Correlation between Odor Concentration and Volatile Organic Compounds (VOC) Composition of Environmental Tobacco Smoke (ETS)
Abstract
:1. Introduction
2. Experimental
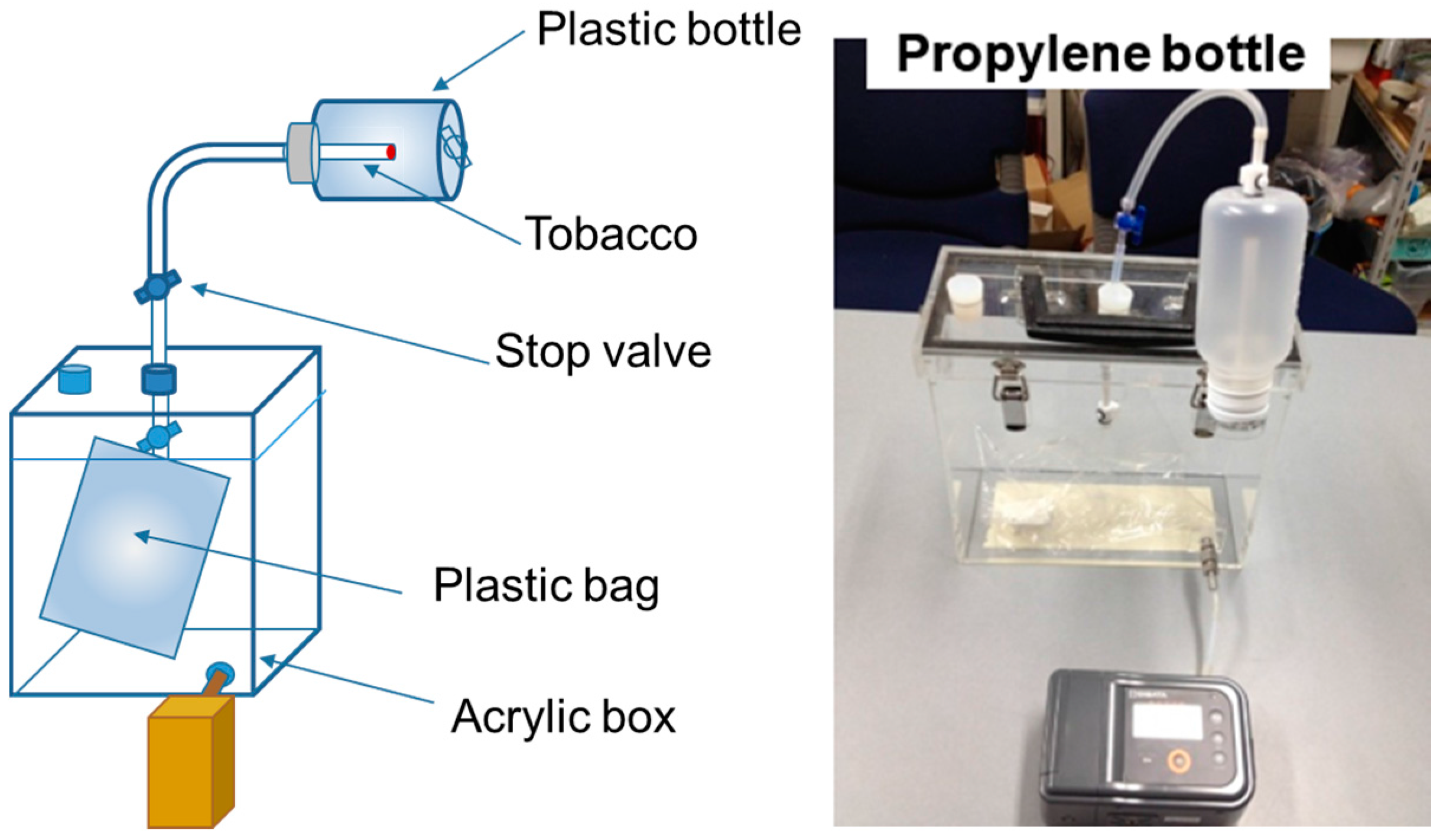
2.1. Preparation of ETS Samples
2.2. Odor Concentration Measurements
2.3. VOC Concentration Measurement
3. Results and Discussion
3.1. Odor Concentration and Odor Index
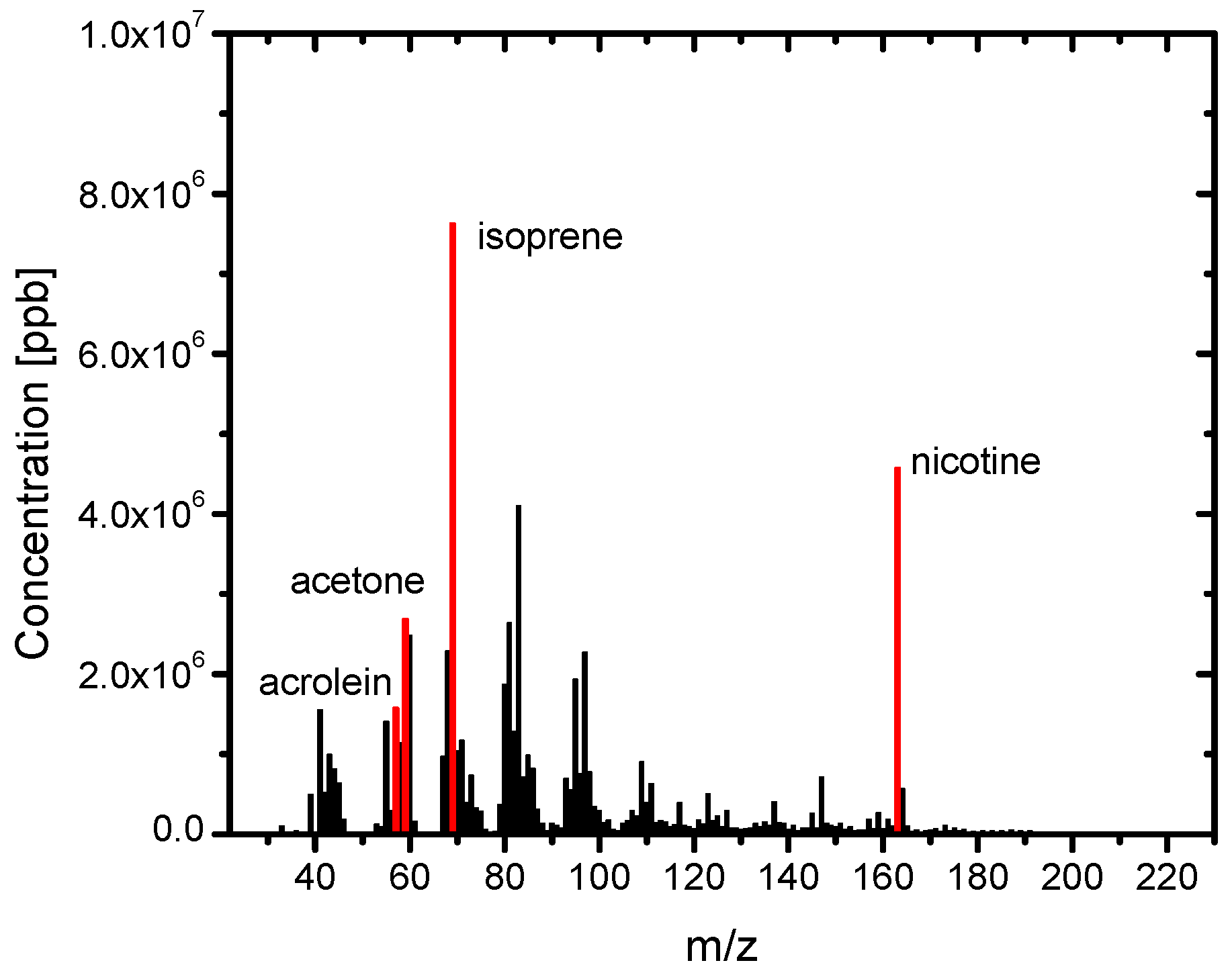
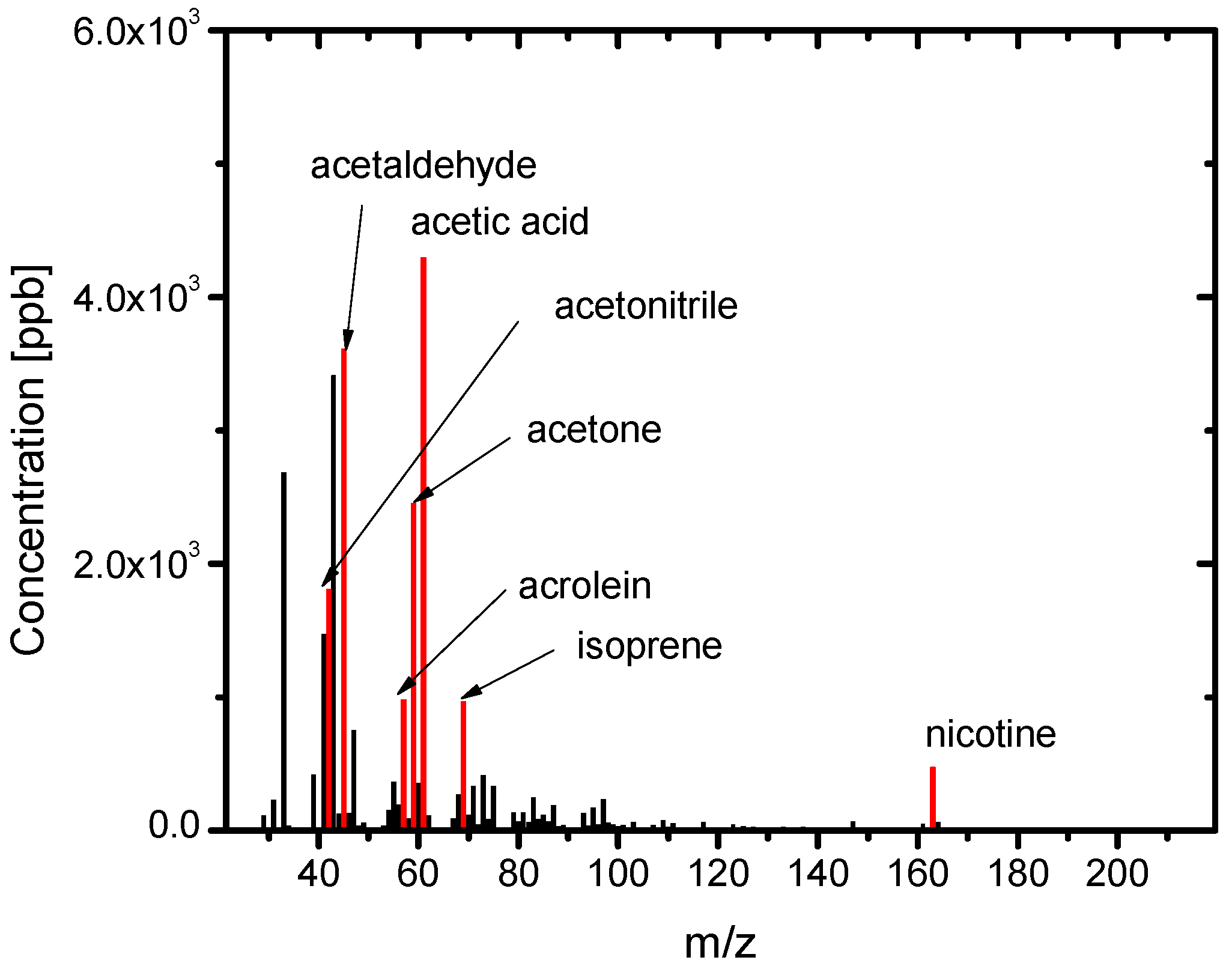
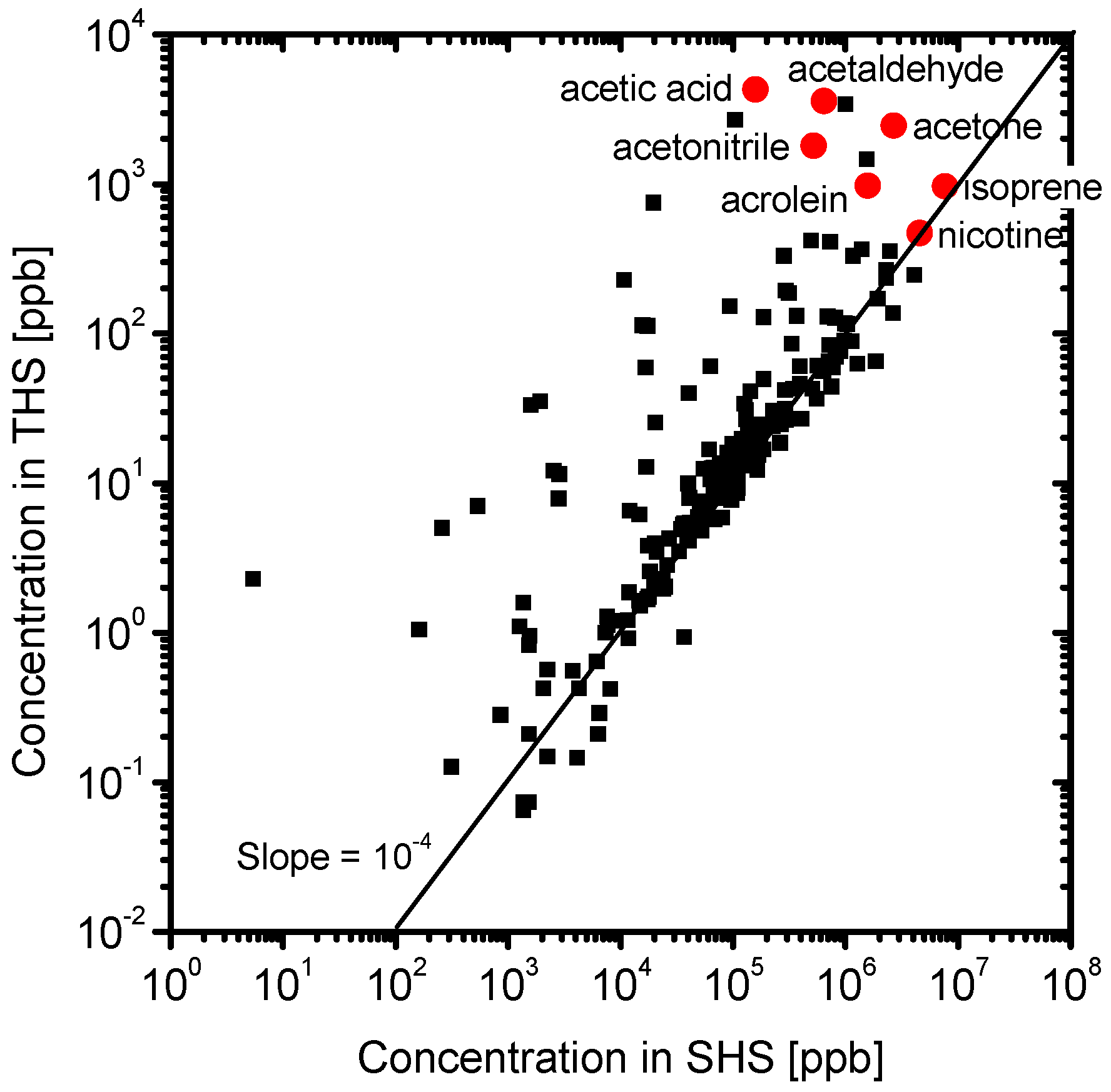
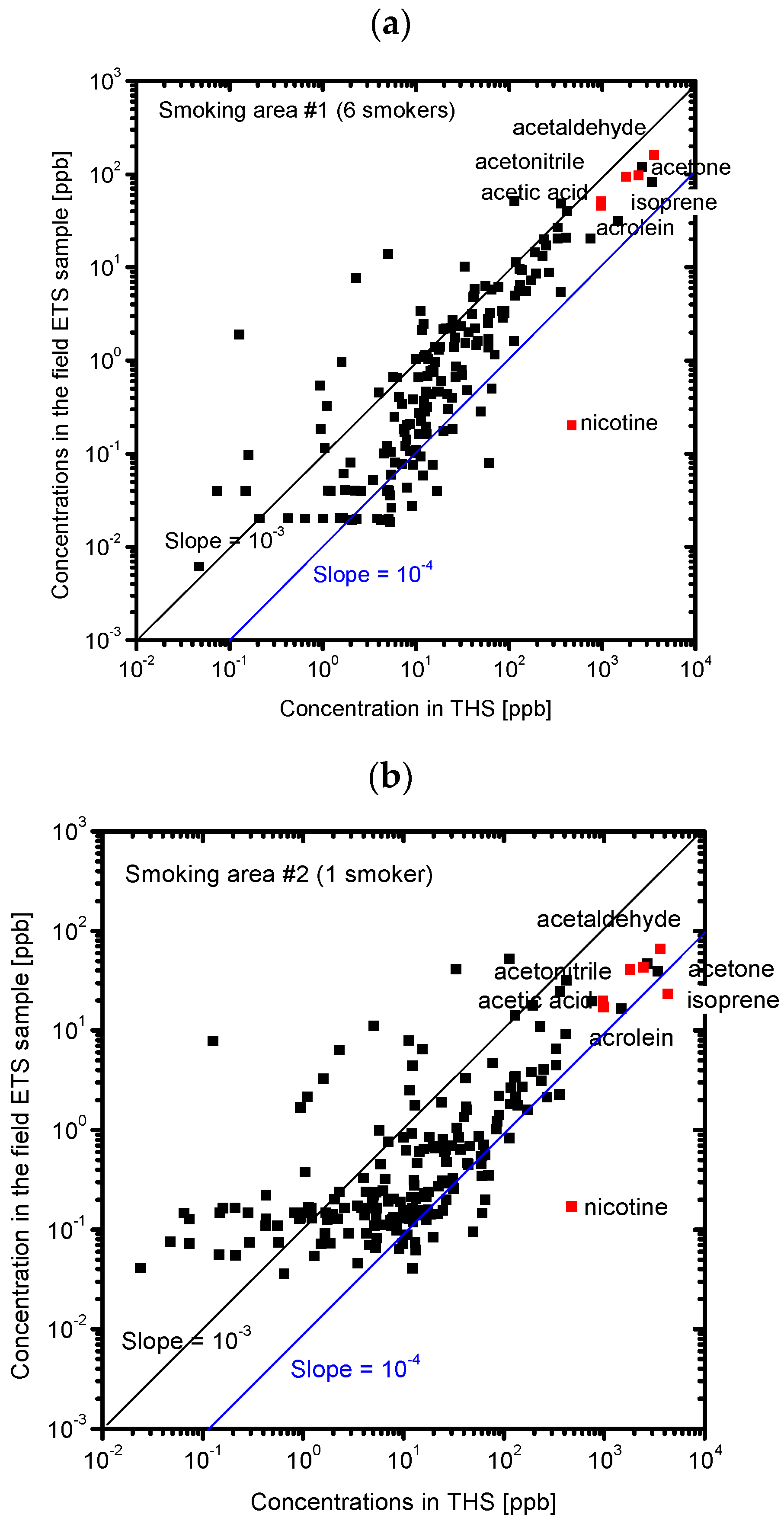
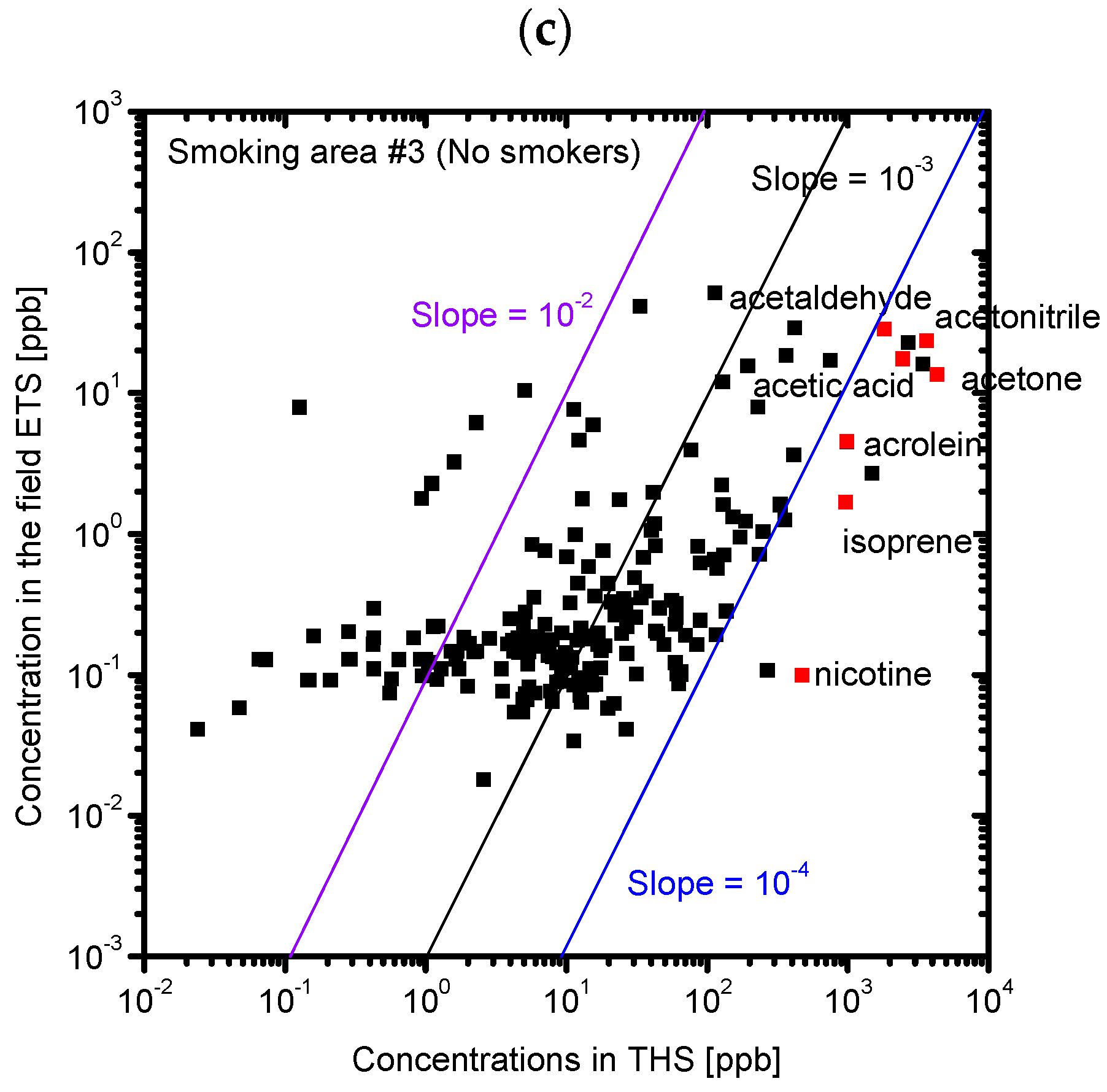
3.2. Composition of the Sample Gases Analyzed by PTR-MS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lebowitz, M.D. Environmental tobacco smoke: A public health issue. Environ. Int. 1989, 15, 11–18. [Google Scholar] [CrossRef]
- Smith, C.J.; Sears, S.B.; Walker, J.C.; DeLuca, P.O. Environmental tobacco smoke: Current assessment and future directions. Toxicol. Pathol. 1992, 20, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.C. Indoor air quality and environmental tobacco smoke: Concentration and exposure. Environ. Int. 1993, 19, 9–40. [Google Scholar] [CrossRef]
- Rickert, W.S. Environmental Tobacco Smoke: Properties, Measurement Techniques and Applications; Background Paper; WHO/NCD/TFI/99.11; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Sleiman, M.; Gundel, L.A.; Pankow, J.F.; Jacob, P., III; Singer, B.C.; Destillats, H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. USA 2010, 107, 6576–6581. [Google Scholar] [CrossRef] [PubMed]
- Petrick, L.M.; Svidovsky, A.; Dubowski, Y. Thirdhand smoke: Heterogeneous oxidation of nicotine and secondary aerosol formation in the indoor environment. Environ. Sci. Technol. 2011, 45, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, M.; Logue, J.M.; Luo, W.; Pankow, J.F.; Gundel, L.A.; Destaillats, H. Inhalable Constituents of thirdhand tobacco smoke: Chemical characterization and health impact considerations. Environ. Sci. Technol. 2014, 48, 13093–13101. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Jacob, P., III; Havel, C.; Schick, S.F.; Talbot, P. Thirdhand cigarette smoke: Factors affecting exposure and remediation. PLoS ONE 2014, 10, e108258. [Google Scholar]
- Acuff, L.; Fristoe, K.; Hamblen, J.; Smith, M.; Chen, J. Third-hand smoke: Old smoke, new concerns. J. Commun. Health 2016, 41, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; Proctor, C.J. The origins and properties of environmental tobacco smoke. Environ. Int. 1990, 16, 231–245. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Dybing, E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. Control 2003, 12, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Dacunto, P.J.; Cheng, K.-C.; Acevedo-Bolton, V.; Kepeis, N.E.; Repace, J.L.; Ott, W.R.; Hindemann, L.M. Identifying and quantifying secondhand smoke in multiunit homes with tobacco smoke odor complaints. Atmos. Environ. 2013, 71, 399–407. [Google Scholar] [CrossRef]
- Jenkins, R.A.; Guerin, M.R.; Tomkins, B.A. The Chemistry of Environmental Tobacco Smoke: Composition and Measurements; Lewis Publishers: Boca Raton, FL, USA, 2000. [Google Scholar]
- Streibel, T.; Mitschke, S.; Adam, T.; Zimmermann, R. Time-resolved analysis of the emission of sidestream smoke (SSS) from cigarettes during smoking by photo ionization/time-of-flight mass spectrometry (PI-TOFMS): Towards a better description of environmental tobacco smoke. Anal. Bioanal. Chem. 2013, 405, 7071–7082. [Google Scholar] [CrossRef] [PubMed]
- Schramm, S.; Carre, V.; Scheffler, J.-L.; Aubriet, F. Active and passive smoking—New insights on the molecular composition of different cigarette smoke aerosols by LDI-FTICRMS. Atmos. Environ. 2014, 92, 411–420. [Google Scholar] [CrossRef]
- Charles, S.M.; Batterman, S.A.; Jia, C. Composition and emissions of VOCs in main- and side-stream smoke of research cigarettes. Atmos. Environ. 2007, 41, 5371–5384. [Google Scholar] [CrossRef]
- Bi, X.; Sheng, G.; Feng, Y.; Fu, J.; Xie, J. Gas- and particulate-phase specific tracer and toxic organic compounds in environmental tobacco smoke. Chemosphere 2005, 61, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Moschandreas, D.J.; Relwani, S.M. Perception of environmental tobacco smoke odors: An olfactory and visual response. Atmos. Environ. 1992, 26, 263–269. [Google Scholar] [CrossRef]
- Nagata, Y. Measurement of Odor Threshold by Triangle Odor Bag Method. Available online: https://www.env.go.jp/en/air/odor/measure/02_3_2.pdf (accessed on 22 January 2016).
- Ueno, H.; Amano, S.; Merecka, B.; Kośmider, J. Difference in the odor concentrations measured by the triangle odor bag method and dynamic olfactometry. Water Sci. Technol. 2009, 59, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- User Manual for the PTR-MS MS; IONICON Analytik Ges. m. b. H.: Innsbruck, Austria, 2009.

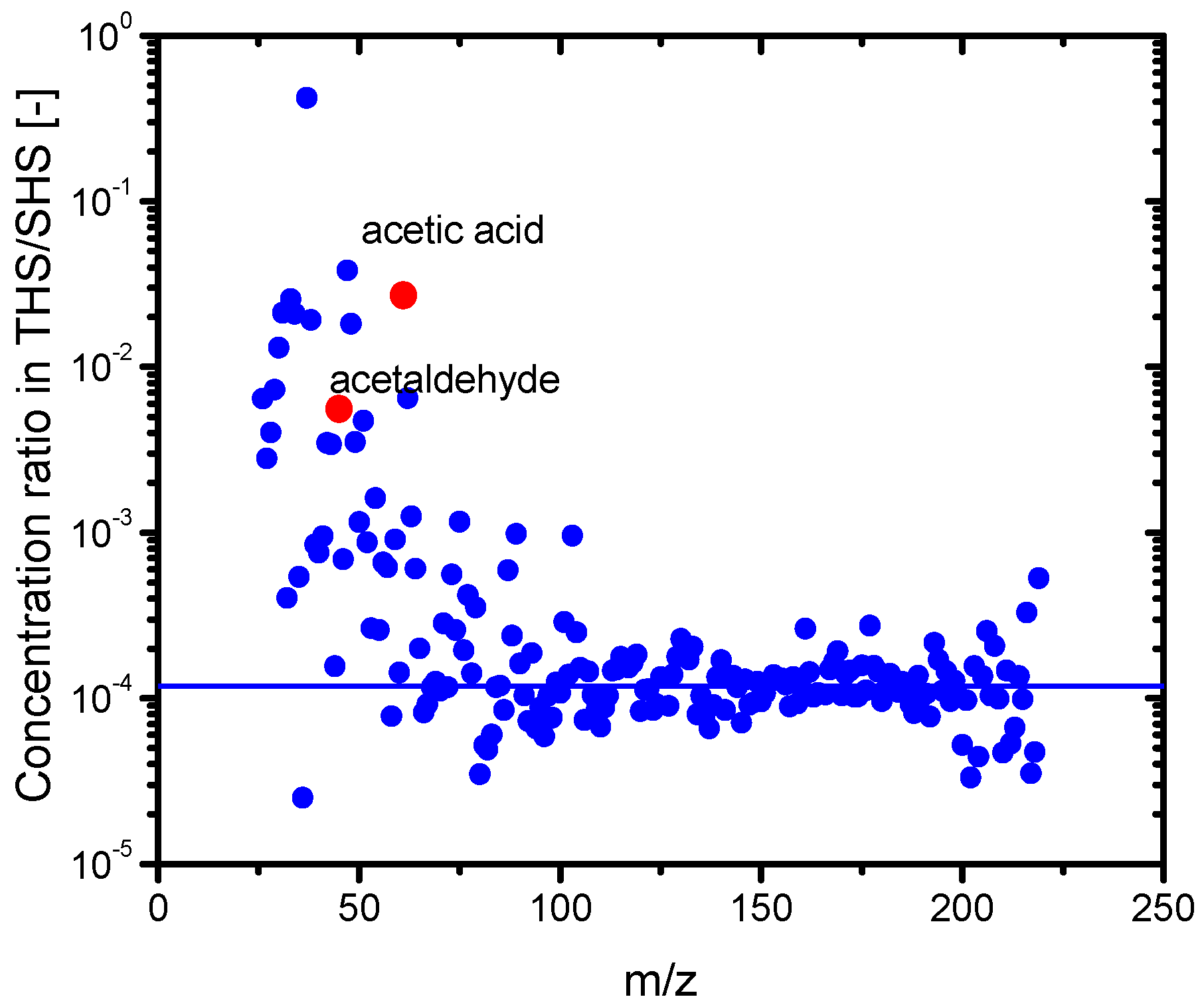
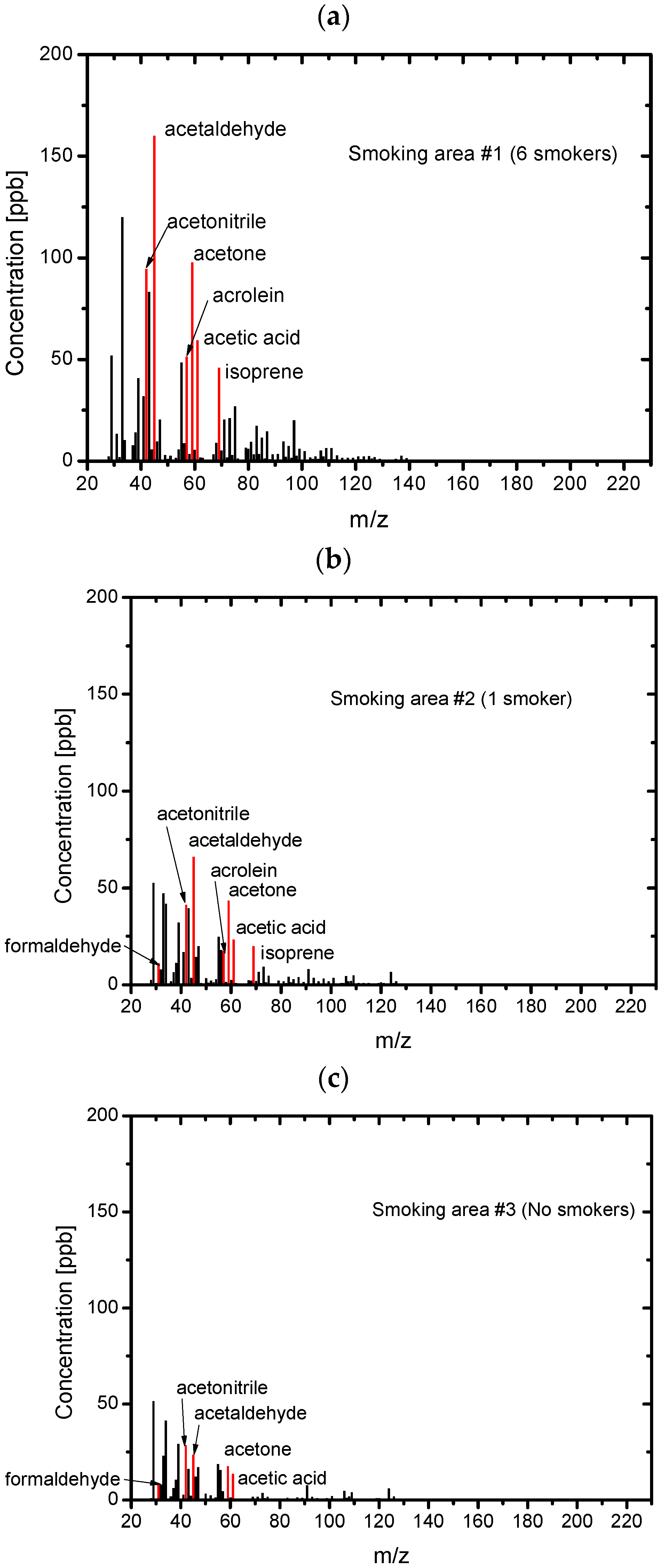
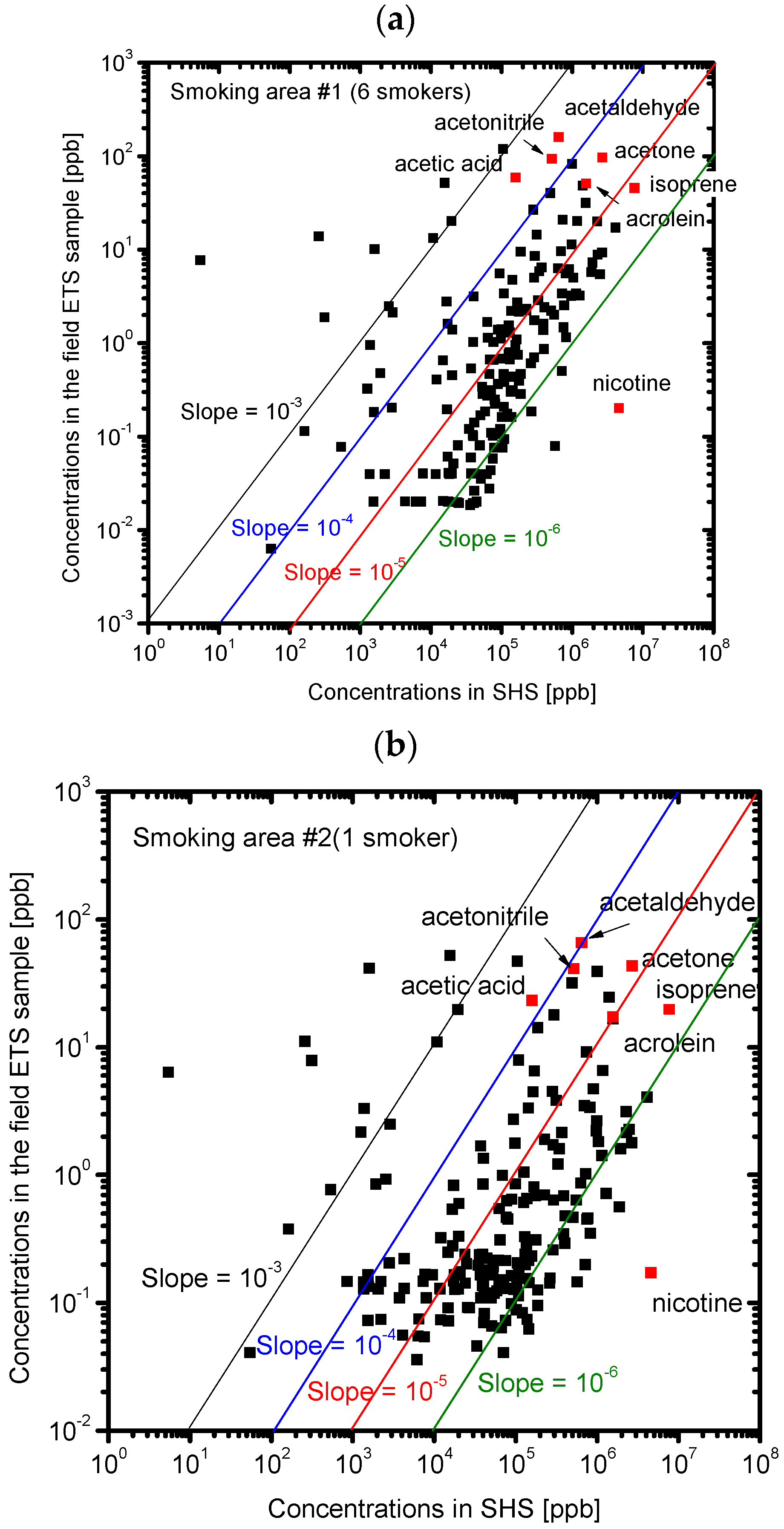
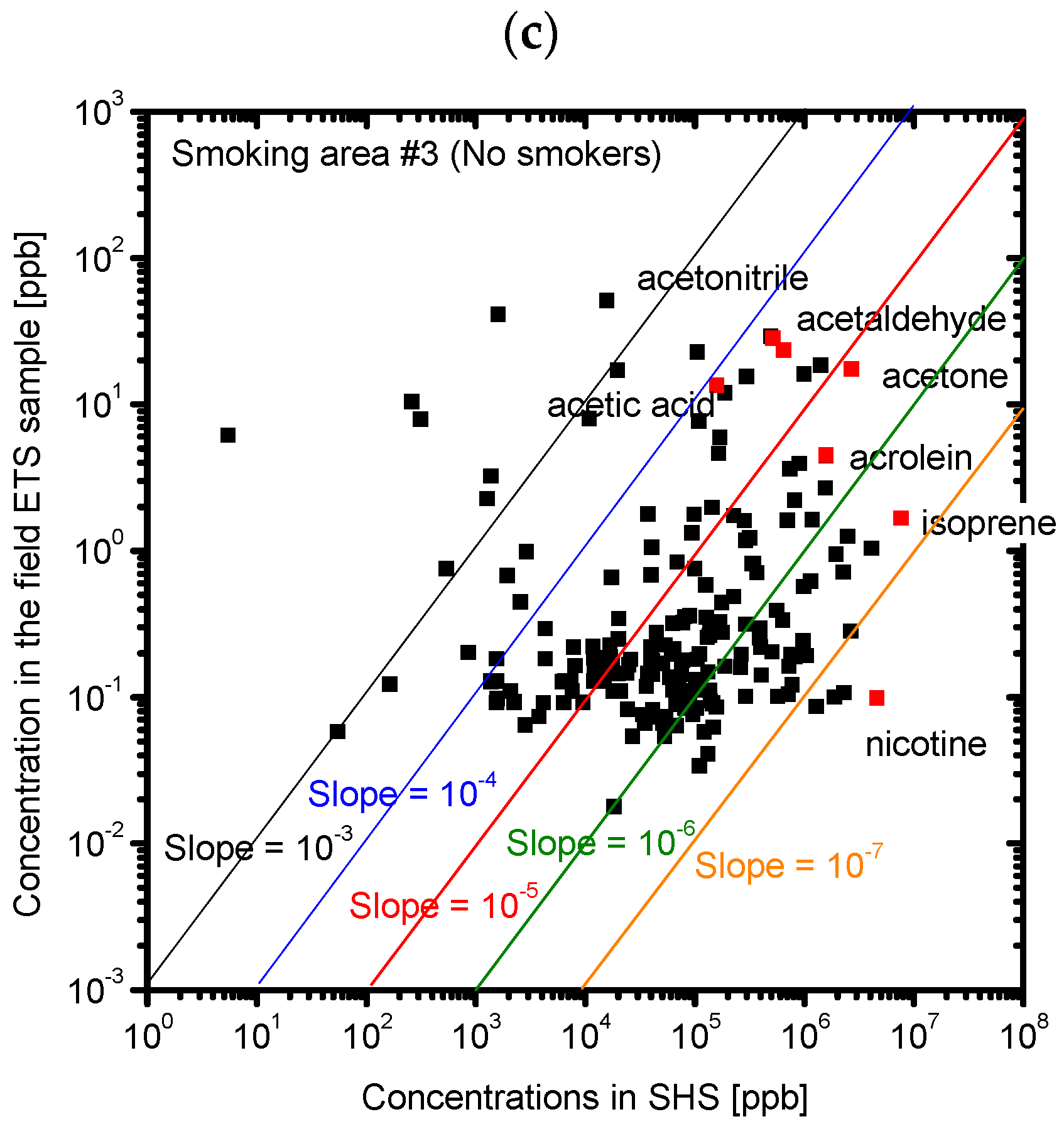
| Sample | Odor Concentration | Odor Index |
|---|---|---|
| Second Hand Smoke (SHS) | 2.7 × 109 | 94 |
| Third Hand Smoke (THS) | 3.2 × 105 | 55 |
| Field ETS #1 (18 July); 6 smokers | 3.2 × 106 | 65 |
| Field ETS #2 (19 July); 1 smoker | 3.2 × 105 | 55 |
| Field ETS #3 (19 July); no smokers | 3.2 × 105 | 55 |
| m/z | Second Hand Smoke (SHS) | Third Hand Smoke (THS) | Smoking Area #1 | Smoking Area #2 | Smoking Area #3 |
|---|---|---|---|---|---|
| 6 Smokers | 1 Smoker | No Smokers | |||
| Concentration (ppb) | Concentration (ppb) | Concentration (ppb) | Concentration (ppb) | Concentration (ppb) | |
| 29 | - | - | 52.0 | 52.4 | 51.5 |
| 31 a | - | - | - | 11 | 8.0 |
| 32 | - | - | - | 7.9 | 7.9 |
| 33 | - | 2682.8 | 120.2 | 47.1 | 23.0 |
| 34 | - | - | - | 41.7 | 41.3 |
| 37 | - | - | - | - | 6.2 |
| 38 | - | - | 13.9 | 11.1 | 10.5 |
| 39 | - | 418.5 | 40.6 | 32 | 29.2 |
| 41 | 1.55 × 106 | 1474.1 | 31.8 | 16.7 | - |
| 42 b | - | 1806.6 | 94.4 | 41.2 | 28.5 |
| 43 | 9.95 × 105 | 3412.6 | 83.1 | 39.4 | 16.1 |
| 45 c | - | 3606.7 | 160.1 | 66 | 23.5 |
| 46 | - | - | - | - | 12.0 |
| 47 | - | 750.3 | 20.4 | 19.7 | 17.1 |
| 55 | 1.40 × 106 | 363.3 | 48.5 | 24.7 | 18.6 |
| 56 | - | - | - | 17.9 | - |
| 57 d | 1.57 × 106 | 976.9 | 51.3 | 17.3 | 15.6 |
| 58 | 1.14 × 106 | - | - | - | - |
| 59 e | 2.69 × 106 | 2451.9 | 97.6 | 43.4 | 17.6 |
| 60 | 2.48 × 106 | 355.9 | - | - | - |
| 61 f | - | 4296.1 | 59.3 | 23.3 | 13.5 |
| 67 | 9.70 × 105 | - | - | - | - |
| 68 | 2.29 × 106 | 267.7 | - | - | - |
| 69 g | 7.63 × 106 | 964.1 | 45.7 | 19.9 | - |
| 70 | 1.04 × 106 | - | - | - | - |
| 71 | 1.17 × 106 | 332.4 | 20.4 | - | - |
| 73 | - | 411.1 | 21.0 | 9.2 | - |
| 75 | - | 330.5 | 26.7 | - | - |
| 80 | 1.87 × 106 | - | - | - | - |
| 81 | 2.64 × 106 | - | - | - | - |
| 82 | 1.28 × 106 | - | - | - | - |
| 83 | 4.10 × 106 | 248.2 | 17.2 | - | - |
| 85 | 9.81 × 105 | - | - | - | - |
| 87 | - | - | 14.5 | ||
| 91 | - | - | - | 7.9 | 7.7 |
| 95 | 1.93 × 106 | - | - | - | - |
| 97 | 2.27 × 106 | 235.6 | 20.0 | - | - |
| 106 | - | - | - | - | 4.7 |
| 124 | - | - | - | - | 6.0 |
| 163 h | 4.58 × 106 | 468.8 | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noguchi, M.; Tanaka, S.; Watanabe, K.; Yamasaki, A. Correlation between Odor Concentration and Volatile Organic Compounds (VOC) Composition of Environmental Tobacco Smoke (ETS). Int. J. Environ. Res. Public Health 2016, 13, 994. https://doi.org/10.3390/ijerph13100994
Noguchi M, Tanaka S, Watanabe K, Yamasaki A. Correlation between Odor Concentration and Volatile Organic Compounds (VOC) Composition of Environmental Tobacco Smoke (ETS). International Journal of Environmental Research and Public Health. 2016; 13(10):994. https://doi.org/10.3390/ijerph13100994
Chicago/Turabian StyleNoguchi, Miyuki, Saya Tanaka, Kaede Watanabe, and Akihiro Yamasaki. 2016. "Correlation between Odor Concentration and Volatile Organic Compounds (VOC) Composition of Environmental Tobacco Smoke (ETS)" International Journal of Environmental Research and Public Health 13, no. 10: 994. https://doi.org/10.3390/ijerph13100994
APA StyleNoguchi, M., Tanaka, S., Watanabe, K., & Yamasaki, A. (2016). Correlation between Odor Concentration and Volatile Organic Compounds (VOC) Composition of Environmental Tobacco Smoke (ETS). International Journal of Environmental Research and Public Health, 13(10), 994. https://doi.org/10.3390/ijerph13100994





