, M.T.B.; Fareleira, P. Annual ryegrass-associated bacteria with potential for plant growth promotion. Microbiol. Res. 2014, 169, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Bezza, F.A.; Chirwa, E.M.N. Biosurfactant from Paenibacillus dendritiformis andits application in assisting polycyclic aromatic hydrocarbon (PAH) and motor oil sludge removal from contaminated soil and sand media. Process Saf. Environ. 2015, 98, 354–364. [Google Scholar] [CrossRef]
- Hadibarata, T.; Tachibana, S.; Itoh, K. Biodegradation of chrysene, an aromatic hydrocarbon by Polyporus sp. S133 in liquid medium. J. Hazard. Mater. 2009, 164, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhu, Q.; Qian, Y.G.; Song, Y.; Yao, J.; Choi, M.M.F. Microcalorimetric investigation of the effect of non-ionic surfactant on biodegradation of pyrene by PAH-degrading bacteria Burkholderia cepacia. Ecotox. Environ. Saf. 2013, 98, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, P.; Megharaj, M.; Naidu, R. Bioremediation of high molecular weight polyaromatic hydrocarbons cocontaminated with metals in liquid and soil slurries by metal tolerant PAHs degrading bacterial consortium. Biodegradation 2012, 23, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zou, S.C.; Lin, L.; Luan, T.G.; Qiu, R.L.; Tam, N.F.Y. Effects of pyrene and fluoranthene on the degradation characteristics of phenanthrene in the cometabolism process by Sphingomonas sp. strain PheB4 isolated from mangrove sediments. Mar. Pollut. Bull. 2010, 60, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Li, Q.X.; Xu, T.; Cui, Z.L.; Sun, Y.; Li, J. Sphingobium sp. FB3 degrades a mixture of polycyclic aromatic hydrocarbons. Int. Biodeterior. Biodegrad. 2014, 87, 44–51. [Google Scholar] [CrossRef]
- Lin, M.; Hu, X.K.; Chen, W.W.; Wang, H.; Wang, C.Y. Biodegradation of phenanthrene by Pseudomonas sp. BZ-3, isolated from crude oil contaminated soil. Int. Biodeterior. Biodegrad. 2014, 94, 176–181. [Google Scholar] [CrossRef]
- Ferguson, S.H.; Powell, S.M.; Snape, I.; Gibson, J.A.E.; Franzmann, P.D. Effect of temperature on the microbial ecology of a hydrocarbon-contaminated Antarctic soil: Implications for high temperature remediation. Cold Reg. Sci. Technol. 2008, 53, 115–129. [Google Scholar] [CrossRef]
- Warshawsky, D.; LaDow, K.; Schneider, J. Enhanced degradation of benzo[a]pyrene by Mycobacterium sp. in conjunction with green alga. Chemosphere 2007, 69, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Syeda, K.; Shann, J.; Yadav, J.S. A novel P450-initiated biphasic process for sustainable biodegradation of benzo[a]pyrene in soil under nutrient-sufficient conditions by the white rot fungus Phanerochaete chrysosporium. J. Hazard. Mater. 2013, 261, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Vauramo, S.; Jaaskelainen, V.; Setala, H. Environmental fate of polycyclic aromatic hydrocarbons under different plant traits in urban soil as affected by nitrogen deposition. Appl. Soil Ecol. 2011, 47, 167–175. [Google Scholar] [CrossRef]
- Ker, K.; Seguin, P.; Driscoll, B.T.; Fyles, J.W.; Smith, D.L. Switchgrass establishment and seeding year production can be improved by inoculation with rhizosphere endophytes. Biomass Bioenergy 2012, 47, 295–301. [Google Scholar] [CrossRef]
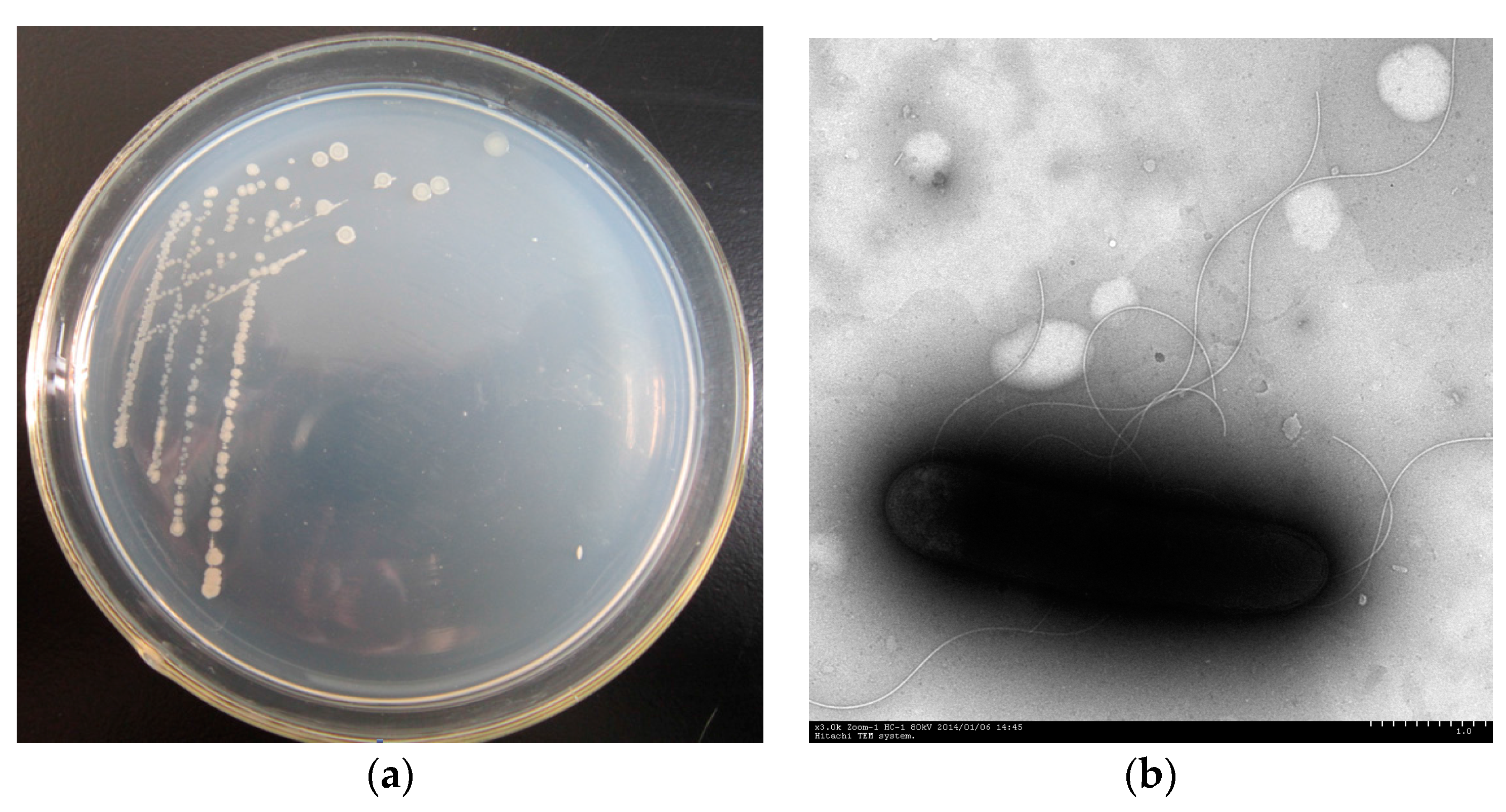

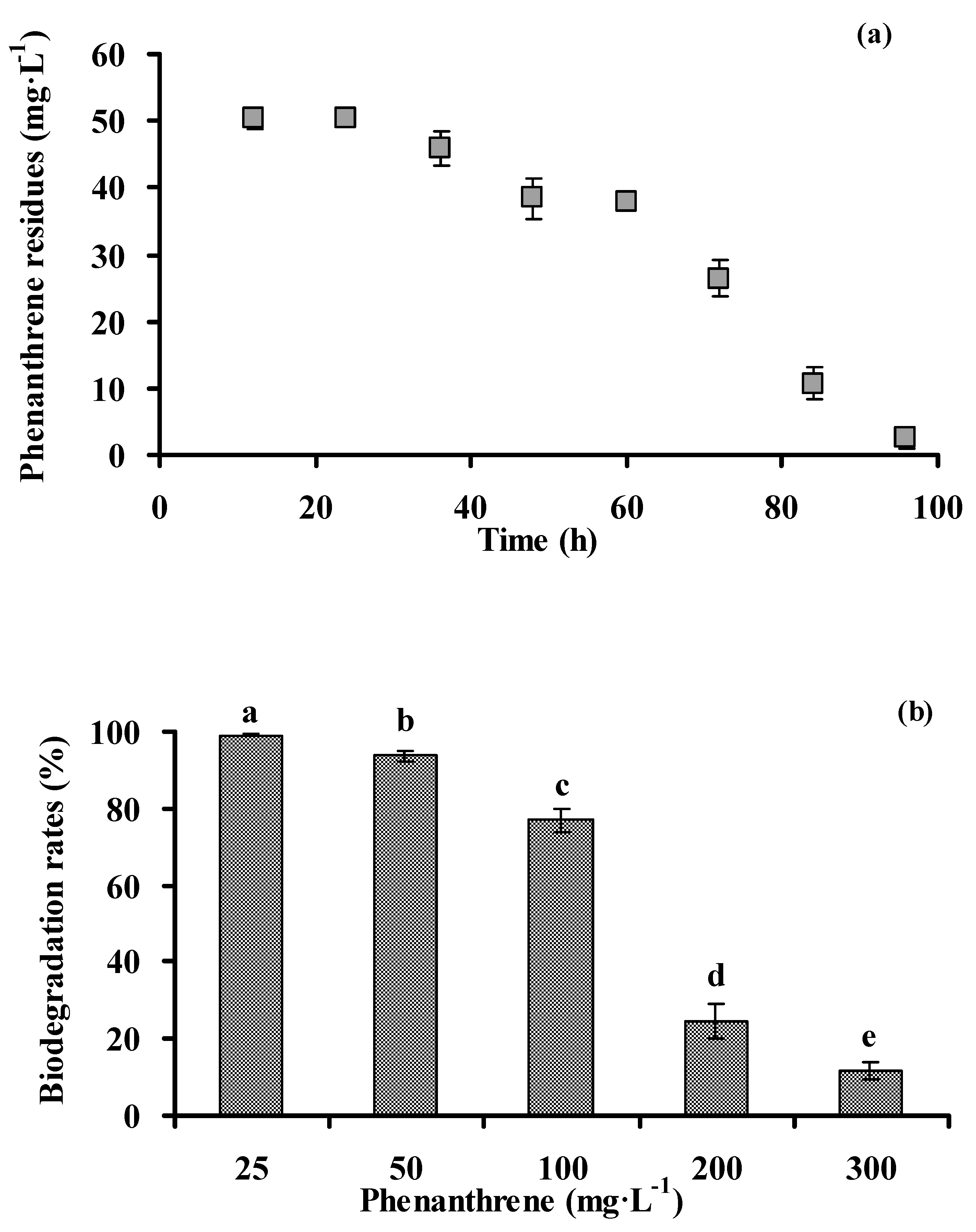
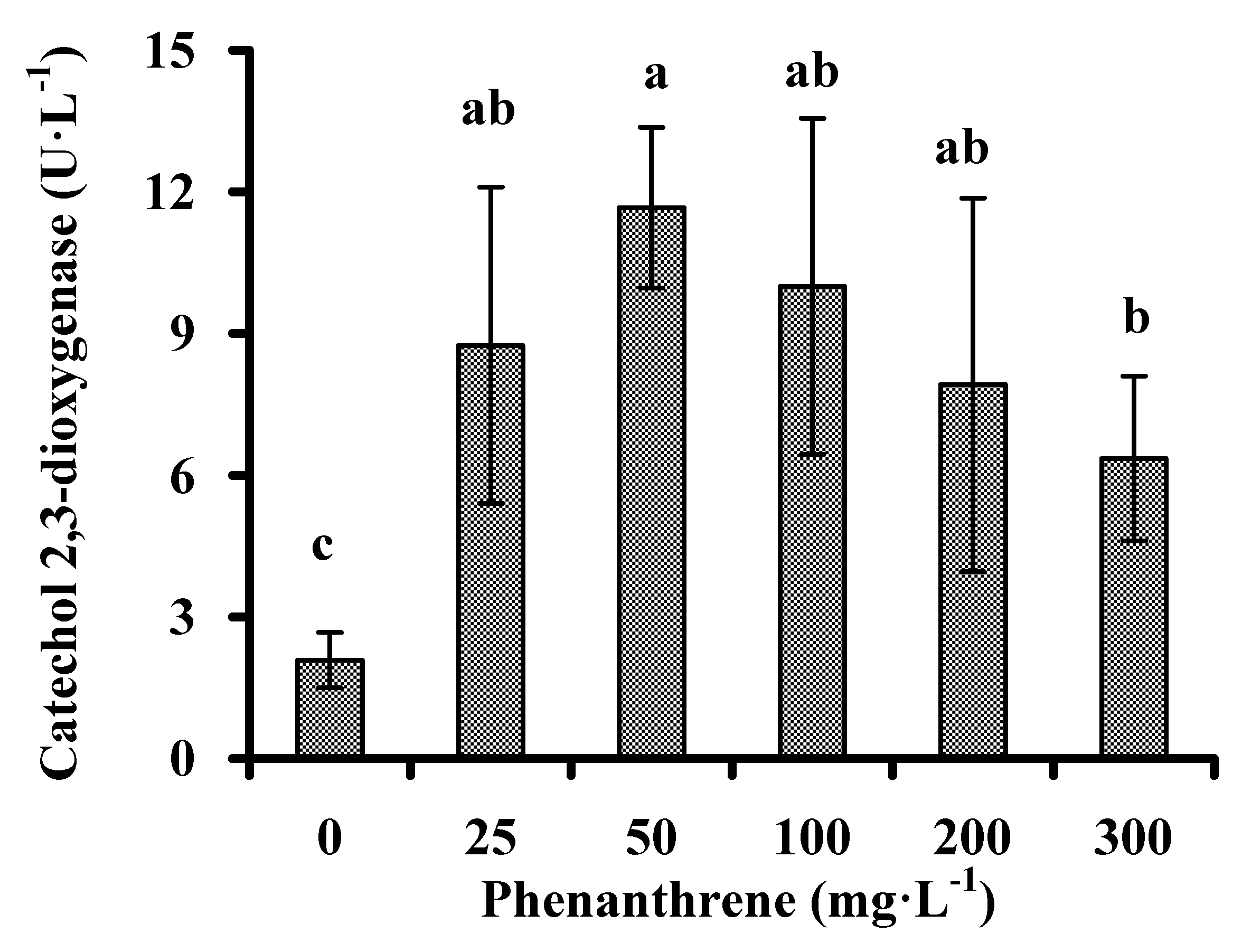
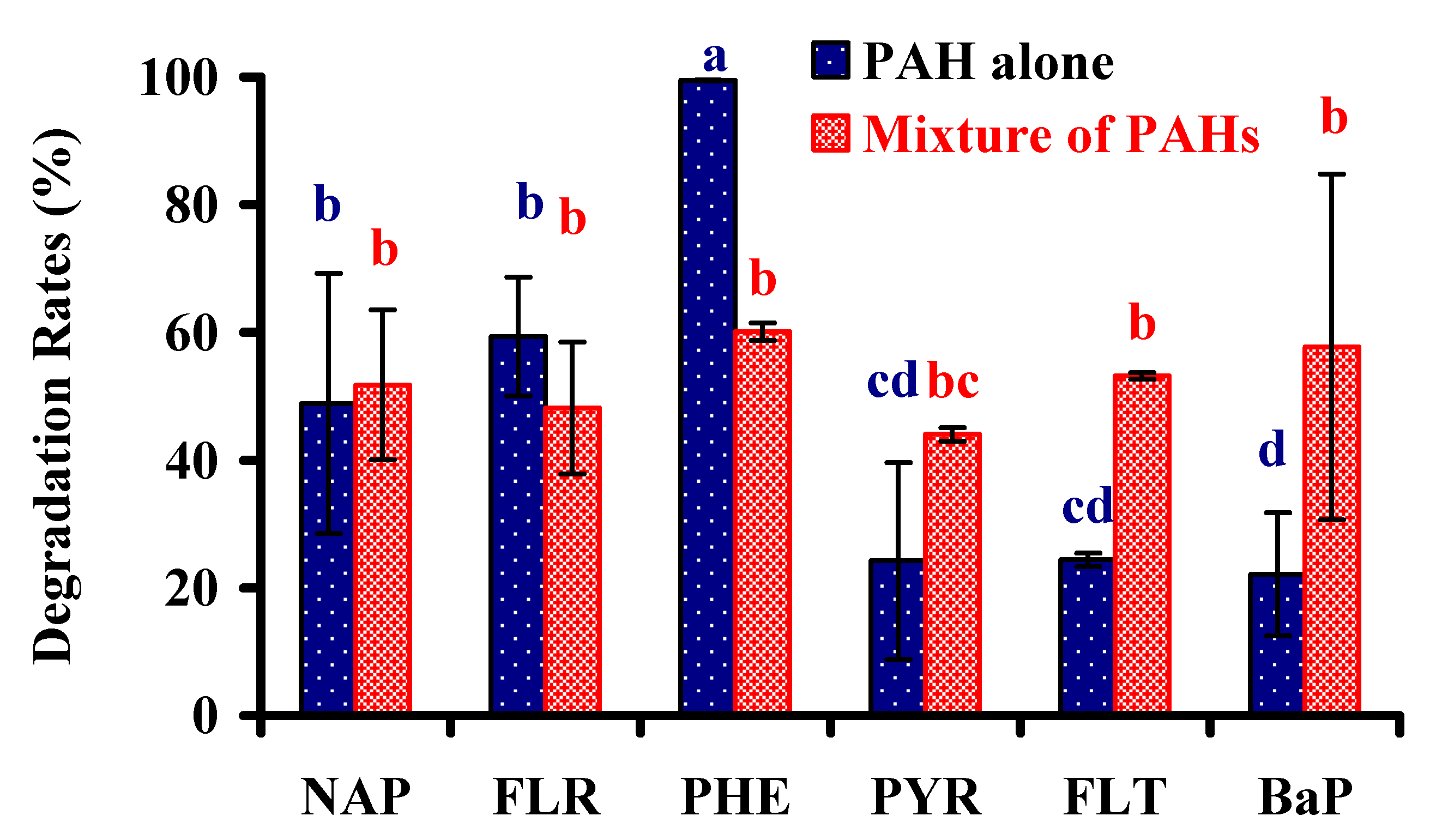
| Reaction | Results | Reaction | Results |
|---|---|---|---|
| Glucose fermentation test | − | Nitrate reaction | − |
| Methyl red staining | − | Voges-Proskauer reaction | − |
| Starch hydrolysis | + | Hydrogen sulfide test | − |
| Indole production | − | Gelatin liquefaction testing | − |
| Citrate utilization test | − | Phenylalanine deaminase | − |
| Carbon Sources | Degradation Rate (%) | Nitrogen Sources | Degradation Rate (%) |
|---|---|---|---|
| CK | 73.12 ± 3.03 d | CK | 73.12 ± 3.03 d |
| Glucose | 95.31 ± 1.05 c | NH4NO3 | 99.77 ± 0.13 a |
| Sucrose | 97.80 ± 1.02 b | (NH4)2SO4 | 99.28 ± 0.38 a |
| Citric acid | 99.41 ± 0.40 a | Glutamate | 99.90 ± 0.03 a |
| Yeast | 99.45 ± 0.44 a | Tryptone | 95.62 ± 0.30 c |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Jin, L.; Sun, K.; Li, S.; Ling, W.; Li, X. Potential of Endophytic Bacterium Paenibacillus sp. PHE-3 Isolated from Plantago asiatica L. for Reduction of PAH Contamination in Plant Tissues. Int. J. Environ. Res. Public Health 2016, 13, 633. https://doi.org/10.3390/ijerph13070633
Zhu X, Jin L, Sun K, Li S, Ling W, Li X. Potential of Endophytic Bacterium Paenibacillus sp. PHE-3 Isolated from Plantago asiatica L. for Reduction of PAH Contamination in Plant Tissues. International Journal of Environmental Research and Public Health. 2016; 13(7):633. https://doi.org/10.3390/ijerph13070633
Chicago/Turabian StyleZhu, Xuezhu, Li Jin, Kai Sun, Shuang Li, Wanting Ling, and Xuelin Li. 2016. "Potential of Endophytic Bacterium Paenibacillus sp. PHE-3 Isolated from Plantago asiatica L. for Reduction of PAH Contamination in Plant Tissues" International Journal of Environmental Research and Public Health 13, no. 7: 633. https://doi.org/10.3390/ijerph13070633
APA StyleZhu, X., Jin, L., Sun, K., Li, S., Ling, W., & Li, X. (2016). Potential of Endophytic Bacterium Paenibacillus sp. PHE-3 Isolated from Plantago asiatica L. for Reduction of PAH Contamination in Plant Tissues. International Journal of Environmental Research and Public Health, 13(7), 633. https://doi.org/10.3390/ijerph13070633






