Temporal Patterns in Bacterioplankton Community Composition in Three Reservoirs of Similar Trophic Status in Shenzhen, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Collection
2.3. Total Bacterioplankton Counts
2.4. Total Microbial DNA Extraction and 16s rDNA-V3 PCR Amplification
2.5. DGGE
2.6. Sequencing and Phylogenetic Analysis of Excised DGGE Bands
3. Results
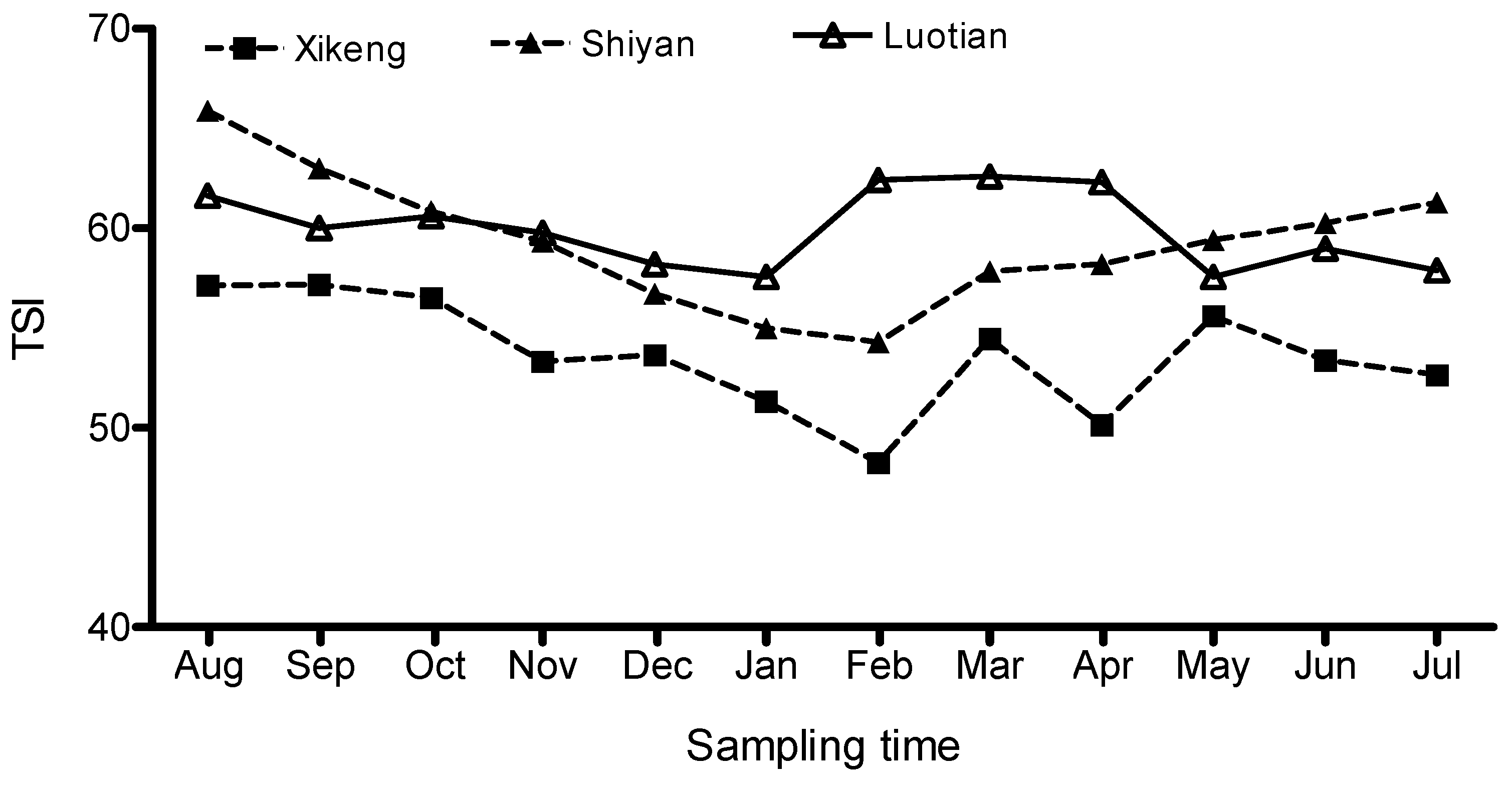
3.1. Seasonal Fluctuations in Trophic State Index (TSI)
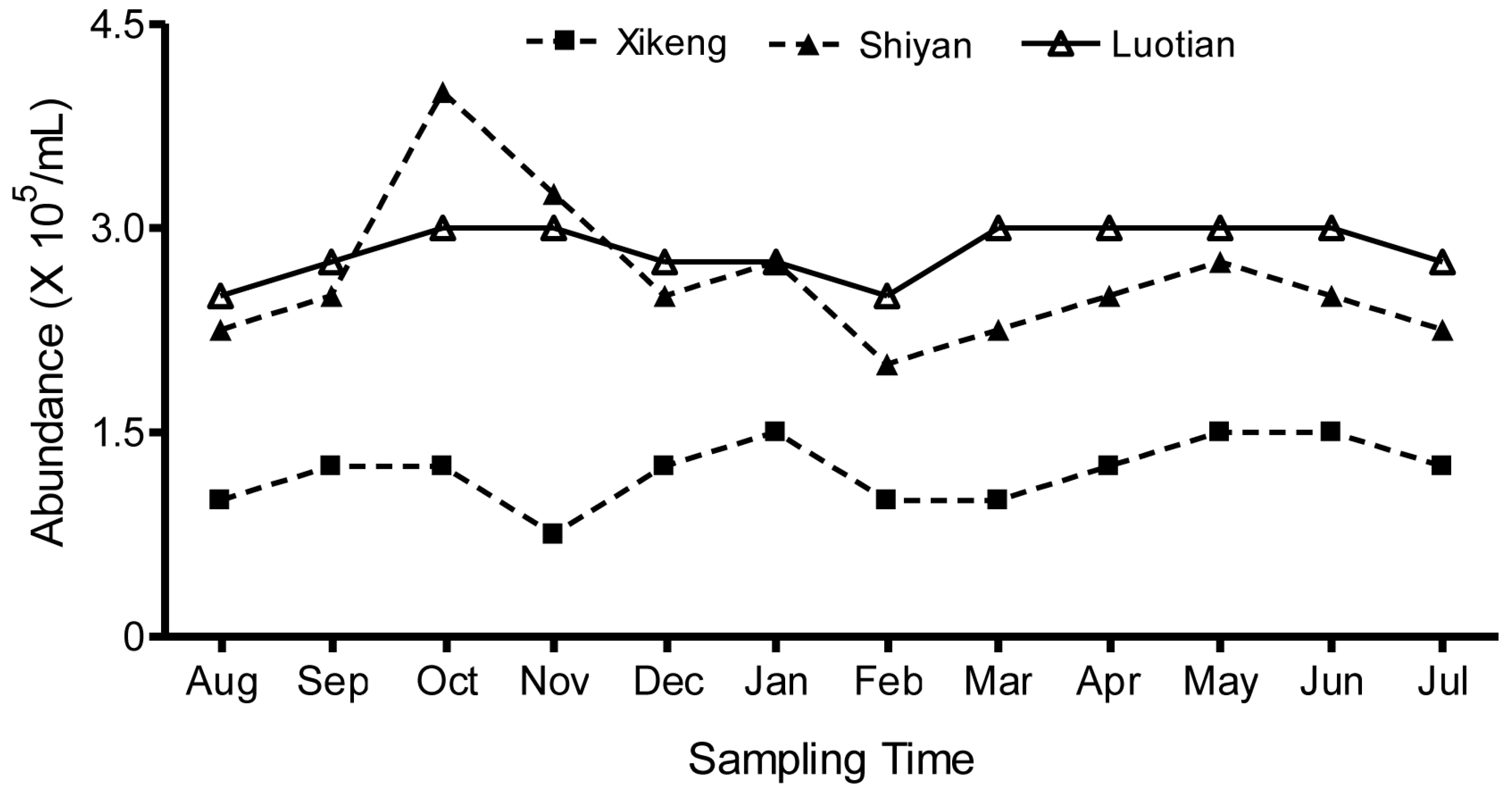
3.2. Seasonal Fluctuations in Total Bacterioplankton Abundance
3.3. Bacterioplankton Community Composition
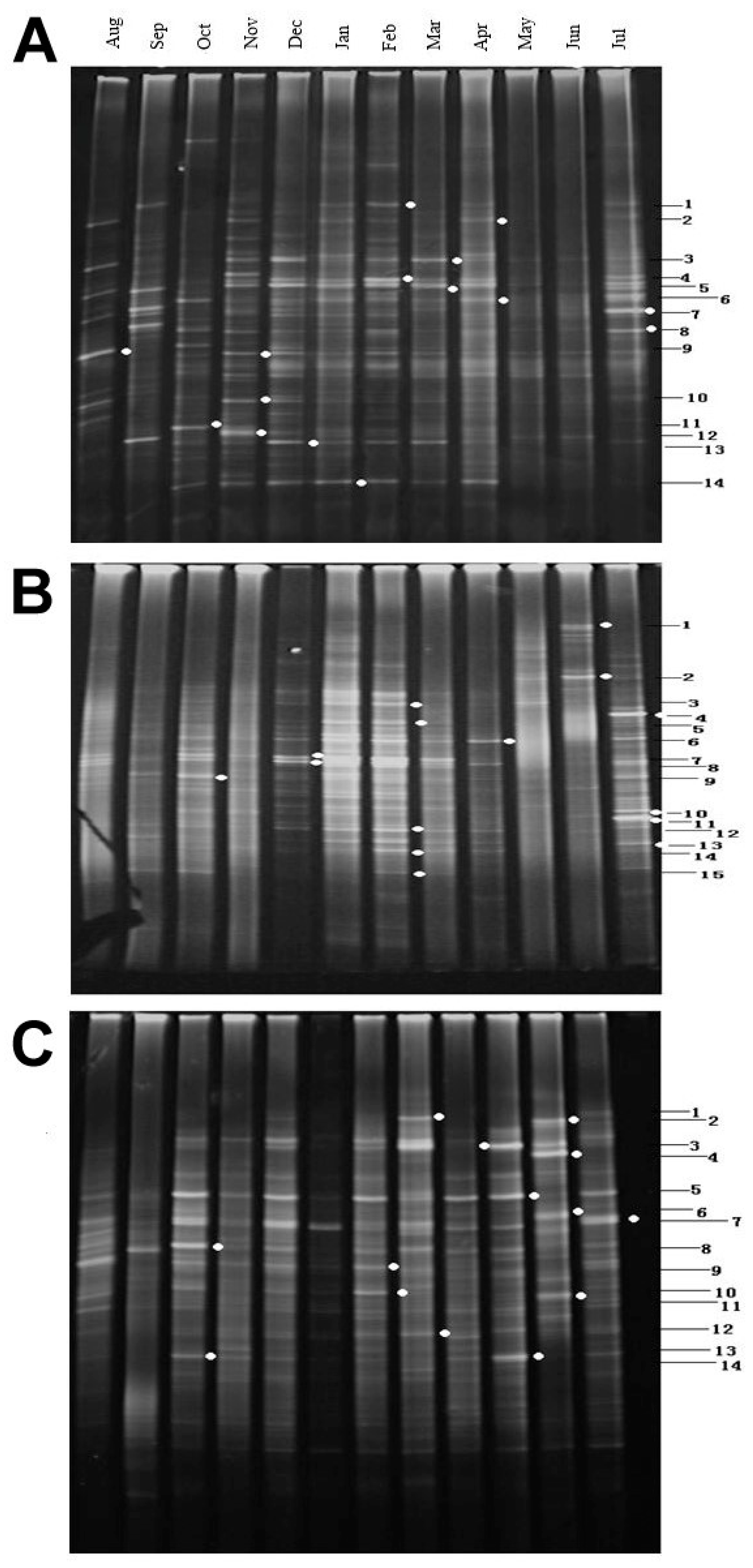
3.3.1. DGGE Fingerprint Patterns
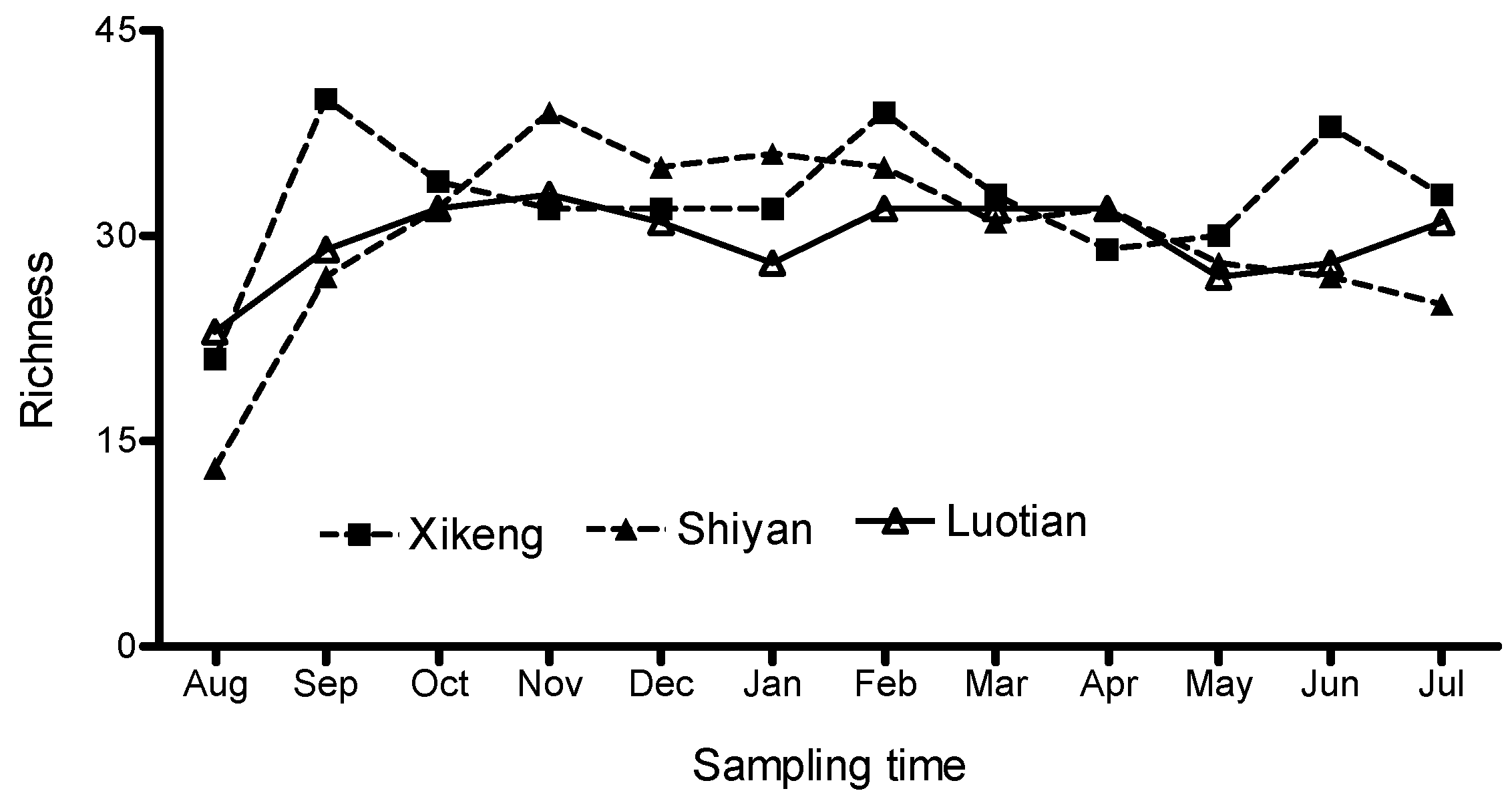
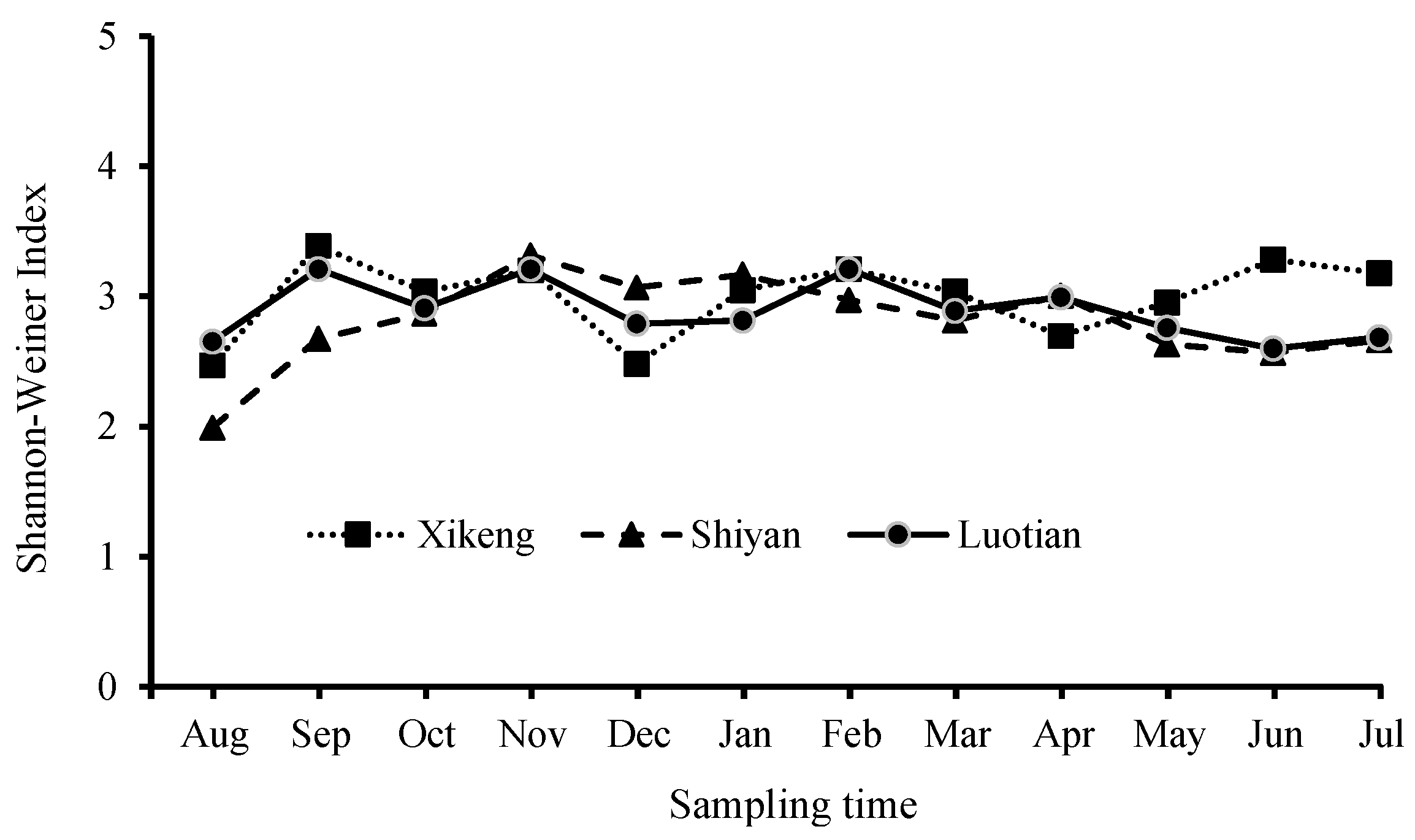
3.3.2. Bacterioplankton Richness and Shannon-Weiner Index
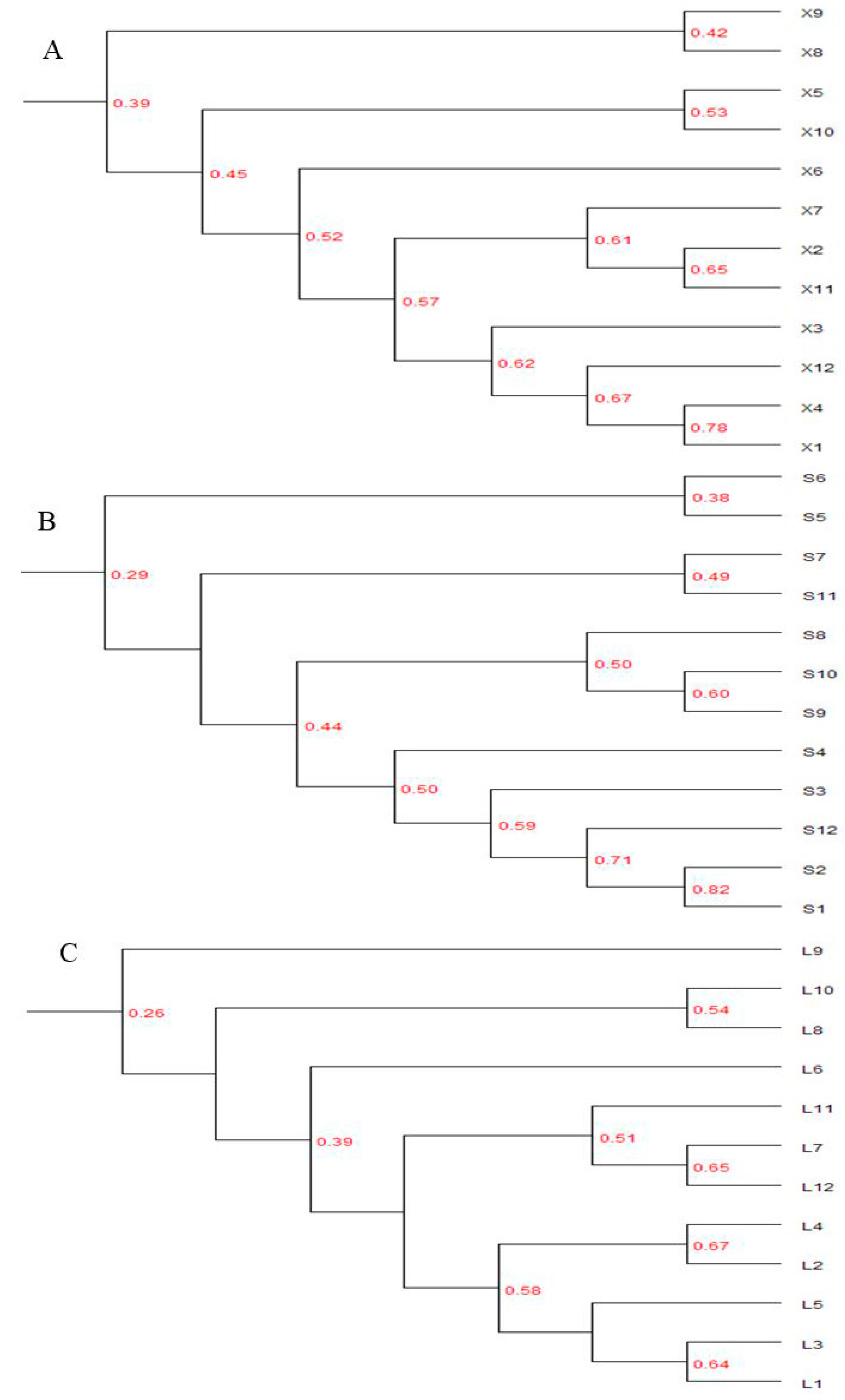
3.3.3. Cluster Analysis
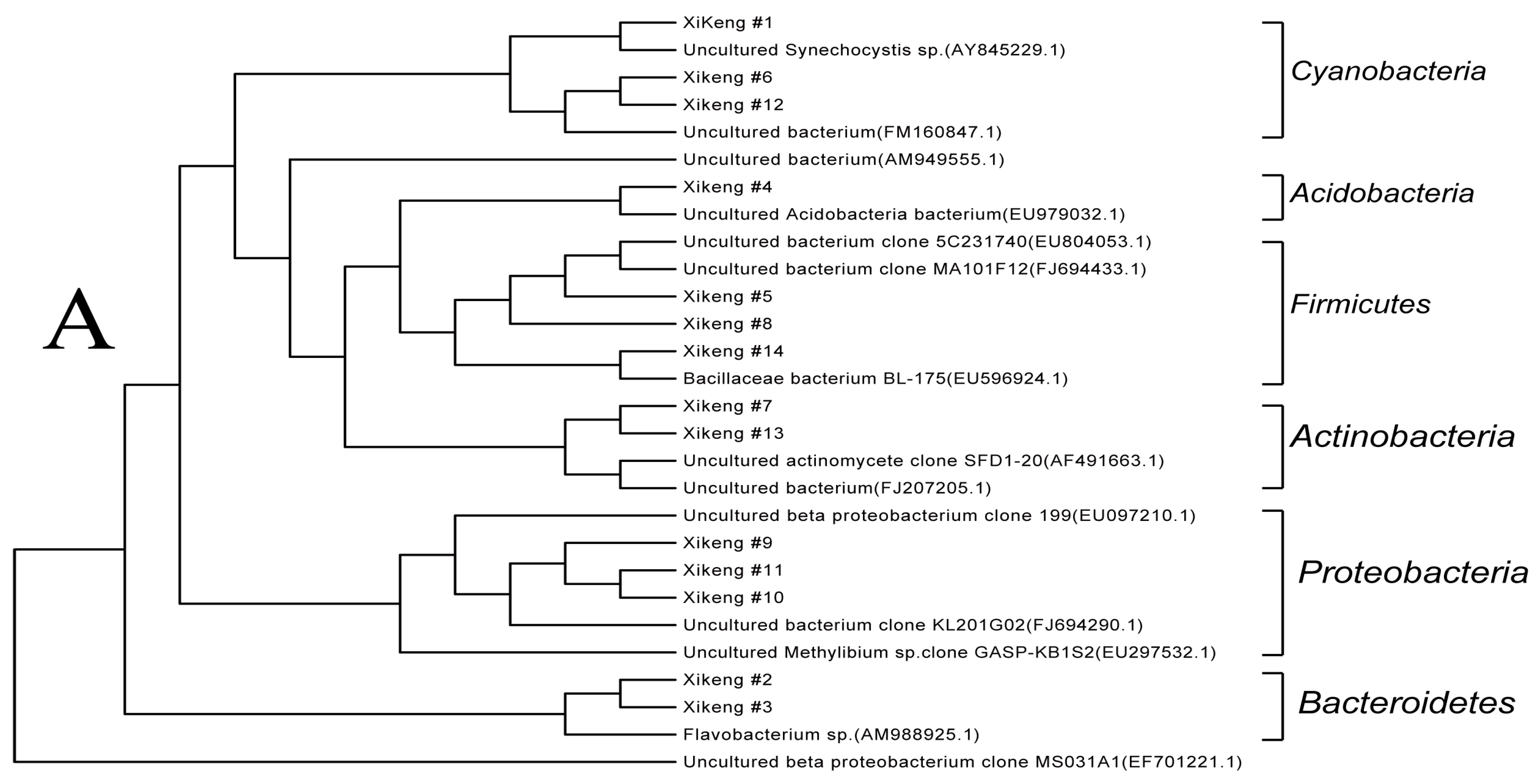
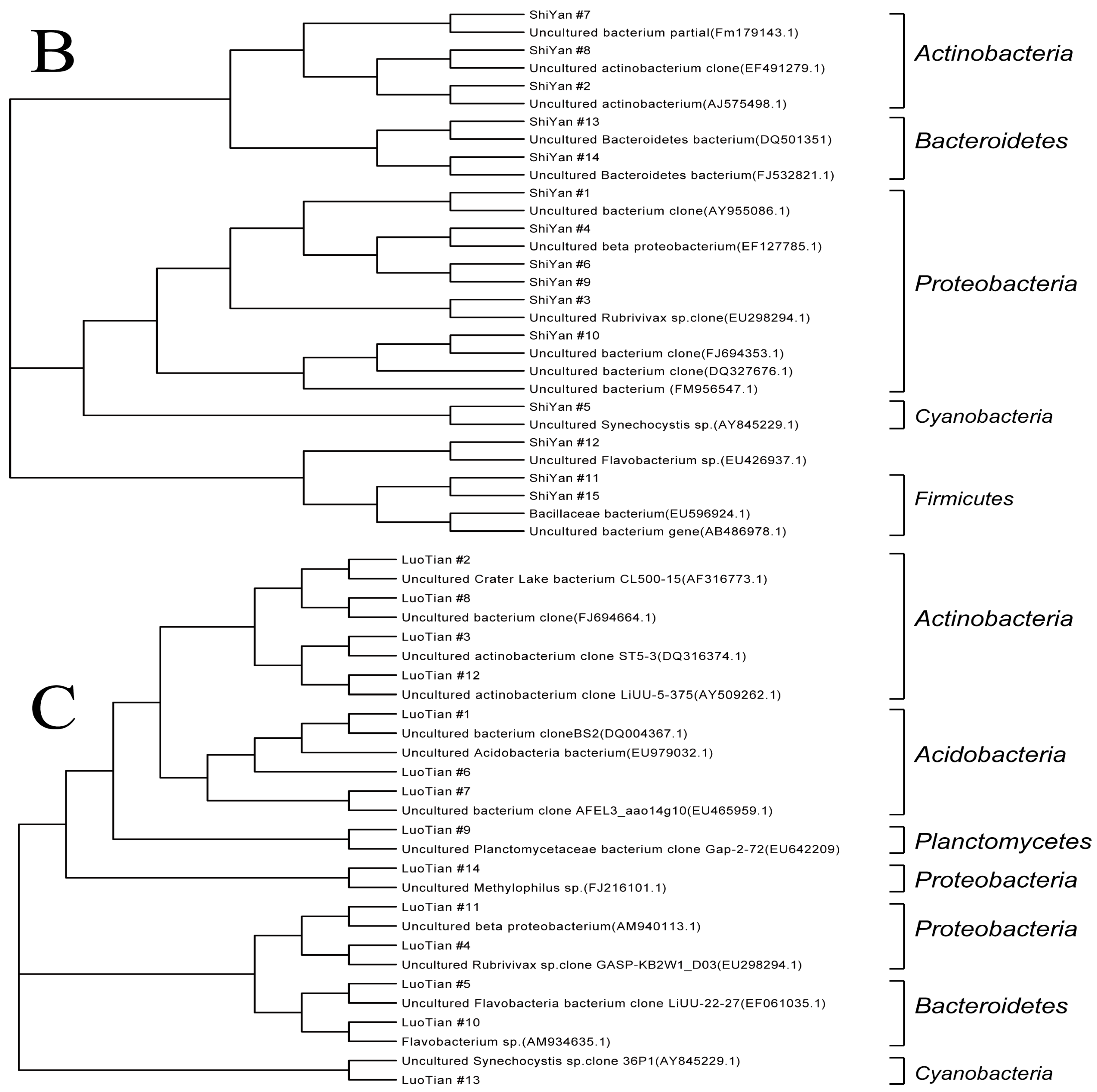
3.3.4. Sequencing and Phylogenetic Analysis
4. Discussion
4.1. Factors Affecting Patterns of BCC
4.2. Effects of PCR-DGGE Techniques on Change Patterns of the BCC
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BCC | Bacterioplankton Community Composition’s |
| DGGE | Denaturing Gradient Gel Electrophoresis |
| TN | Total Nitrogen |
| TP | Total Phosphate |
| VBNC | Viable But Not Culturable |
| T-RFLP | Terminal Restriction Fragment Length Polymorphism |
| DOC | Dissolved Organic Carbon |
| UPGMA | Unweighted Pair-Group Method with Arithmetic |
| PCR | Polymerase Chain Reaction |
References
- Matveev, V.; Robson, B.J. Aquatic food web structure and the flow of carbon. Freshw. Rev. 2014, 7, 1–24. [Google Scholar] [CrossRef]
- Beisner, B.E. Plankton community structure in fluctuating environments and the role of productivity. Oikos 2001, 95, 496–510. [Google Scholar] [CrossRef]
- Bianchi, F.; Acri, F.; Aubry, F.B.; Berton, A.; Boldrin, A.; Camatti, E.; Cassin, D.; Comaschi, A. Can plankton communities be considered as bio-indicators of water quality in the Lagoon of Venice? Mar. Pollut. Bull. 2003, 46, 964–971. [Google Scholar] [CrossRef]
- Lai, X.T.; Zeng, X.F.; Fang, S.; Huang, Y.L.; Cao, L.X.; Zhou, S.N. Denaturing gradient gel electrophoresis (DGGE) analysis of bacterial community composition in deep-sea sediments of the South China Sea. World J. Microb. Biot. 2006, 22, 1337–1345. [Google Scholar] [CrossRef]
- Eiler, A.; Bertilsson, S. Composition of freshwater bacterial communities associated with Cyanobacterial blooms in four Swedish lakes. Environ. Microbiol. 2007, 9, 838–838. [Google Scholar] [CrossRef]
- Lindstrom, E.S. Bacterioplankton community composition in five lakes differing in trophic status and humic content. Microb. Ecol. 2000, 40, 104–113. [Google Scholar] [PubMed]
- Yannarell, A.C.; Kent, A.D.; Lauster, G.H.; Kratz, T.K.; Triplett, E.W. Temporal patterns in bacterial communities in three temperate lakes of different trophic status. Microb. Ecol. 2003, 46, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Bao, S.M.; Zhu, X.Y.; Huang, X.M. Spatio-temporal variations of the bacterioplankton community composition in Chaohu Lake, China. Prog. Nat. Sci. 2008, 18, 1115–1122. [Google Scholar] [CrossRef]
- Lymperopoulou, D.S.; Kormas, K.A.; Karagouni, A.D. Variability of prokaryotic community structure in a drinking water reservoir (Marathonas, Greece). Microb. Environ. 2012, 27, 1–8. [Google Scholar] [CrossRef]
- Fisher, M.M.; Klug, J.L.; Lauster, G.; Newton, M.; Triplett, E.W. Effects of resources and trophic interactions on freshwater bacterioplankton diversity. Microb. Ecol. 2000, 40, 125–138. [Google Scholar] [PubMed]
- Rösel, S.; Allgaier, M.; Grossart, H.P. Long-term characterization of free-living and particle-associated bacterial communities in Lake Tiefwaren Reveals distinct seasonal patterns. Microb. Ecol. 2012, 64, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J.; Mcmahon, K.D. Seasonal differences in bacterial community composition following nutrient additions in a eutrophic lake. Environ. Microbiol. 2011, 13, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, T.; Ghosh, A.; Pazhani, G.P.; Shinoda, S. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front. Public Health 2014, 2, 103–103. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H. Microbial diversity: Facts, problems and prospects. Syst. Appl. Microbiol. 2004, 27, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lemke, M.J.; Leff, L.G. Culturability of stream bacteria assessed at the assemblage and population levels. Microb. Ecol. 2006, 51, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.C.; Cleary, D.F.; Almeida, A.; Cunha, A.; Dealtry, S.; Mendonça-Hagler, L.C.; Smalla, K.; Gomes, N.C. Denaturing gradient gel electrophoresis and barcoded pyrosequencing reveal unprecedented archaeal diversity in mangrove sediment and rhizosphere samples. Appl. Environ. Microbiol. 2012, 78, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Palmer, M.W.; Melcher, U. Community terminal restriction fragment length polymorphisms reveal insights into the diversity and dynamics of leaf endophytic bacteria. BMC Microbiol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.M.; Moore, E.R.B.; Timmis, K.N. An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis for the study of microbial community structure and dynamics. Environ. Microbiol. 2000, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.Y.; Yu, Y.H.; Feng, W.S.; Yu, Z.G.; Chen, H.T. Plankton community composition in the Three Gorges Reservoir Region revealed by PCR-DGGE and its relationships with environmental factors. J. Environ. Sci. Chin. 2008, 20, 732–738. [Google Scholar] [CrossRef]
- Dar, S.A.; Kuenen, J.G.; Muyzer, G. Nested PCR-denaturing gradient gel electrophoresis approach to determine the diversity of sulfate-reducing bacteria in complex microbial communities. Appl. Environ. Microb. 2005, 71, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Gilcreas, F.W. Standard methods for the examination of water and wastewater. Am. J. Public Health Nations Health 1966, 56, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microb. 1993, 59, 695–700. [Google Scholar]
- Juck, D.; Charles, T.; Whyte, L.; Greer, C. Polyphasic microbial community analysis of petroleum hydrocarbon-contaminated soils from two northern Canadian communities. FEMS Microbiol. Ecol. 2000, 33, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rölleke, S.; Muyzer, G.; Wawer, C.; Wanner, G.; Lubitz, W. Identification of bacteria in a biodegraded wall painting by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microb. 1996, 62, 2059–2065. [Google Scholar]
- McCaig, A.E.; Glover, L.A.; Prosser, J.I. Numerical analysis of grassland bacterial community structure under different land management regimens by using 16S ribosomal DNA sequence data and denaturing gradient gel electrophoresis banding patterns. Appl. Environ. Microb. 2001, 67, 4554–4559. [Google Scholar] [CrossRef]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.J. Cosmopolitan Freshwater Bacterial Dynamics in Lakes across Time and Space; ProQuest: Madison, WI, USA, 2008. [Google Scholar]
- Luo, W.; Chen, H.; Lei, A.; Lu, J.; Hu, Z. Estimating Cyanobacteria community dynamics and its relationship with environmental factors. Int. J. Environ. Res. Public Health 2014, 11, 1141–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, S.; Cassio, F.; Pascoal, C. Denaturing gradient gel electrophoresis (DGGE) in microbial ecology—Insights from freshwaters. Gel Electrophor. Princ. Basics 2012, 4. [Google Scholar] [CrossRef]
- Tatiana, V.; Edward, T.; Gerard, M.; Valérie, M.; Gisèle, L.; Annabel, R.; Guy, S. Evaluation of denaturing gradient gel electrophoresis in the detection of 16S rDNA sequence variation in rhizobia and methanotrophs. FEMS Microbiol. Ecol. 1997, 24, 279–285. [Google Scholar]
- Hang, M.; Xiao-Yu, X.; Zhen-Mei, L.; He, L. Comparison of DNA extraction methods for PCR-DGGE analysis of the soil bacterial community. Chin. J. Agric. Biotechnol. 2006, 3, 1–6. [Google Scholar] [CrossRef]
- Tian, J.; Lu, J.; Zhang, Y.; Li, J.C.; Sun, L.C.; Hu, Z.L. Microbial community structures and dynamics in the O3/BAC drinking water treatment process. Int. J. Environ. Res. Public Health 2014, 11, 6281–6290. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Physico-Chemical Characteristics | Reservoirs | ||
|---|---|---|---|
| Shi-Yan | Xi-Keng | Luo-Tian | |
| Surface area (km2) | 6.33 | 3.87 | 3.28 |
| Mean depth (m) | 4.6 | 3.5 | 3.1 |
| Total phosphorus (mg/L) | 0.04 | 0.03 | 0.03 |
| Total nitrogen (mg/L) | 1.26 | 0.93 | 0.95 |
| Chlorophyll a (μg/L) | 27.01 | 13.39 | 39.52 |
| Transparency (cm) | 55.3 | 82.7 | 65.8 |
| Dissolved organic carbon (DOC) (mg/L) | 8.27 | 7.95 | 8.39 |
| Permanganate Index (mg/L) | 2.76 | 1.72 | 1.95 |
| Temperature (°C) | 25.48 | 25.24 | 25.95 |
| Karlson TSIM (modified tropic state index) | 59.49 | 53.99 | 60.17 |
| Bacteria | Reservoirs | ||
|---|---|---|---|
| Shi-Yan | Xi-Keng | Luo-Tian | |
| Proteobacteria | 40% | 21.4% | 21.4% |
| Actinobacteria | 20% | 14.3% | 28.6% |
| Acidobacteria | / | 7.1% | 21.4% |
| Bacteroidetes | 20% | 14.3% | 14.3% |
| Cyanobacteria | 6.7% | 21.4% | 7.1% |
| Planctomycetes | / | / | 7.1% |
| Firmicutes | 13.3% | 21.4% | / |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, C.; Lu, J.; Lei, A.; Hu, Z. Temporal Patterns in Bacterioplankton Community Composition in Three Reservoirs of Similar Trophic Status in Shenzhen, China. Int. J. Environ. Res. Public Health 2016, 13, 599. https://doi.org/10.3390/ijerph13060599
Li J, Chen C, Lu J, Lei A, Hu Z. Temporal Patterns in Bacterioplankton Community Composition in Three Reservoirs of Similar Trophic Status in Shenzhen, China. International Journal of Environmental Research and Public Health. 2016; 13(6):599. https://doi.org/10.3390/ijerph13060599
Chicago/Turabian StyleLi, Jiancheng, Cheng Chen, Jun Lu, Anping Lei, and Zhangli Hu. 2016. "Temporal Patterns in Bacterioplankton Community Composition in Three Reservoirs of Similar Trophic Status in Shenzhen, China" International Journal of Environmental Research and Public Health 13, no. 6: 599. https://doi.org/10.3390/ijerph13060599
APA StyleLi, J., Chen, C., Lu, J., Lei, A., & Hu, Z. (2016). Temporal Patterns in Bacterioplankton Community Composition in Three Reservoirs of Similar Trophic Status in Shenzhen, China. International Journal of Environmental Research and Public Health, 13(6), 599. https://doi.org/10.3390/ijerph13060599








