Effects of Sachet Water Consumption on Exposure to Microbe-Contaminated Drinking Water: Household Survey Evidence from Ghana
Abstract
:1. Introduction
2. Experimental Section
2.1. Data
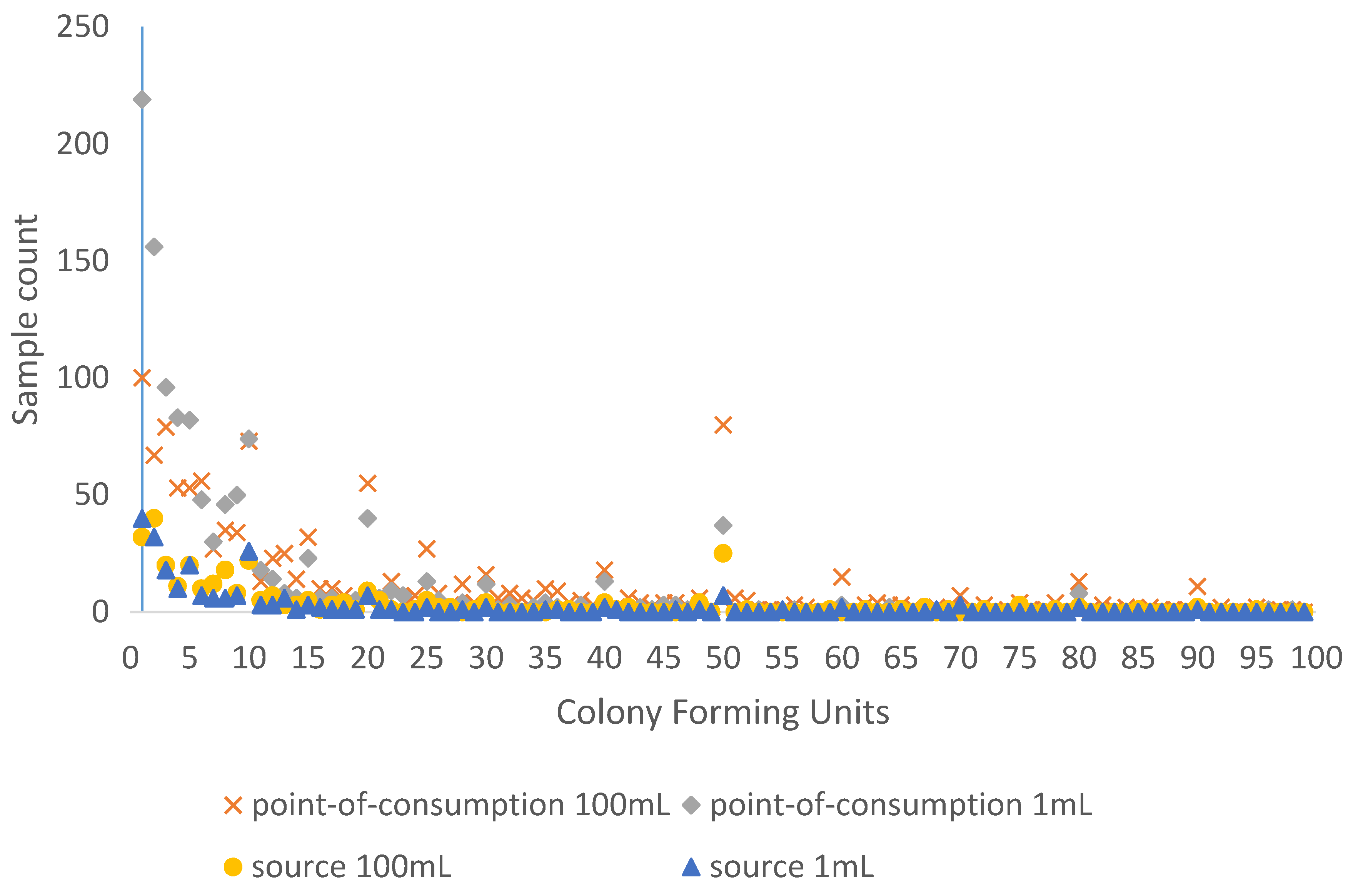
2.2. Preliminary Evaluation of E. coli Counts
2.3. Assessing Risk Factors for Water Contamination at the Point of Consumption
2.4. Risk Factors for Purchasing of Contaminated Sachet Water
3. Results
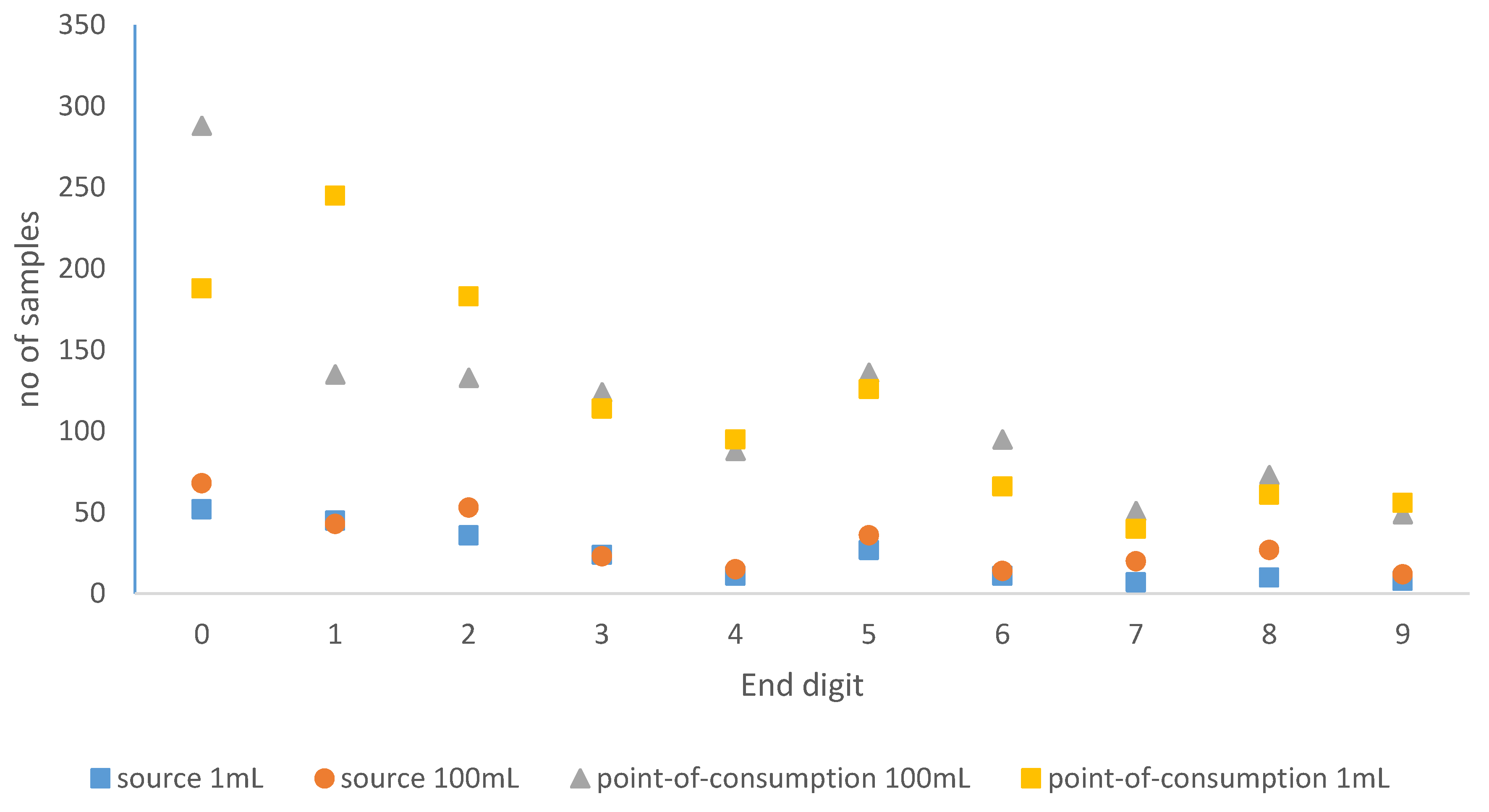
3.1. Digit Preference in Bacterial Counts
3.2. Risk Factors for Contaminated Water at Point of Consumption
3.3. Risk Factors for Purchasing Contaminated Sachet Water
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- UN-Habitat. The State of African Cities 2014: Re-Imagining Sustainable Urban Transitions. Available online: https://issuu.com/unpublications/docs/9789210575614 (accessed on 4 December 2015).
- Stoler, J.; Fink, G.; Weeks, J.R.; Otoo, R.A.; Ampofo, J.A.; Hill, A.G. When urban taps run dry: Sachet water consumption and health effects in low income neighborhoods of Accra, Ghana. Health Place 2012, 18, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Kumpel, E.; Nelson, K.L. Comparing microbial water quality in an intermittent and continuous piped water supply. Water Res. 2013, 47, 5176–5188. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Gundry, S.; Conroy, R. Household drinking water in developing countries: A systematic review of microbiological contamination between source and point-of-use. Trop. Med. Int. Health 2004, 9, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Shields, K.; Bain, R.E.S.; Cronk, R.; Wright, J.; Bartram, J. Association of supply type with fecal contamination of source water and household stored drinking water in developing countries: A systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bain, R.; Bartram, J.; Gundry, S.; Pedley, S.; Wright, J. Water safety and inequality in access to drinking-water between rich and poor households. Environ. Sci. Technol. 2013, 47, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ghana Statistical Service. Ghana Living Standards Survey Round 6 Main Report. Available online: http://www.statsghana.gov.gh/docfiles/glss6/GLSS6_Main%20Report.pdf (accessed on 4 December 2015).
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef] [PubMed]
- Stoler, J.; Weeks, J.R.; Fink, G. Sachet drinking water in Ghana’s Accra-Tema metropolitan area: Past, present, and future. J. Water Sanit. Hyg Dev. 2012, 2, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Stoler, J.; Tutu, R.A.; Ahmed, H.; Frimpong, L.A.; Bello, M. Sachet water quality and brand reputation in two low-income urban communities in Greater Accra, Ghana. Am. J. Trop. Med. Hyg. 2014, 90, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.; Amoako-Mensah, S. Water Quality Module of the Ghana Living Standards Survey VI: Monitoring Household Drinking Water Quality through a Household Survey. Available online: http://whconference.unc.edu/files/2014/11/johnston-03.pdf (accessed on 2 March 2016).
- Edberg, S.C.; Rice, E.W.; Karlin, R.J.; Allen, M.J. Escherichia coli: The best biological drinking water indicator for public health protection. J. Appl. Microbiol. 2000, 88, 106S–116S. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Kirkpatrick, C.; Parker, D.; Zhang, Y.-F. An empirical analysis of state and private-sector provision of water services in Africa. World Bank Econ. Rev. 2006, 20, 143–163. [Google Scholar] [CrossRef]
- Oluwasanya, G.; Smith, J.; Carter, R. Self supply systems: Urban dug wells in Abeokuta, Nigeria. Water Sci. Technol. 2011, 11, 172–178. [Google Scholar] [CrossRef]
- Litchfield, J.; Waddington, H. Migration and Poverty in Ghana: Evidence from the Ghana Living Standards Survey. Available online: http://repository.forcedmigration.org/show_metadata.jsp?pid=fmo:5625 (accessed on 4 December 2015).
- Christenson, E.; Bain, R.; Wright, J.; Aondoakaa, S.; Hossain, R.; Bartram, J. Examining the influence of urban definition when assessing relative safety of drinking-water in Nigeria. Sci. Total Environ. 2014, 490, 301–312. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality. Vol. 3—Surveillance and Control of Community Supplies; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- World Health Organization, UNICEF. Progress on Sanitation and Drinking-Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Fisher, M.B.; Williams, A.R.; Jalloh, M.F.; Saquee, G.; Bain, R.E.S.; Bartram, J.K. Microbiological and chemical quality of packaged sachet water and household stored drinking water in Freetown, Sierra Leone. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Spoorenberg, T. Quality of age reporting: Extension and application of a modified Whipple’s index. Population 2007, 62, 847–859. [Google Scholar] [CrossRef]
- Graves, J.W.; Bailey, K.R.; Grossardt, B.R.; Gullerud, R.E.; Meverden, R.A.; Grill, D.E.; Sheps, S.G. The impact of observer and patient factors on the occurrence of digit preference for zero in blood pressure measurement in a hypertension specialty clinic: Evidence for the need of continued observation. Am. J. Hypertens. 2006, 19, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.K.; Jayasinghe, G.; van der Hoek, W.; Cairncross, S.; Dalsgaard, A. Is there an association between bacteriological drinking water quality and childhood diarrhoea in developing countries? Trop. Med. Int. Health 2004, 9, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Proum, S.; Sobsey, M.D. Escherichia coli in household drinking water and diarrheal disease risk: Evidence from Cambodia. Water Sci. Technol. 2008, 58, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Gundry, S.; Wright, J.; Conroy, R. A systematic review of the health outcomes related to household water quality in developing countries. J. Water Health 2004, 2, 1–13. [Google Scholar] [PubMed]
- Gundry, S.; Wright, J.; Conroy, R.; du Preez, M.; Genthe, B.; Mutisi, C.; Ndamba, J.; Potgieter, N. Contamination of drinking water between source and point-of-use in rural households of South Africa and Zimbabwe: Implications for monitoring the Millennium Development Goal for water. Water Pract. Technol. 2006, 1. [Google Scholar] [CrossRef]
- Gruber, J.S.; Ercumen, A.; Colford, J.M., Jr. Coliform bacteria as indicators of diarrheal risk in household drinking water: Systematic review and meta-analysis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Eilers, R.H.C.; Borgdorff, M.W. Modeling and correction of digit preference in tuberculin surveys. Int. J. Tuberc. Lung Dis. 2004, 8, 232–239. [Google Scholar] [PubMed]
- Gleeson, C.; Gray, N. The Coliform Index and Water-Borne Disease: Problems of Microbial Drinking-Water Assessment; CRC Press: London, UK, 2002; p. 208. [Google Scholar]
- Wu, J.; Long, S.C.; Das, D.; Dorner, S.M. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J. Water Health 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Savichtcheva, O.; Okabe, S. Alternative indicators of fecal pollution: Relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res. 2006, 40, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Bain, R.; Bartram, J.; Elliott, M.; Matthews, R.; McMahan, L.; Tung, R.; Chuang, P.; Gundry, S. A summary catalogue of microbial drinking water tests for low and medium resource settings. Int. J. Environ. Res. Public Health 2012, 9, 1609–1625. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.E.; Lescano, A.G.; Bern, C.; Calderon, M.M.; Cabrera, L.; Gilman, R.H. Fecal contamination of drinking water within peri-urban households, Lima, Peru. Am. J. Trop. Med. Hyg. 2007, 77, 699–704. [Google Scholar] [PubMed]
- Howard, G.; Pedley, S.; Barrett, M.; Nalubega, M.; Johal, K. Risk factors contributing to microbiological contamination of shallow groundwater in Kampala, Uganda. Water Res. 2003, 37, 3421–3429. [Google Scholar] [CrossRef]
| Variable | Category | Contamination Level * (Rows Sum to 100%) | n | ||
|---|---|---|---|---|---|
| Uncontaminated | Medium | High | n | ||
| Total | 28.0% | 37.3% | 34.7% | 2972 | |
| Source contamination risk factors | |||||
| Water source | Piped to premises | 34.5% | 49.2% | 16.4% | 240 |
| Standpipe, tanker or neighbours tap | 13.8% | 42.2% | 44.0% | 500 | |
| Protected well | 11.8% | 31.1% | 57.2% | 101 | |
| Unprotected well or spring | 12.2% | 36.9% | 50.9% | 142 | |
| Rainwater | 7.1% | 51.4% | 41.6% | 57 | |
| Surface water | 5.1% | 17.1% | 77.8% | 315 | |
| Packaged water | 68.8% | 29.2% | 2.0% | 590 | |
| Community borehole | 10.7% | 44.1% | 45.2% | 565 | |
| NGO borehole | 13.6% | 41.9% | 44.6% | 207 | |
| Other borehole | 7.9% | 42.7% | 49.4% | 218 | |
| Missing | 9.7% | 64.5% | 25.9% | 37 | |
| Distance between latrine and water source | Within 30 m | 8.6% | 38.2% | 53.2% | 488 |
| More than 30 m | 10.0% | 36.3% | 53.7% | 1097 | |
| Not ground water | 42.5% | 37.6% | 19.9% | 1387 | |
| Urban | No | 15.5% | 36.0% | 48.6% | 1667 |
| Yes | 38.4% | 38.4% | 23.3% | 1305 | |
| Recontamination risk factors | |||||
| Improved sanitation | No | 13.6% | 37.6% | 48.8% | 2076 |
| Yes | 31.5% | 37.1% | 31.5% | 890 | |
| Missing | 6.3% | 73.0% | 20.7% | 6 | |
| Soap observed | No | 19.3% | 38.7% | 42.1% | 1765 |
| Yes | 32.5% | 36.6% | 30.9% | 1207 | |
| Water obtained from source or vessel | From source | 47.1% | 33.1% | 19.8% | 891 |
| From covered vessel | 17.4% | 41.5% | 41.1% | 1667 | |
| From uncovered vessel | 6.5% | 27.6% | 65.9% | 295 | |
| Missing | 23.4% | 41.1% | 35.5% | 119 | |
| Water storage container | Plastic bucket or container | 24.7% | 37.7% | 37.7% | 1963 |
| Pot or earthenware vessel | 7.6% | 41.6% | 50.7% | 499 | |
| Metal container | 12.7% | 30.3% | 57.1% | 137 | |
| Other (including sachets) | 61.7% | 34.1% | 4.2% | 361 | |
| Missing | 59.8% | 40.2% | 0% | 12 | |
| Expenditure (GH₵) | Mean | 13.3 | 9.0 | 6.5 | NA |
| Median | 9.0 | 6.7 | 4.9 | NA | |
| Range (min–max) | 0.70−227.9 | 0.33−103.3 | 0.18–60.1 | NA | |
| Covariate | n | Category | Medium Contamination (1 to 72 CFU) | High Contamination (73+ CFU) | ||
|---|---|---|---|---|---|---|
| RRR * (95% CI) | p-value | RRR * (95% CI) | p-value | |||
| Water source | 2935 | Piped to premises | Reference | Reference | ||
| Standpipe, tanker or neighbours tap | 2.14 (1.26 to 3.64) | 0.005 | 6.69 (3.57 to 12.55) | <0.001 | ||
| Protected well | 1.85 (0.76 to 4.50) | 0.17 | 10.24 (4.30 to 24.39) | <0.001 | ||
| Unprotected well or spring | 2.11 (0.66 to 6.74) | 0.21 | 8.76 (2.67 to 28.76) | <0.001 | ||
| Rainwater | 5.10 (1.43 to 18.15) | 0.01 | 12.38 (3.13 to 48.94) | <0.001 | ||
| Surface water | 2.36 (1.02 to 5.49) | 0.05 | 32.28 (13.63 to 76.48) | <0.001 | ||
| Packaged water | 0.30 (0.18 to 0.49) | <0.001 | 0.06 (0.03 to 0.13) | <0.001 | ||
| Community-managed borehole | 2.88 (1.62 to 5.15) | <0.001 | 8.89 (4.68 to 16.88) | <0.001 | ||
| NGO-managed borehole | 2.17 (1.14 to 4.11) | 0.02 | 6.93 (3.14 to 15.27) | <0.001 | ||
| Other borehole | 3.77 (1.80 to 7.92) | <0.001 | 13.12 (5.86 to 29.37) | <0.001 | ||
| Expenditure | 2972 | (GH₵ per day) ** | 0.97 (0.95 to 0.98) | <0.001 | 0.89 (0.87 to 0.92) | <0.001 |
| Improved sanitation | 2966 | No | Reference | Reference | ||
| Yes | 0.43 (0.31 to 0.59) | <0.001 | 0.28 (0.20 to 0.39) | <0.001 | ||
| Soap observed | 2972 | No | Reference | Reference | ||
| Yes | 0.56 (0.42 to 0.76) | <0.001 | 0.44 (0.33 to 0.58) | <0.001 | ||
| Water obtained from source or vessel | 2853 | From source | Reference | Reference | ||
| From covered vessel | 3.40 (2.48 to 4.67) | <0.001 | 5.62 (3.99 to 7.91) | <0.001 | ||
| From uncovered vessel | 6.11 (2.56 to 14.60) | <0.001 | 24.29 (10.05 to 58.71) | <0.001 | ||
| Urban | 2972 | No | Reference | Reference | ||
| Yes | 0.43 (0.32 to 0.58) | <0.001 | 0.19 (0.14 to 0.26) | <0.001 | ||
| Water storage container | 2960 | Plastic bucket or container | Reference | Reference | ||
| Pot or earthenware vessel | 3.57 (2.23 to 5.72) | <0.001 | 4.35 (2.69 to 7.02) | <0.001 | ||
| Metal container | 1.56 (0.81 to 2.99) | 0.18 | 2.94 (1.60 to 5.38) | 0.001 | ||
| Other (including sachets) | 0.36 (0.25 to 0.53) | <0.001 | 0.04 (0.02 to 0.09) | <0.001 | ||
| Distance between latrine and water source | 2972 | Within 30 m | Reference | Reference | ||
| More than 30 m | 0.82 (0.48 to 1.39) | 0.45 | 0.87 (0.52 to 1.45) | 0.60 | ||
| Not ground water | 0.20 (0.12 to 0.32) | <0.001 | 0.08 (0.05 to 0.12) | <0.001 | ||
| Covariate | Category | Medium Contamination (1 to 72 CFU) | High Contamination (73+ CFU) | ||
|---|---|---|---|---|---|
| RRR * (95% CI) | p-Value | RRR * (95% CI) | p-Value | ||
| Constant | 2.79 (1.36 to 5.74) | 0.005 | 1.14 (0.45 to 2.94) | 0.78 | |
| Water source | Piped to premises | Reference | Reference | ||
| Standpipe, tanker or neighbours tap | 2.37 (1.17 to 4.83) | 0.02 | 8.37 (3.44 to 20.34) | <0.001 | |
| Protected well | 0.96 (0.28 to 3.33) | 0.95 | 9.32 (2.07 to 41.91) | 0.004 | |
| Unprotected well or spring | 2.13 (0.66 to 6.90) | 0.21 | 10.27 (2.81 to 37.46) | <0.001 | |
| Rainwater | 2.67 (0.16 to 44.15) | 0.49 | 15.73 (0.90 to 276.53) | 0.06 | |
| Surface water | 2.17 (0.73 to 6.45) | 0.16 | 42.01 (12.73 to 138.63) | <0.001 | |
| Packaged water | 0.18 (0.09 to 0.37) | <0.001 | 0.04 (0.01 to 0.15) | <0.001 | |
| Community-managed borehole | 2.02 (0.97 to 4.21) | 0.06 | 6.36 (2.49 to 16.26) | <0.001 | |
| NGO-managed borehole | 2.85 (1.01 to 8.03) | 0.05 | 6.86 (1.78 to 26.52) | 0.005 | |
| Other borehole | 1.77 (0.40 to 7.90) | 0.46 | 8.68 (1.76 to 42.82) | 0.008 | |
| Expenditure | (GH₵ per day) ** | 0.98 (0.95 to 1.00) | 0.08 | 0.98 (0.94 to 1.01) | 0.22 |
| Water source * expenditure interaction | Piped to premises | Reference | Reference | ||
| Standpipe, tanker or neighbours tap | 0.98 (0.95 to 1.01) | 0.24 | 0.97 (0.92 to 1.02) | 0.20 | |
| Protected well | 1.05 (0.94 to 1.17) | 0.41 | 0.96 (0.84 to 1.01) | 0.61 | |
| Unprotected well or spring | 1.03 (0.91 to 1.18) | 0.60 | 1.01 (0.89 to 1.15) | 0.87 | |
| Rainwater | 1.04 (0.78 to 1.40) | 0.78 | 0.92 (0.67 to 1.25) | 0.59 | |
| Surface water | 0.97 (0.89 to 1.06) | 0.52 | 0.89 (0.82 to 0.97) | 0.009 | |
| Packaged water | 1.02 (1.00 to 1.05) | 0.10 | 1.01 (0.93 to 1.10) | 0.85 | |
| Community-managed borehole | 1.01 (0.98 to 1.04) | 0.43 | 1.00 (0.94 to 1.06) | 0.97 | |
| NGO-managed borehole | 0.91 (0.82 to 1.01) | 0.08 | 0.93 (0.82 to 1.06) | 0.29 | |
| Other borehole | 1.08 (0.90 to 1.30) | 0.41 | 1.02 (0.86 to 1.23) | 0.79 | |
| Improved sanitation | No | Reference | Reference | ||
| Yes | 0.82 (0.58 to 1.15) | 0.26 | 0.90 (0.62 to 1.30) | 0.56 | |
| Water obtained from source or vessel | From source | Reference | Reference | ||
| From covered vessel | 0.86 (0.59 to 1.23) | 0.40 | 0.60 (0.39 to 0.93) | 0.02 | |
| From uncovered vessel | 1.86 (0.76 to 4.56) | 0.17 | 2.82 (1.14 to 6.96) | 0.03 | |
| Variable | Category | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Constant | 0.12 (0.02 to 0.76) | 0.03 | |
| Urban | No | Reference | |
| Yes | 2.26 (0.48 to 10.74) | 0.30 | |
| Region | Greater Accra | Reference | |
| Central | 2.55 (0.58 to 11.28) | 0.22 | |
| Other | 0.49 (0.13 to 1.83) | 0.29 | |
| Expenditure | 0.99 (0.94 to 1.05) | 0.85 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, J.; Dzodzomenyo, M.; Wardrop, N.A.; Johnston, R.; Hill, A.; Aryeetey, G.; Adanu, R. Effects of Sachet Water Consumption on Exposure to Microbe-Contaminated Drinking Water: Household Survey Evidence from Ghana. Int. J. Environ. Res. Public Health 2016, 13, 303. https://doi.org/10.3390/ijerph13030303
Wright J, Dzodzomenyo M, Wardrop NA, Johnston R, Hill A, Aryeetey G, Adanu R. Effects of Sachet Water Consumption on Exposure to Microbe-Contaminated Drinking Water: Household Survey Evidence from Ghana. International Journal of Environmental Research and Public Health. 2016; 13(3):303. https://doi.org/10.3390/ijerph13030303
Chicago/Turabian StyleWright, Jim, Mawuli Dzodzomenyo, Nicola A. Wardrop, Richard Johnston, Allan Hill, Genevieve Aryeetey, and Richard Adanu. 2016. "Effects of Sachet Water Consumption on Exposure to Microbe-Contaminated Drinking Water: Household Survey Evidence from Ghana" International Journal of Environmental Research and Public Health 13, no. 3: 303. https://doi.org/10.3390/ijerph13030303
APA StyleWright, J., Dzodzomenyo, M., Wardrop, N. A., Johnston, R., Hill, A., Aryeetey, G., & Adanu, R. (2016). Effects of Sachet Water Consumption on Exposure to Microbe-Contaminated Drinking Water: Household Survey Evidence from Ghana. International Journal of Environmental Research and Public Health, 13(3), 303. https://doi.org/10.3390/ijerph13030303






