Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
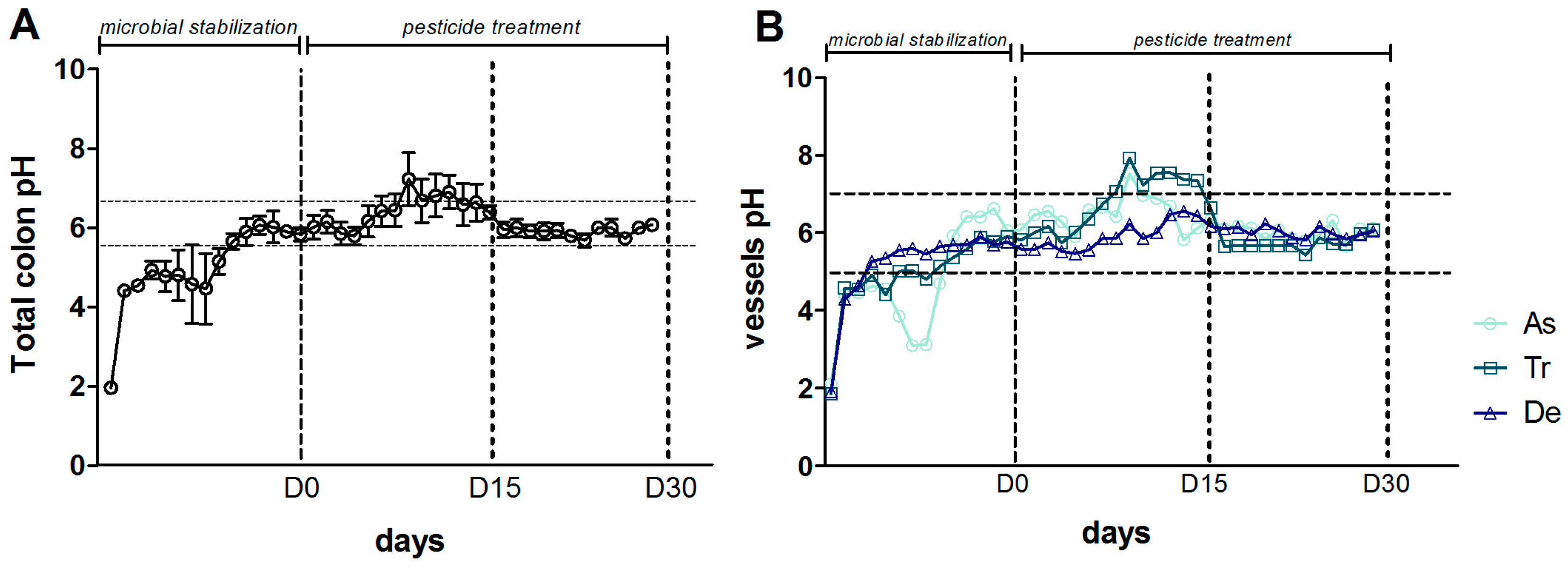
2.2. Description of the SHIME®
2.3. Plate Counts
2.4. SCFA Assays
2.5. l- and d-Lactate Assay
2.6. Extraction and Amplification of DNA from SHIME® Samples
2.7. Temporal Temperature Gradient Gel Electrophoresis (TTGE)
2.8. Real-Time Quantitative PCR (RT-qPCR) Analyses of Bacterial 16S rDNA Genes
2.9. Statistical Analysis
3. Results
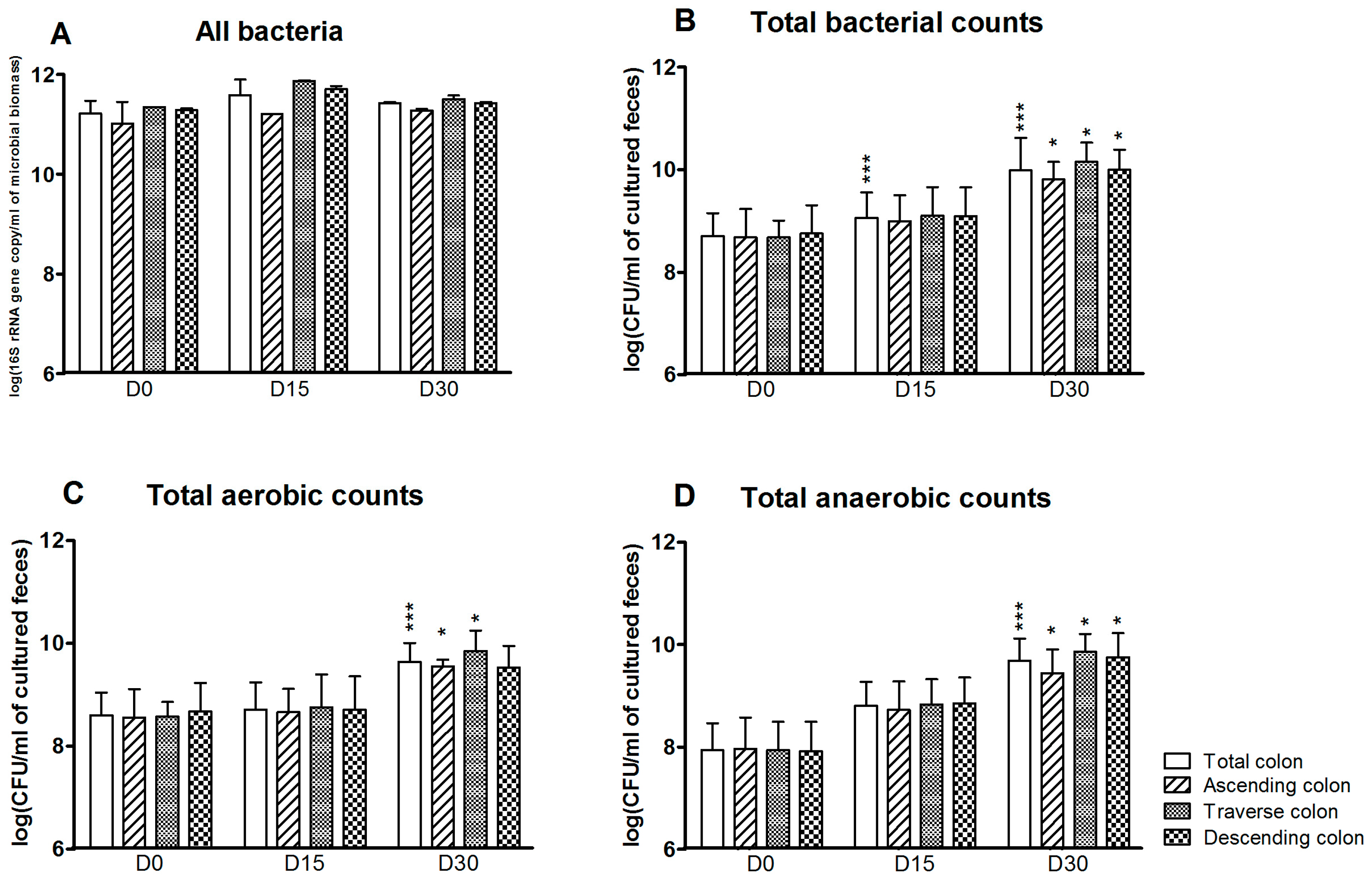
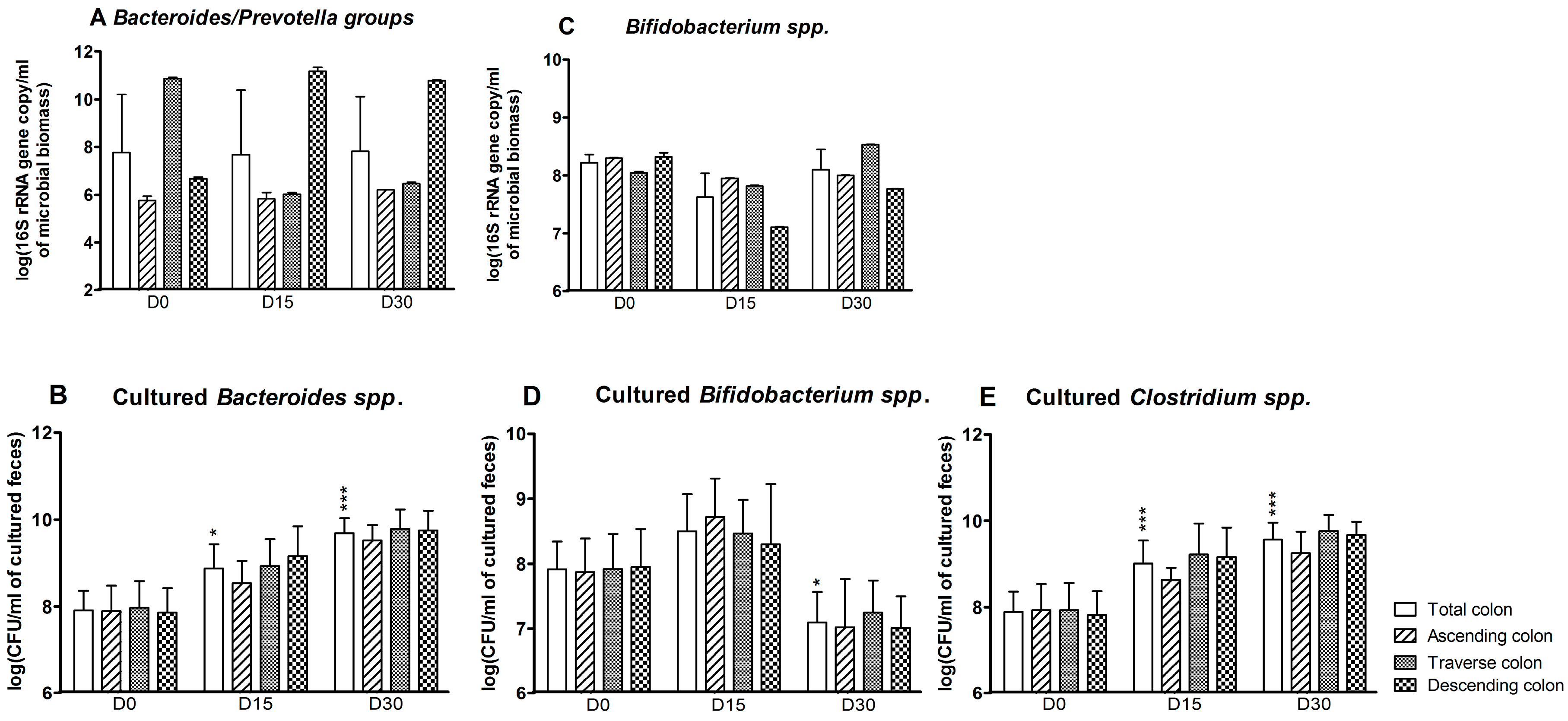
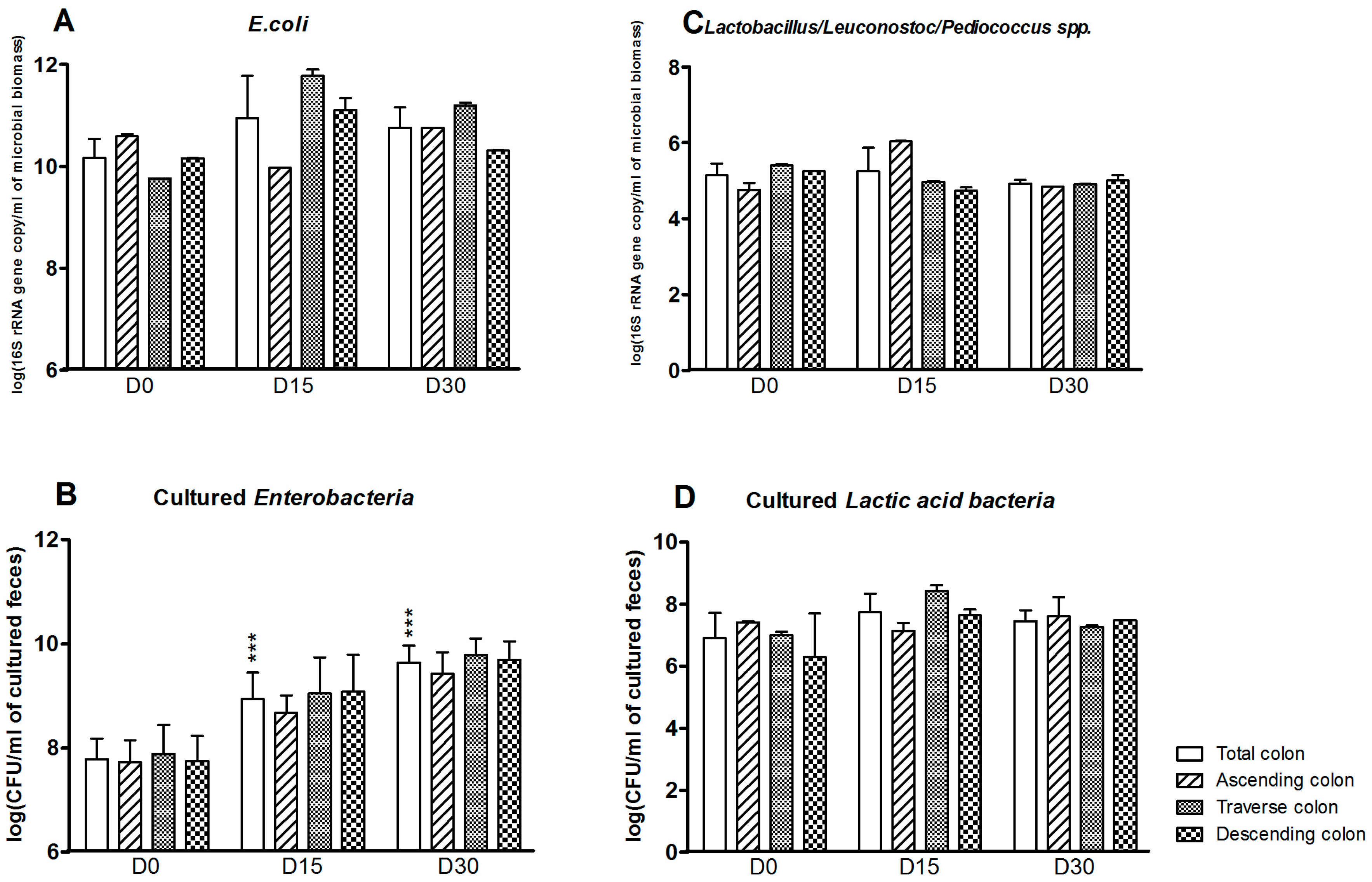
3.1. Bacterial Composition
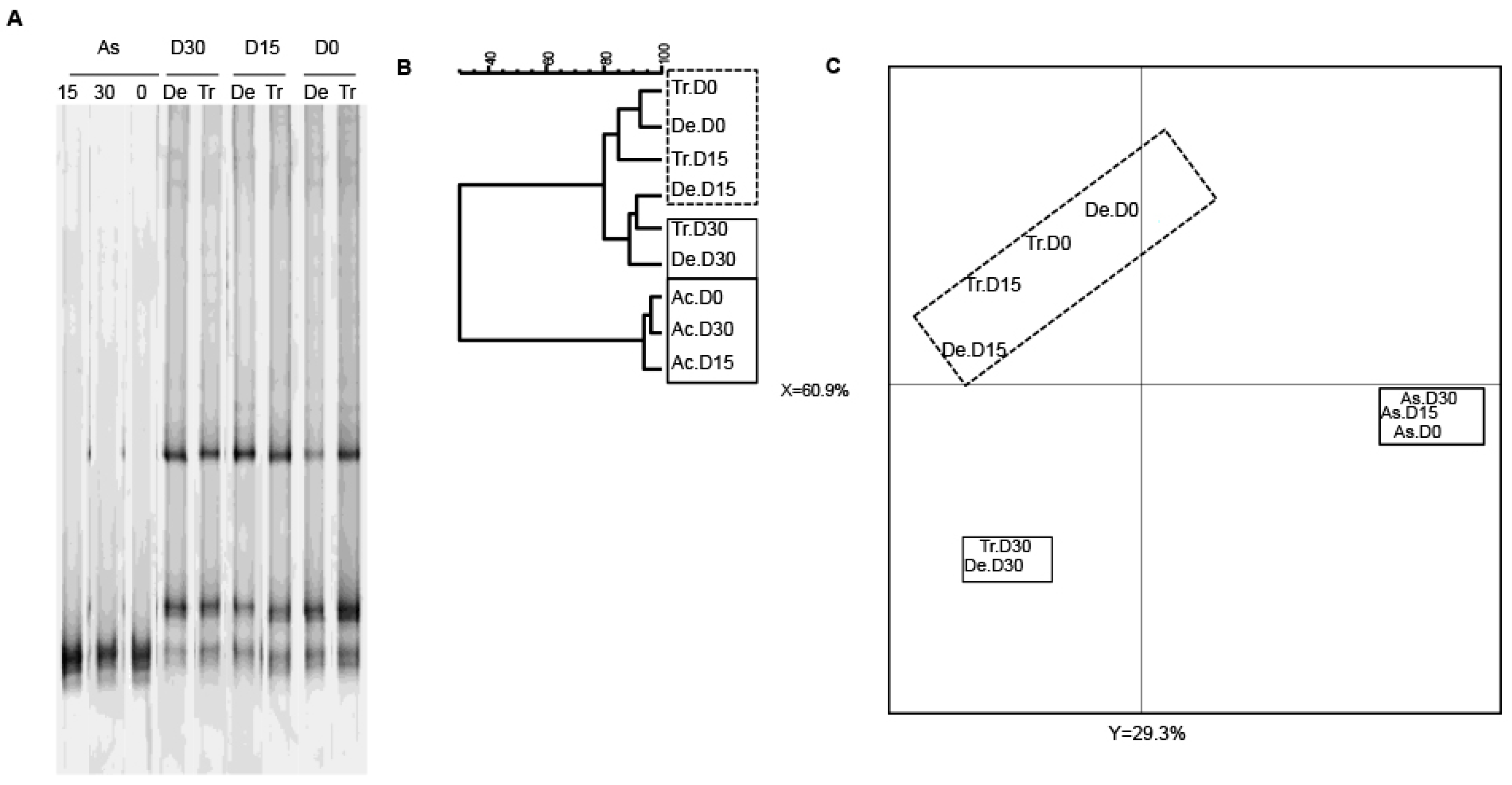
3.2. Bacterial Diversity
3.3. Levels of Bacterial Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaacks, L.M.; Staimez, L.R. Association of persistent organic pollutants and non-persistent pesticides with diabetes and diabetes-related health outcomes in Asia: A systematic review. Environ. Int. 2015, 76, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Nougadère, A.; Sirot, V.; Kadar, A.; Fastier, A.; Truchot, E.; Vergnet, C.; Hommet, F.; Baylé, J.; Gros, P.; Leblanc, J.-C. Total diet study on pesticide residues in France: Levels in food as consumed and chronic dietary risk to consumers. Environ. Int. 2012, 45, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, S.; Liang, D.; Shi, X.; Wang, F.; Liu, W.; Zhang, L.; Chen, L.; Gu, Y.; Tian, Y. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: A birth cohort study in Shenyang, China. PLoS ONE 2014, 9, e88491. [Google Scholar] [CrossRef] [PubMed]
- Kwong, T.C. Organophosphate pesticides: Biochemistry and clinical toxicology. Ther. Drug Monit. 2002, 24, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008, 38, 1–125. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Magnanti, B.L.; Correia Carreira, S.; Yang, A.; Alamo-Hernández, U.; Riojas-Rodriguez, H.; Calamandrei, G.; Koppe, J.G.; Krayer von Krauss, M.; Keune, H.; et al. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Venerosi, A.; Tait, S.; Stecca, L.; Chiarotti, F.; De Felice, A.; Cometa, M.F.; Volpe, M.T.; Calamandrei, G.; Ricceri, L. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring—A mouse study. Environ. Health 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.J.; Shenoy, S.S. Intestinal permeability of chlorpyrifos using the single-pass intestinal perfusion method in the rat. Toxicology 2003, 184, 125–133. [Google Scholar] [CrossRef]
- Leoni, C.; Balduzzi, M.; Buratti, F.M.; Testai, E. The contribution of human small intestine to chlorpyrifos biotransformation. Toxicol. Lett. 2012, 215, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Joly Condette, C.; Khorsi-Cauet, H.; Morlière, P.; Zabijak, L.; Reygner, J.; Bach, V.; Gay-Quéheillard, J. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS ONE 2014, 9, e102217. [Google Scholar] [CrossRef] [PubMed]
- Joly Condette, C.; Bach, V.; Mayeur, C.; Gay-Quéheillard, J.; Khorsi-Cauet, H. Chlorpyrifos Exposure During Perinatal Period Affects Intestinal Microbiota Associated with Delay of Maturation of Digestive Tract in Rats. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 30–40. [Google Scholar] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. The gut microbiota and obesity: From correlation to causality. Nat. Rev. Microbiol. 2013, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; Gilijamse, P.W.; Pai, N.; Kaplan, L.M. Role of the Microbiome in Energy Regulation and Metabolism. Gastroenterology 2014, 146, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiol. Read. Engl. 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.F.; Macfarlane, S.; Macfarlane, G.T. Effects of Antibiotics on Bacterial Species Composition and Metabolic Activities in Chemostats Containing Defined Populations of Human Gut Microorganisms. Antimicrob. Agents Chemother. 2013, 57, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Joly, C.; Gay-Quéheillard, J.; Léké, A.; Chardon, K.; Delanaud, S.; Bach, V.; Khorsi-Cauet, H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) and in the rat. Environ. Sci. Pollut. Res. 2013, 20, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, V.; Catone, T.; Turco, L.; Di Consiglio, E.; Testai, E.; De Angelis, I. Effects of the pesticide clorpyrifos on an in vitro model of intestinal barrier. Toxicol. In Vitro 2007, 21, 308–313. [Google Scholar] [CrossRef] [PubMed]
- De Wiele, T.V.; Boon, N.; Possemiers, S.; Jacobs, H.; Verstraete, W. Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 51, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, M.; Vilchez-Vargas, R.; Bussche, J.V.; Truchado, P.; Jauregui, R.; El Hage, R.A.; Pieper, D.H.; Vanhaecke, L.; Van de Wiele, T. High-fiber and high-protein diets shape different gut microbial communities, which ecologically behave similarly under stress conditions, as shown in a gastrointestinal simulator. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Morales, M.L.V.; Saad, S.M.I.; Adorno, M.A.T.; Sakamoto, I.K.; Rossi, E.A. Prebiotic effect of fructooligosaccharide in the simulator of the human intestinal microbial ecosystem (SHIME® model). J. Med. Food 2014, 17, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.; Rivière, A.; Moens, F.; Van den Abbeele, P.; Geirnaert, A.; Rogelj, I.; Leroy, F.; De Vuyst, L. Inulin-type fructan fermentation by bifidobacteria depends on the strain rather than the species and region in the human intestine. Appl. Microbiol. Biotechnol. 2016, 100, 4097–4107. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, L.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Chemopreventive effects from prebiotic inulin towards microbial 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine bioactivation. J. Appl. Microbiol. 2009, 106, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- De Boever, P.; Deplancke, B.; Verstraete, W. Fermentation by gut microbiota cultured in a simulator of the human intestinal microbial ecosystem is improved by supplementing a soygerm powder. J. Nutr. 2000, 130, 2599–2606. [Google Scholar] [PubMed]
- Possemiers, S.; Verthé, K.; Uyttendaele, S.; Verstraete, W. PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 49, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Djouzi, Z.; Andrieux, C.; Degivry, M.C.; Bouley, C.; Szylit, O. The association of yogurt starters with Lactobacillus casei DN 114.001 in fermented milk alters the composition and metabolism of intestinal microflora in germ-free rats and in human flora-associated rats. J. Nutr. 1997, 127, 2260–2266. [Google Scholar] [PubMed]
- Mayeur, C.; Gratadoux, J.-J.; Bridonneau, C.; Chegdani, F.; Larroque, B.; Kapel, N.; Corcos, O.; Thomas, M.; Joly, F. Faecal d/l lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS ONE 2013, 8, e54335. [Google Scholar] [CrossRef] [PubMed]
- Godon, J.J.; Zumstein, E.; Dabert, P.; Habouzit, F.; Moletta, R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 1997, 63, 2802–2813. [Google Scholar] [PubMed]
- Gérard, P.; Béguet, F.; Lepercq, P.; Rigottier-Gois, L.; Rochet, V.; Andrieux, C.; Juste, C. Gnotobiotic rats harboring human intestinal microbiota as a model for studying cholesterol-to-coprostanol conversion. FEMS Microbiol. Ecol. 2004, 47, 337–343. [Google Scholar] [CrossRef]
- De Boever, P.; Wouters, R.; Vermeirssen, V.; Boon, N.; Verstraete, W. Development of a six-stage culture system for simulating the gastrointestinal microbiota of weaned infants. Microb. Ecol. Health Dis. 2001, 13, 111–123. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Grootaert, C.; Possemiers, S.; Verstraete, W.; Verbeken, K.; Van de Wiele, T. In vitro model to study the modulation of the mucin-adhered bacterial community. Appl. Microbiol. Biotechnol. 2009, 83, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef] [PubMed]
- Suau, A.; Bonnet, R.; Sutren, M.; Godon, J.J.; Gibson, G.R.; Collins, M.D.; Doré, J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 1999, 65, 4799–4807. [Google Scholar] [PubMed]
- Harishankar, M.K.; Sasikala, C.; Ramya, M. Efficiency of the intestinal bacteria in the degradation of the toxic pesticide, chlorpyrifos. 3 Biotech 2013, 3, 137–142. [Google Scholar] [CrossRef]
- Cho, K.M.; Math, R.K.; Islam, S.M.A.; Lim, W.J.; Hong, S.Y.; Kim, J.M.; Yun, M.G.; Cho, J.J.; Yun, H.D. Biodegradation of chlorpyrifos by lactic acid bacteria during kimchi fermentation. J. Agric. Food Chem. 2009, 57, 1882–1889. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Walker, A.; Morgan, J.A.W.; Wright, D.J. Effects of Soil pH on the Biodegradation of Chlorpyrifos and Isolation of a Chlorpyrifos-Degrading Bacterium. Appl. Environ. Microbiol. 2003, 69, 5198–5206. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.B.; Sharma, S.; Saini, H.S.; Chadha, B.S. Biosurfactant production by Pseudomonas sp. and its role in aqueous phase partitioning and biodegradation of chlorpyrifos. Lett. Appl. Microbiol. 2009, 49, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandez-Garayzabal, J.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A. The phylogeny of the genus Clostridium: Proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 1994, 44, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Anderson, S.E.; Lobley, G.E.; Flint, H.J. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 2007, 73, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, V.L.; Reiter, N.; Perner, A. Luminal concentrations of l- and d-lactate in the rectum may relate to severity of disease and outcome in septic patients. Crit. Care 2006, 10. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Oh, M.S.; Carroll, H.J. d-lactic acidosis: A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine 1998, 77, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Holtrop, G.; Duncan, S.H.; Anderson, S.E.; Calder, A.G.; Flint, H.J.; Lobley, G.E. Rates of production and utilization of lactate by microbial communities from the human colon. FEMS Microbiol. Ecol. 2011, 77, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.S.; Briceño, G.E.; Saez, J.M.; Benimeli, C.S.; Diez, M.C.; Amoroso, M.J. Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Tsao, C.-Y.; Wu, H.-C.; Quan, D.N.; Payne, G.F.; Rubloff, G.W.; Bentley, W.E. Distal modulation of bacterial cell–cell signalling in a synthetic ecosystem using partitioned microfluidics. Lab Chip 2015, 15, 1842–1851. [Google Scholar] [CrossRef] [PubMed]

| Reactor | Volume (mL) | Residence Time (h) | pH |
|---|---|---|---|
| R1: Stomach | 200 | 3 | 2 |
| R2: Duodenum/Jejunum | 300 | 3 | 7 |
| R3: Ileum/Caecum | 300 | 4 | 7 |
| R4: Ascending Colon | 1000 | 20 | 5.5–6.0 |
| R5: Transverse Colon | 1600 | 32 | 6.0–6.4 |
| R6: Descending Colon | 1200 | 24 | 6.4–6.8 |
| Bacterial Group | Medium | Condition |
|---|---|---|
| Total Aerobes | Columbia Agar a | Aerobic |
| Total Anaerobes | Blood Columbia Agar a | Anaerobic |
| Bacteroides spp. | Schaedler Agar a | Anaerobic |
| Clostridium spp. | Shahidi-Ferguson Perfringens a | Anaerobic |
| Enterobacteria | Bromocresol Purple a | Aerobic |
| Lactic Acid Bacteria | de Man Rogosa Sharpe b | Aerobic |
| Bifidobacteria | Bereens | Anaerobic |
| Primer | Temperature-Time Program | Specificity |
|---|---|---|
| Bact 968-GC-f Bact 1401-r | (1) 15Ë′; 95 °C | Bacteria |
| (2) 1′; 97 °C/1′; 58 °C/1′30; 72 °C (×30) | ||
| (3) 15′; 72 °C | ||
| Bif 164-f Bif 662-GC-r | (1) 15′; 95 °C | Bifidobacterium spp. |
| (2) 1′; 97 °C/1′; 58°C/1′30; 72 °C (×30) | ||
| (3) 15′; 72 °C |
| Vessel | Ascending | Transverse | Descending | Colon | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | D0 | D15 | D30 | D0 | D15 | D30 | D0 | D15 | D30 | D0 | D15 | D30 |
| Total SCFA (mM) | 15.3 ± 0.1 | 17.1 ± 0.3 | 19.3 ± 0.2 | 49.7 ± 0.1 | 6.6 ± 0.5 | 38.6 ± 0.1 | 29 ± 0.6 | 22.8 ± 0.003 | 58.7 ± 0.02 | 31.3 ± 12.3 | 15.7 ± 6.1 | 38.9 ± 8.9 |
| Acetate | 14.2 ± 0.1 | 16.4 ± 1.1 | 18.2 ± 0.2 | 39.7 ± 0.02 | 5.6 ± 0.5 | 35.1 ± 0.03 | 27 ± 0.7 | 14.7 ± 0.7 | 47.6 ± 0.02 | 27 ± 8.7 | 15.5 ± 4.4 | 33.6 ± 10.2 |
| Propionate | 0.8 ± 0.01 | 1.34 ± 0 | 0.9 ± 0.04 | 7.9 ± 0.01 | 0.9 ± 0.1 | 1.2 ± 0.001 | 0.8 ± 0.08 | 8 ± 0.07 | 9.2 ± 0.08 | 3.2 ± 3.1 | 4.7 ± 3.1 | 3.8 ± 3.6 |
| Butyrate | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.004 | 2 ± 0.03 | 0.1 ± 0.004 | 2.2 ± 0.03 | 1.2 ± 0.02 | 0.1 ± 0.01 | 1.8 ± 0.01 | 1.1 ± 07 | 0.1 ± 0.01 | 1.4 ± 0.8 |
| Valerate | 0.06 ± 0.01 | 0.0 ± 0.0 | 0.01 ± 0.0 | 0.1 ± 0.001 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.01 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.02 | 0.05 ± 0.02 | 0.0 ± 0.0 * | 0.1 ± 0.05 |
| Caproate | 0.08 ± 0.02 | 0.0 ± 0.0 | 0.02 ± 0.0 | 0.05 ± 0.01 | 0.0 ± 0.0 | 0.04 ± 0.003 | 0.04 ± 0.0 | 0.0 ± 0.0 | 0.04 ± 0.002 | 0.06 ± 0.01 | 0.0 ± 0.0 ** | 0.04 ± 0.001 ** |
| Total Branched SCFA | 0.1 ± 0.02 | 0.01 ± 0.01 | 0.07 ± 0.02 | 0.8 ± 0.01 | 0.0 ± 0.0 | 0.7 ± 0.01 | 0.1 ± 0.1 | 0.6 ± 0.001 | 0.7 ± 0.01 | 0.4 ± 0.3 | 0.3 ± 0.3 | 0.3 ± 0.004 |
| l-lactate (mM) | 1.4 ± 0.1 | 2 ± 0.1 * | 0.3 ± 0.1 * | 0.07 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.03 | 0.5 ± 0.02 | 0.0 ± 0.0 * | 0.0 ± 0.0 * | 0.7 ± 0.5 | 2 ± 0.1 | 0.3 ± 0.1 ** |
| d-lactate (mM) | 1.7 ± 0.1 | 3.2 ± 0.1 * | 7.3 ± 0.2 * | 0.2 ± 0.1 | 0.2 ± 0.04 | 2.4 ± 0.1 * | 0.6 ± 0.03 | 0.2 ± 0.1 * | 0.5 ± 0.1 * | 0.8 ± 0.6 | 1.2 ± 1.4 | 3.4 ± 2.6 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reygner, J.; Joly Condette, C.; Bruneau, A.; Delanaud, S.; Rhazi, L.; Depeint, F.; Abdennebi-Najar, L.; Bach, V.; Mayeur, C.; Khorsi-Cauet, H. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. Int. J. Environ. Res. Public Health 2016, 13, 1088. https://doi.org/10.3390/ijerph13111088
Reygner J, Joly Condette C, Bruneau A, Delanaud S, Rhazi L, Depeint F, Abdennebi-Najar L, Bach V, Mayeur C, Khorsi-Cauet H. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. International Journal of Environmental Research and Public Health. 2016; 13(11):1088. https://doi.org/10.3390/ijerph13111088
Chicago/Turabian StyleReygner, Julie, Claire Joly Condette, Aurélia Bruneau, Stéphane Delanaud, Larbi Rhazi, Flore Depeint, Latifa Abdennebi-Najar, Veronique Bach, Camille Mayeur, and Hafida Khorsi-Cauet. 2016. "Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model" International Journal of Environmental Research and Public Health 13, no. 11: 1088. https://doi.org/10.3390/ijerph13111088
APA StyleReygner, J., Joly Condette, C., Bruneau, A., Delanaud, S., Rhazi, L., Depeint, F., Abdennebi-Najar, L., Bach, V., Mayeur, C., & Khorsi-Cauet, H. (2016). Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. International Journal of Environmental Research and Public Health, 13(11), 1088. https://doi.org/10.3390/ijerph13111088






