Simultaneous Detection of Five Pathogens from Cerebrospinal Fluid Specimens Using Luminex Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viral and Bacterial Strains
2.2. Clinical Specimens
2.3. Primer
| Organism | GenBank Accession No. | Target Gene | Oligonucleotide Sequence of Primer (5’–3’) | Length (bp) | Position (5’–3’) |
|---|---|---|---|---|---|
| M. tuberculosis | CP009480.1 | IS986 | F- CTTAACATTTAACTTCTATAACAC-12C-CGTGAGGGCATCGAGGTGGCR-biotin-GCGTAGGCGTCGGTGACAAA | 245 | 889650–889894 |
| C . neoformans | L38588.1 | URA | F- CACTTAATTCATTCTAAATCTATC-12C-TGTCCTAACCAGTGCGACAGCGATGR-biotin-GTACTTCCTGACCTCTTGCAGCTCC | 360 | 390–749 |
| S. pneumoniae | AF467249.1 | lytA | F- ATTAAACAACTCTTAACTACACAA-12C-CGCAATCTAGCAGATGAAGCAGGTT R-biotin-AAGGGTCAACGTGGTCTGAGTGGTT | 124 | 328–451 |
| HSV-1 | X14112.1 | Gene 42 | F- TACATTCAACACTCTTAAATCAAA-12C-GCCGTTGAGCTAGCCAGCGA R-biotin-GTGCTGGTGCTGGACGACAC | 257 | 93557–93813 |
| HSV-2 | Z86099.2 | TK | F- ACTACTTATTCTCAAACTCTAATA-12C-GTAAGCGCGGGCCAAAGGATR-biotin-TCAAACACGGAAGCCCGAAC | 234 | 46635–46868 |
2.4. Plasmid Preparation
2.5. DNA Extraction and PCR Amplification
2.6. PCR Product Identification and Luminex Assay
2.7. Determination of Cutoff Values and Data Analysis
3. Results
3.1. Specificity of Primers
3.2. Determination of Amplification Conditions for Five-Plex PCR

3.3. Analytical Specificity of PCR-Luminex Assay
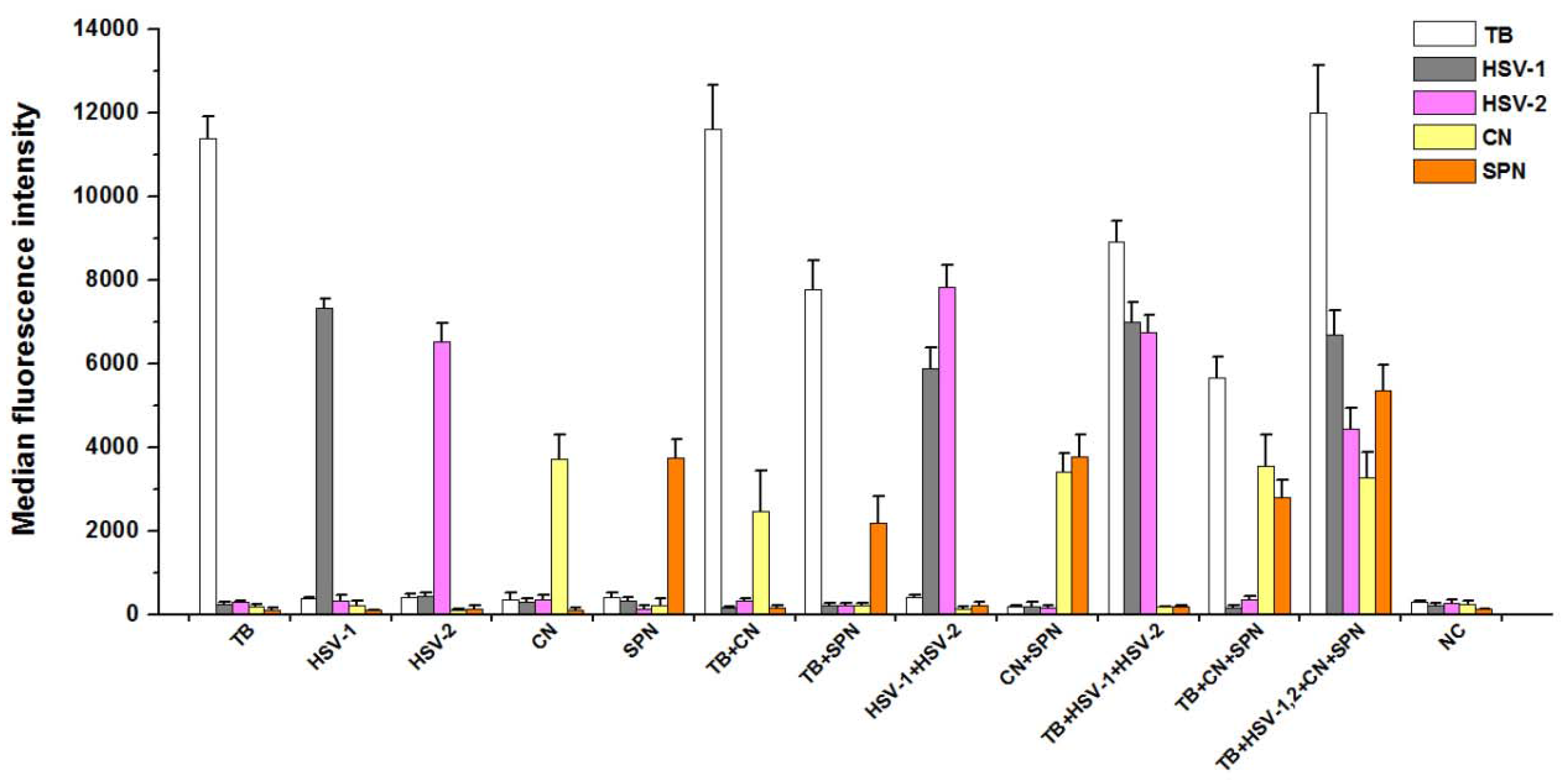
3.4. Analytical Sensitivity of PCR-Luminex Assay
| Organism | Plasmid Quantity (Copies) | cMFI |
|---|---|---|
| M. t uberculosis | 108 | 16.95 ± 0.45 |
| 106 | 10.06 ± 0.22 | |
| 104 | 7.49 ± 0.30 | |
| 102 | 6.79 ± 0.20 | |
| 101 | 0.93 ± 0.07 | |
| HSV-1 | 102 | 11.41 ± 0.72 |
| 50 | 6.56 ± 0.20 | |
| 10 | 5.30 ± 0.31 | |
| 5 | 2.30 ± 0.12 | |
| 1 | 2.45 ± 0.15 | |
| HSV-2 | 108 | 17.72 ± 0.59 |
| 106 | 7.14 ± 0.38 | |
| 104 | 6.19 ± 0.14 | |
| 102 | 3.49 ± 0.07 | |
| 101 | 2.25 ± 0.02 | |
| C. neoformans | 108 | 34.80 ± 0.21 |
| 106 | 31.86 ± 0.18 | |
| 104 | 28.29 ± 0.45 | |
| 102 | 9.62 ± 0.12 | |
| 101 | 1.23 ± 0.10 | |
| S. pneumoniae | 108 | 8.78 ± 0.47 |
| 106 | 6.15 ± 0.36 | |
| 104 | 4.94 ± 0.29 | |
| 102 | 3.77 ± 0.22 | |
| 101 | 2.18 ± 0.14 |
3.5. Assessment of PCR-Luminex Performance Using CSF Specimens
| PCR-Luminex | Smear Positive | Smear Negative | Sensitivity | Specificity | Kappa |
|---|---|---|---|---|---|
| (+) | 49 | 1 | 90.7% | 99.1% | 0.9153 |
| (−) | 5 | 108 |
| PCR-Luminex | MGG Positive | MGG Negative | Sensitivity | Specificity | Kappa |
|---|---|---|---|---|---|
| (+) | 23 | 4 | 92% | 97.1% | 0.8628 |
| (−) | 2 | 134 |
| PCR-Luminex | ELISA Positive | ELISA Negative | Sensitivity | Specificity | Kappa | |
|---|---|---|---|---|---|---|
| HSV-1 | (+) | 21 | 8 | 80.8% | 94.2% | 0.7158 |
| (−) | 5 | 129 | ||||
| HSV-2 | (+) | 1 | 1 | 100% | 99% | 0.6639 |
| (−) | 0 | 161 | ||||
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
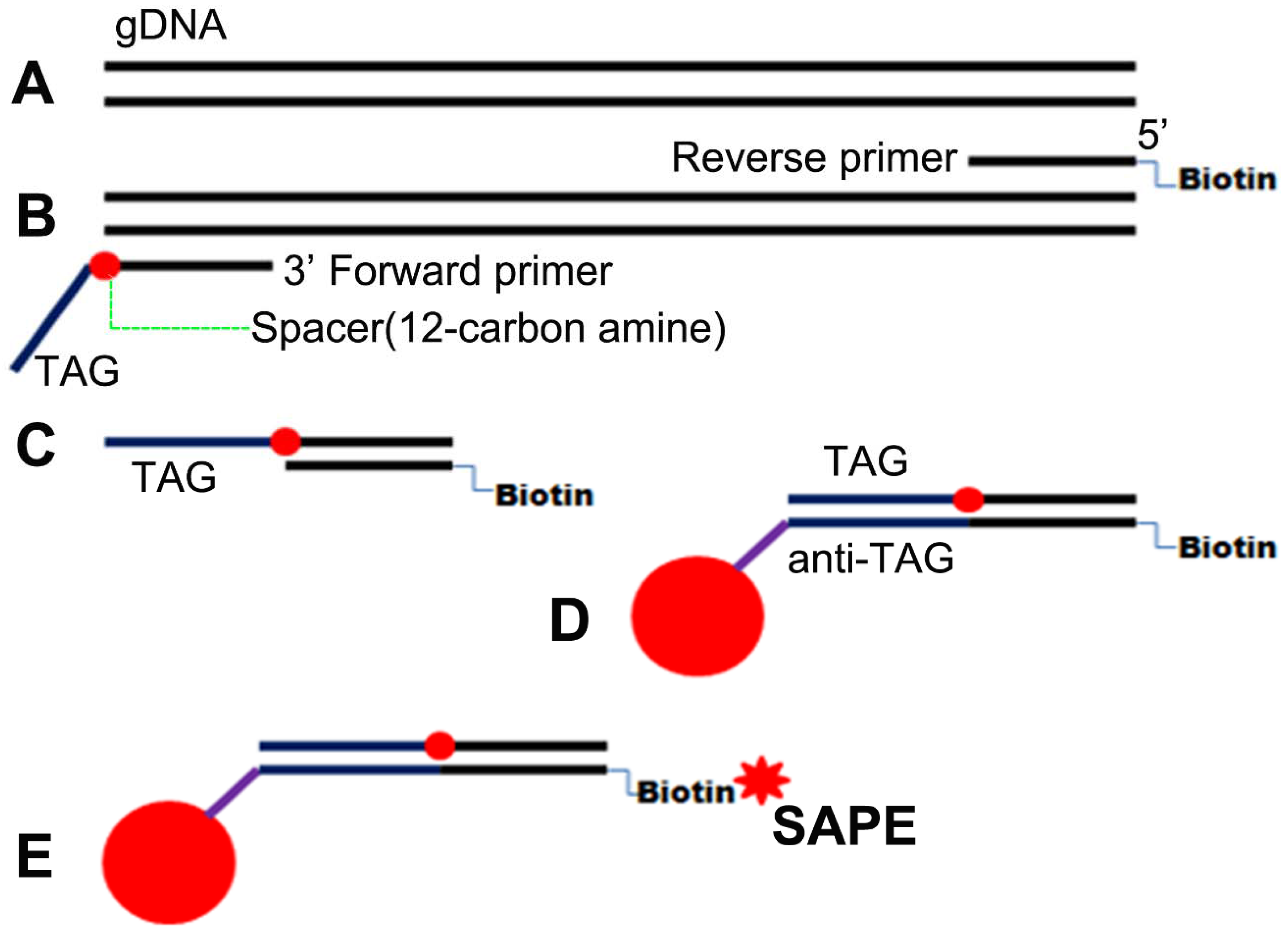
References
- Bautista, C. Central nervous system infections. Crit. Care Nurs. Clin. N. Am. 2013, 25, ix. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Gill, A.K.; Kim, K.S. Treatment strategies for central nervous system infections: An update. Expert Opin. Pharmacother. 2015, 16, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Yansouni, C.P.; Bottieau, E.; Lutumba, P.; Winkler, A.S.; Lynen, L.; Buscher, P.; Jacobs, J.; Gillet, P.; Lejon, V.; Alirol, E.; et al. Rapid diagnostic tests for neurological infections in Central Africa. Lancet. Infect. Dis. 2013, 13, 546–558. [Google Scholar] [CrossRef]
- Thomson, R.B., Jr.; Bertram, H. Laboratory diagnosis of central nervous system infections. Infect. Dis. Clin. N. Am. 2001, 15, 1047–1071. [Google Scholar] [CrossRef]
- Kamei, S. Bacterial meningitis: Determination of pathogens and therapeutic management. Clin. Neurol. 2004, 44, 846–848. [Google Scholar]
- Miner, J.R.; Heegaard, W.; Mapes, A.; Biros, M. Presentation, time to antibiotics, and mortality of patients with bacterial meningitis at an urban county medical center. J. Emerg. Med. 2001, 21, 387–392. [Google Scholar] [CrossRef]
- Nemeth, J.; Oesch, G.; Kuster, S.P. Bacteriostatic versus bactericidal antibiotics for patients with serious bacterial infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2015, 70, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Morita, A. Clinical and laboratory diagnosis of central nervous system infections. Brain Nerve 2015, 67, 777–785. [Google Scholar] [PubMed]
- Sussmuth, S.D.; Brettschneider, J.; Spreer, A.; Wick, M.; Jesse, S.; Lewerenz, J.; Otto, M.; Tumani, H. Current cerebrospinal fluid diagnostics for pathogen-related diseases. Der Nervenarzt 2013, 84, 229–244. [Google Scholar] [PubMed]
- Cambanis, A.; Ramsay, A.; Wirkom, V.; Tata, E.; Cuevas, L.E. Investing time in microscopy: An opportunity to optimise smear-based case detection of tuberculosis. Int. J. Tuberc. Lung Dis. 2007, 11, 40–45. [Google Scholar] [PubMed]
- Qing, X. Diagnosis and treatment of bacterial meningitis. Chin. J. Contemp. Neurol. Neurosurg. 2004, 8, 210–211. [Google Scholar]
- Zhao, J.; Zhao, Y.; Ge, C.X.; Yu, M.H.; Ma, H.L. Comparison of several methods in the diagnosis of cryptococcal meningitis. J. Qiqihar Univ. Med. 2015, 11, 4442–4443. [Google Scholar]
- Philip, N.; William, T.; William, D.V. Diagnosis of tuberculous meningitis: Challenges and promises. Malaysian J. Pathol. 2015, 37, 1–9. [Google Scholar]
- Jaaskelainen, A.J.; Piiparinen, H.; Lappalainen, M.; Vaheri, A. Improved multiplex-PCR and microarray for herpesvirus detection from CSF. J. Clin. Virol. 2008, 42, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Kusum, S.; Aman, S.; Pallab, R.; Kumar, S.S.; Manish, M.; Sudesh, P.; Subhash, V.; Meera, S. Multiplex PCR for rapid diagnosis of tuberculous meningitis. J. Neurol. 2011, 258, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Theodore, M.J.; Mair, R.; Trujillo-Lopez, E.; Du Plessis, M.; Wolter, N.; Baughman, A.L.; Hatcher, C.; Vuong, J.; Lott, L.; et al. Clinical validation of multiplex real-time PCR assays for detection of bacterial meningitis pathogens. J. Clin. Microbiol. 2012, 50, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, H.; Guo, Z.; Yang, R.; Zhang, L. Advances in research on multiple biological detecting technologies. Milit. Med. Sci. 2012, 36, 713–717. [Google Scholar] [CrossRef]
- Fraiture, M.A.; Herman, P.; Taverniers, I.; De Loose, M.; Deforce, D.; Roosens, N.H. Current and new approaches in GMO detection: Challenges and solutions. BioMed Res. Int. 2015, 2015, 392872. [Google Scholar] [CrossRef] [PubMed]
- Korczak, B.; Frey, J.; Schrenzel, J.; Pluschke, G.; Pfister, R.; Ehricht, R.; Kuhnert, P. Use of diagnostic microarrays for determination of virulence gene patterns of Escherichia coli K1, a major cause of neonatal meningitis. J. Clin. Microbiol. 2005, 43, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, S.A.; Van der Zee, C.A.; Oliver, K.G.; Karem, K.L.; Jacobson, J.W. Quantitative, multiplexed detection of bacterial pathogens: DNA and protein applications of the Luminex LabMAP system. J. Microbiol. Meth. 2003, 53, 245–252. [Google Scholar] [CrossRef]
- Tait, B.D.; Hudson, F.; Cantwell, L.; Brewin, G.; Holdsworth, R.; Bennett, G.; Jose, M. Review article: Luminex technology for HLA antibody detection in organ transplantation. Nephrology 2009, 14, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.E.; Sanchez, A.M.; D’Souza, M.P.; Rountree, W.; Denny, T.N.; Kalos, M.; Sempowski, G.D. Development and implementation of a proficiency testing program for luminex bead-based cytokine assays. J. Immunol. Meth. 2014, 409, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, Z.; Ji, S.; Gottschalk, M.; Zheng, H.; Xu, J. Simultaneous detection of 33 streptococcus suis serotypes using the luminex xTAG(r) assay. J. Microbiol. Meth. 2015, 117, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, S.A. Applications of luminex xmap technology for rapid, high-throughput multiplexed nucleic acid detection. Int. J. Clin. Chem. 2006, 363, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Moller, J.K. Detection of neisseria meningitidis in cerebrospinal fluid using a multiplex PCR and the luminex detection technology. Meth. Mol. Biol. 2012, 799, 37–53. [Google Scholar]
- Pabbaraju, K.; Wong, S.; Tokaryk, K.L.; Fonseca, K.; Drews, S.J. Comparison of the luminex xTAG respiratory viral panel with xtag respiratory viral panel fast for diagnosis of respiratory virus infections. J. Clin. Microbiol. 2011, 49, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Verweij, J.J.; Noor, Z.; Sobuz, S.U.; Lieshout, L.; Petri, W.A., Jr.; Haque, R.; Houpt, E.R. High throughput multiplex PCR and probe-based detection with luminex beads for seven intestinal parasites. Am. J. Tropic. Med. Hyg. 2011, 84, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Duan, Z.J. Application of luminex xMAP technology in infectious diseases. Chin. J. Virol. 2010, 26, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shi, M.; Feng, G.D.; Liu, J.Y.; Wang, B.J.; Shi, X.D.; Ma, L.; Liu, X.D.; Yang, Y.N.; Dai, W.; et al. A highly efficient ziehl-neelsen stain: Identifying de novo intracellular Mycobacterium tuberculosis and improving detection of extracellular M. tuberculosis in cerebrospinal fluid. J. Clin. Microbiol. 2012, 50, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.D.; Shi, M.; Ma, L.; Chen, P.; Wang, B.J.; Zhang, M.; Chang, X.L.; Su, X.C.; Yang, Y.N.; Fan, X.H.; et al. Diagnostic accuracy of intracellular mycobacterium tuberculosis detection for tuberculous meningitis. Am. J. Respir. Critic. Care Med. 2014, 189, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Feng, G.D.; Yang, Y.N.; Dai, W.; Zhao, G. Diagnostic value of may grunwald giemsa staining of cerebrospinal fluid in patients with cryptococcal meningitis. J. Int. Neurol. Neurosurg. 2013, 40, 220–222. [Google Scholar]
- Marais, S.; Thwaites, G.; Schoeman, J.F.; Torok, M.E.; Misra, U.K.; Prasad, K.; Donald, P.R.; Wilkinson, R.J.; Marais, B.J. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect. Dis. 2010, 10, 803–812. [Google Scholar] [CrossRef]
- Boving, M.K.; Pedersen, L.N.; Moller, J.K. Eight-plex PCR and liquid-array detection of bacterial and viral pathogens in cerebrospinal fluid from patients with suspected meningitis. J. Clin. Microbiol. 2009, 47, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, M.; Verweij, J.J.; Sethabutr, O.; Bodhidatta, L.; Garcia, L.; Maro, A.; Kumburu, H.; Gratz, J.; Kibiki, G.; Houpt, E.R. Multiplex polymerase chain reaction method to detect Cyclospora, Cystoisospora, and Microsporidia in stool samples. Diagn.Microbiol. Infect. Dis. 2011, 71, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; Grindle, K.; Pappas, T.; Marshall, D.J.; Moser, M.J.; Beaty, E.L.; Shult, P.A.; Prudent, J.R.; Gern, J.E. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J. Clin. Microbiol. 2007, 45, 2626–2634. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlugt, R.A.; Van Raaij, H.; De Weerdt, M.; Bergervoet, J.H. Multiplex detection of plant pathogens through the luminex magplex bead system. Meth. Mol. Biol. 2015, 1302, 283–299. [Google Scholar]
- Booth, S.A.; Drebot, M.A.; Martin, I.E.; Ng, L.K. Design of oligonucleotide arrays to detect point mutations: Molecular typing of antibiotic resistant strains of Neisseria gonorrhoeae and Hantavirus infected deer mice. Mol. Cell. Probe. 2003, 17, 77–84. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, C.H.; Lee, T.T.; Peck, K. Optimization of probe length and the number of probes per gene for optimal microarray analysis of gene expression. Nucl. Acid. Res. 2004, 32, e99. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Preibisch, S. Specific and nonspecific hybridization of oligonucleotide probes on microarrays. Biophys. J. 2005, 89, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.B.; Levicky, R.L.; Herne, T.M.; Tarlo, M.J. Immobilization of nucleic acids at solid surfaces: Effect of oligonucleotide length on layer assembly. Biophys. J. 2000, 79, 975–981. [Google Scholar] [CrossRef]
- Wilson, W.J.; Erler, A.M.; Nasarabadi, S.L.; Skowronski, E.W.; Imbro, P.M. A multiplexed PCR-coupled liquid bead array for the simultaneous detection of four biothreat agents. Mol. Cell. Prob. 2005, 19, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jokela, P.; Piiparinen, H.; Mannonen, L.; Auvinen, E.; Lappalainen, M. Performance of the luminex xTAG respiratory viral panel fast in a clinical laboratory setting. J. Virol. Meth. 2012, 182, 82–86. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wu, R.; Shi, X.; Feng, D.; Feng, G.; Yang, Y.; Dai, W.; Bian, T.; Liu, T.; He, Y.; et al. Simultaneous Detection of Five Pathogens from Cerebrospinal Fluid Specimens Using Luminex Technology. Int. J. Environ. Res. Public Health 2016, 13, 193. https://doi.org/10.3390/ijerph13020193
Zhou L, Wu R, Shi X, Feng D, Feng G, Yang Y, Dai W, Bian T, Liu T, He Y, et al. Simultaneous Detection of Five Pathogens from Cerebrospinal Fluid Specimens Using Luminex Technology. International Journal of Environmental Research and Public Health. 2016; 13(2):193. https://doi.org/10.3390/ijerph13020193
Chicago/Turabian StyleZhou, Linfu, Rui Wu, Xiaodan Shi, Dongyun Feng, Guodong Feng, Yining Yang, Wen Dai, Ting Bian, Tingting Liu, Ying He, and et al. 2016. "Simultaneous Detection of Five Pathogens from Cerebrospinal Fluid Specimens Using Luminex Technology" International Journal of Environmental Research and Public Health 13, no. 2: 193. https://doi.org/10.3390/ijerph13020193
APA StyleZhou, L., Wu, R., Shi, X., Feng, D., Feng, G., Yang, Y., Dai, W., Bian, T., Liu, T., He, Y., Shi, M., & Zhao, G. (2016). Simultaneous Detection of Five Pathogens from Cerebrospinal Fluid Specimens Using Luminex Technology. International Journal of Environmental Research and Public Health, 13(2), 193. https://doi.org/10.3390/ijerph13020193





