Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
| Characteristics | RIF Women with TAI (n = 21) | RIF Women without TAI (n = 51) | p |
|---|---|---|---|
| Age (years) | 33.0 (31.0–37.0) | 34.0 (31.0–38.0) | 0.490 |
| BMI (kg/m2) | 21.8 (19.5–23.4) | 21.4 (20.0–22.1) | 0.848 |
| TSH (uIU/mL) | 2.2 (2.0–2.5) | 2.2 (1.7–2.5) | 0.376 |
| Reproductive hormone | |||
| FSH (mIU/L) | 6.2 (5.7–7.2) | 6.2 (4.8–6.6) | 0.299 |
| LH (mIU/L) | 4.0 (2.7–4.3) | 3.6 (2.6–4.0) | 0.587 |
| E2 (pg/mL) | 38.0 (29.5–65.7) | 47.0 (30.0–64.5) | 0.828 |
| PRL (ng/mL) | 30.0 (16.3–33.5) | 22.1 (15.2–33.5) | 0.554 |
| T (ng/mL) | 1.1 (0.3–3.0) | 0.7 (0.3–3.0) | 0.693 |
2.2. Thyroid Function Test
2.3. Anti-Thyroid Antibodies Assay
2.4. Peripheral Blood Lymphocytes Assay
2.5. Statistical Analysis
3. Results
3.1. Prevalence of TAI in Women with RIF
3.2. Association of TAI with Thyroid Function Abnormality in Women with RIF
| RIF Women with TAI (n = 21) | RIF Women without TAI (n = 51) | χ2 | p | |
|---|---|---|---|---|
| Thyroid function abnormality | 23.8% (5) | 13.7% (7) | 0.484 | 0.487 |
| Thyroid function normality | 76.2% (16) | 86.3% (44) |
3.3. Association of TAI with Lymphocytes in Women with RIF
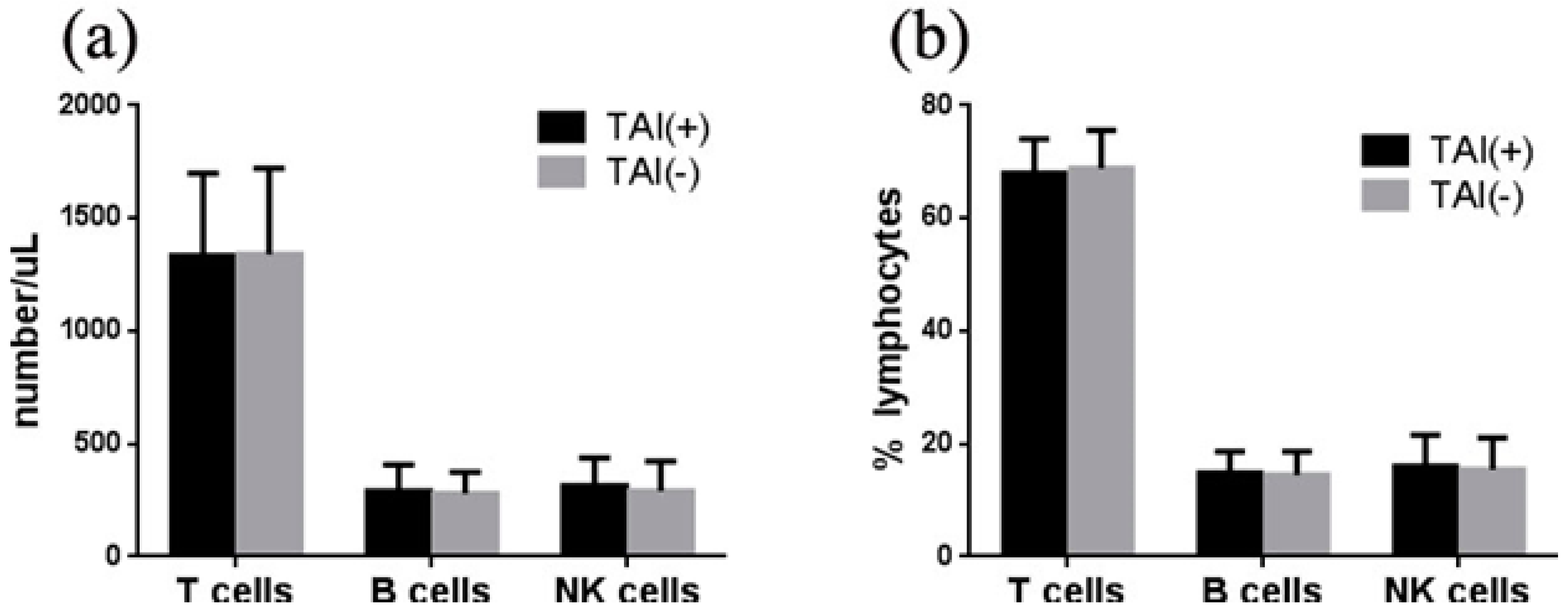
3.4. Association of TAI with T Subpopulations in Women with RIF

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carp, H.J.; Selmi, C.; Shoenfeld, Y. The autoimmune bases of infertility and pregnancy loss. J. Autoimmun. 2012, 38, J266–J274. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Margalioth, E.J.; Ben-Chetrit, A.; Gal, M.; Eldar-Geva, T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum. Reprod. 2006, 21, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Vandekerckhove, P.; Kennedy, R.; Keay, S.D. Investigation and current management of recurrent ivf treatment failure in the UK. BJOG 2005, 112, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Ostensen, M.; Andreoli, L.; Brucato, A.; Cetin, I.; Chambers, C.; Clowse, M.E.; Costedoat-Chalumeau, N.; Cutolo, M.; Dolhain, R.; Fenstad, M.H.; et al. State of the art: Reproduction and pregnancy in rheumatic diseases. Autoimmun. Rev. 2015, 14, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Cleary-Goldman, J.; Malone, F.D.; Lambert-Messerlian, G.; Sullivan, L.; Canick, J.; Porter, T.F.; Luthy, D.; Gross, S.; Bianchi, D.W.; D’Alton, M.E. Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynaecol. 2008, 112, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiras, G.; Faria, R.; Braga, J.; Vasconcelos, C. Fetal outcome in autoimmune diseases. Autoimmun. Rev. 2012, 11, A520–A530. [Google Scholar] [CrossRef] [PubMed]
- Prummel, M.F.; Wiersinga, W.M. Thyroid autoimmunity and miscarriage. Eur. J. Endocrinol. 2004, 150, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Soares, S.R.; Alvarez, C.; Munoz, E.; Ramirez, A.; Rubio, C.; Serra, V.; Remohi, J.; Pellicer, A. The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure and recurrent spontaneous abortion. Hum. Reprod. 2008, 23, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Formoso, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J. Clin. Endocrinol. Metab. 2006, 91, 2587–2591. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Ng, H.P.; Lau, K.S.; Liu, W.M.; O, W.S.; Yeung, W.S.; Kung, A.W. Increased fetal abortion rate in autoimmune thyroid disease is related to circulating TPO autoantibodies in an autoimmune thyroiditis animal model. Fertil. Steril. 2009, 91, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Cho, H.J.; Kim, H.Y.; Yang, K.M.; Ahn, H.K.; Thornton, S.; Park, J.C.; Beaman, K.; Gilman-Sachs, A.; Kwak-Kim, J. Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am. J. Reprod. Immunol. 2011, 65, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Shina, A.; Amital, H.; Shoenfeld, Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J. Autoimmune. 2012, 38, J275–J281. [Google Scholar] [CrossRef] [PubMed]
- Stassi, G.; de Maria, R. Autoimmune thyroid disease: New models of cell death in autoimmunity. Nat. Rev. Immunol. 2002, 2, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Psarra, K.; Kapsimali, V.; Tarassi, K.; Dendrinos, S.; Athanasiadis, T.; Botsis, D.; Kreatsas, G.; Papasteriades, C. Tcr Gamma Delta T Lymphocytes in unexplained recurrent spontaneous abortions. Am. J. Reprod. Immunol. 2001, 45, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Tong, Y.; You, Y.; Li, P.; Huo, T.; Tu, W.; Mao, M. Prospective cohort study about the lymphocyte subpopulations’ change and impact on the pregnancy outcome in fetal growth restriction. J. Matern. Fetal. Neonatal. Med. 2012, 25, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakova-Dzhambazova, E.; Milchev, N.; Pavlov, P.; Dimitrakov, D. Lymphocyte populations and subpopulations in peripheral blood of pregnant women with pre-eclampsia. Akush. Ginekol. 2005, 44, 40–44. [Google Scholar]
- Baretic, M. 100 Years of hashimoto thyroiditis, still an intriguing disease. Acta. Med. Croatica. 2011, 65, 453–457. [Google Scholar] [PubMed]
- Ringelstein, M.; Harmel, J.; Distelmaier, F.; Ingwersen, J.; Menge, T.; Hellwig, K.; Kieseier, B.; Mayatepek, E.; Hartung, H.P.; Kuempfel, T.; et al. Neuromyelitis optica and pregnancy during therapeutic B cell depletion: Infant exposure to anti-AQP4 antibody and prevention of rebound relapses with low-dose rituximab postpartum. Mult. Scler. 2013, 19, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Sangle, S.R.; Lutalo, P.M.; Davies, R.J.; Khamashta, M.A.; D’Cruz, D.P. B-cell depletion therapy and pregnancy outcome in severe, refractory systemic autoimmune diseases. J. Autoimmun. 2013, 43, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Coulam, C.B.; Roussev, R.G. Correlation of NK cell activation and inhibition markers with nk cytoxicity among women experiencing immunologic implantation failure after in vitro fertilization and embryo transfer. J. Assist. Reprod. Genet. 2003, 20, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Emmer, P.M.; Nelen, W.L.; Steegers, E.A.; Hendriks, J.C.; Veerhoek, M.; Joosten, I. Peripheral natural killer cytotoxicity and CD56posCD16pos cells increase during early pregnancy in women with a history of recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Ntrivalas, E.I.; Kwak-Kim, J.Y.; Gilman-Sachs, A.; Chung-Bang, H.; Ng, S.C.; Beaman, K.D.; Mantouvalos, H.P.; Beer, A.E. Status of peripheral blood natural killer cells in women with recurrent spontaneous abortions and infertility of unknown aetiology. Hum. Reprod. 2001, 16, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.F.; Drexhage, H.A.; Berghout, A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: Recent insights and consequences for antenatal and postnatal care. Endocr. Rev. 2001, 22, 605–630. [Google Scholar] [CrossRef] [PubMed]
- Poppe, K.; Velkeniers, B.; Glinoer, D. The role of thyroid autoimmunity in fertility and pregnancy. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A.; Glinoer, D. Thyroid autoimmunity and the risk of miscarriage. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Matalon, S.T.; Blank, M.; Ornoy, A.; Shoenfeld, Y. The association between anti-thyroid antibodies and pregnancy loss. Am. J. Reprod. Immunol. 2001, 45, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, E.; Lazzarin, N.; Caserta, D.; Valensise, H.; Baldi, M.; Moscarini, M.; Arduini, D. Diagnostic evaluation of women experiencing repeated in vitro fertilization failure. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 125, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bussen, S.; Steck, T.; Dietl, J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum. Reprod. 2000, 15, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Bartha, J.L.; Comino-Delgado, R.; Martinez-del Fresno, P.; Ortega, M.J.; Fernandez-Lorente, J.R.; Cabello, J.M. Lymphocyte subpopulations after normal pregnancy and spontaneous abortion in primigravidas. Int. J. Reprod. Med. 2000, 45, 567–571. [Google Scholar]
- Bossowski, A.; Urban, M.; Stasiak-Barmuta, A. Analysis of changes in the percentage of B (CD19) and T (CD3) lymphocytes, subsets CD4, CD8 and their memory (CD45RO), and naive (CD45RA) T cells in children with immune and non-immune thyroid diseases. J. Pediatr. Endocrinol. Metab. 2003, 16, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Stagnaro-Green, A.; Roman, S.H.; Cobin, R.H.; El-Harazy, E.; Wallenstein, S.; Davies, T.F. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: Evidence of a T-cell etiology for postpartum thyroid dysfunction. J. Pediatr. Endocrinol. Metab. 1992, 74, 645–653. [Google Scholar]
- Xia, N.; Zhou, S.; Liang, Y.; Xiao, C.; Shen, H.; Pan, H.; Deng, H.; Wang, N.; Li, Q.Q. Cd4+ T cells and the Th1/Th2 imbalance are implicated in the pathogenesis of graves’ ophthalmopathy. Int. J. Mol. Med. 2006, 17, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Gilman-Sachs, A.; Thaker, P.; Beaman, K.D.; Beer, A.E.; Kwak-Kim, J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IEF/ET or normal pregnancy. Am. J. Reprod. Immunol. 2002, 48, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Turhan Iyidir, O.; Konca Degertekin, C.; Sonmez, C.; Atak Yucel, A.; Erdem, M.; Akturk, M.; Ayvaz, G. The effect of thyroid autoimmunity on T-cell responses in early pregnancy. J. Reprod. Immunol. 2015, 110, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Joachim, R.; Kandil, J.; Margni, R.; Tometten, M.; Klapp, B.F.; Arck, P.C. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J. Immunol. 2004, 172, 5893–5899. [Google Scholar] [CrossRef] [PubMed]
- Muzzio, D.; Zenclussen, A.C.; Jensen, F. The role of b cells in pregnancy: The good and the bad. Am. J. Reprod. Immunol. 2013, 69, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Diamond, B. Autoimmune diseases. N. Engl. J. Med. Overseas. Ed. 2001, 345, 340–350. [Google Scholar]
- Fukui, A.; Funamizu, A.; Yokota, M.; Yamada, K.; Nakamua, R.; Fukuhara, R.; Kimura, H.; Mizunuma, H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J. Reprod. Immunol. 2011, 90, 105–110. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Liang, P.; Diao, L.; Liu, C.; Chen, X.; Li, G.; Chen, C.; Zeng, Y. Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure. Int. J. Environ. Res. Public Health 2015, 12, 10352-10361. https://doi.org/10.3390/ijerph120910352
Huang C, Liang P, Diao L, Liu C, Chen X, Li G, Chen C, Zeng Y. Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure. International Journal of Environmental Research and Public Health. 2015; 12(9):10352-10361. https://doi.org/10.3390/ijerph120910352
Chicago/Turabian StyleHuang, Chunyu, Peiyan Liang, Lianghui Diao, Cuicui Liu, Xian Chen, Guangui Li, Cong Chen, and Yong Zeng. 2015. "Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure" International Journal of Environmental Research and Public Health 12, no. 9: 10352-10361. https://doi.org/10.3390/ijerph120910352
APA StyleHuang, C., Liang, P., Diao, L., Liu, C., Chen, X., Li, G., Chen, C., & Zeng, Y. (2015). Thyroid Autoimmunity is Associated with Decreased Cytotoxicity T Cells in Women with Repeated Implantation Failure. International Journal of Environmental Research and Public Health, 12(9), 10352-10361. https://doi.org/10.3390/ijerph120910352





