Biosurfactant Production by Bacillus salmalaya for Lubricating Oil Solubilization and Biodegradation
Abstract
:1. Introduction
| Microorganism | Biosurfactant |
|---|---|
| Pseudomonas sp. | Rhamnolipids |
| Rhodococcus sp. | Trehalose lipids |
| Candida sp. | Mannosyl erythritol lipid |
| Candida bogoriensis | Sophorolipid |
| Corynebacterium lepus | Corynemycolic acids |
| Candida petrophilum | Peptidolipid |
| Bacillus subtilis | Cyclic lipopeptide |
| Bacillus licheniformis | Cyclic lipopeptide |
| Candida tropicalis | Mannan-fatty acid complex |
2. Materials and Methods
2.1. Microorganism Isolation
2.2. Culture Medium and Biosurfactant Preparation
2.3. Determination of Biomass
2.4. Drop Collapse and Oil Displacement Tests
2.5. Determination of Emulsification Activity
2.6. Determination of Bacterial Growth and Surface Tension Reduction
2.7. Critical Micelle Concentration of the Biosurfactant
2.8. Fourier Transform Infrared Spectroscopy
2.9. Stability Analyses
2.9.1. pH and Temperature
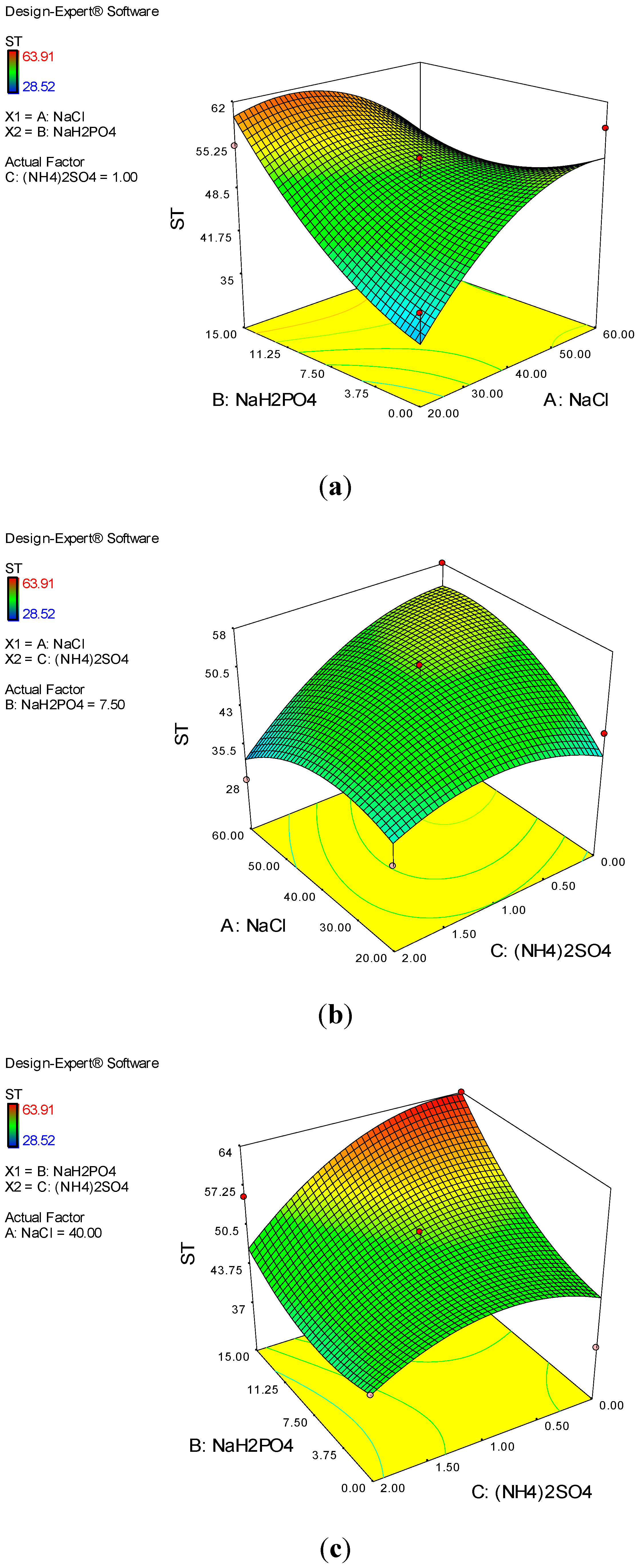
2.9.2. Carbon, Nitrogen, and Phosphorus Sources
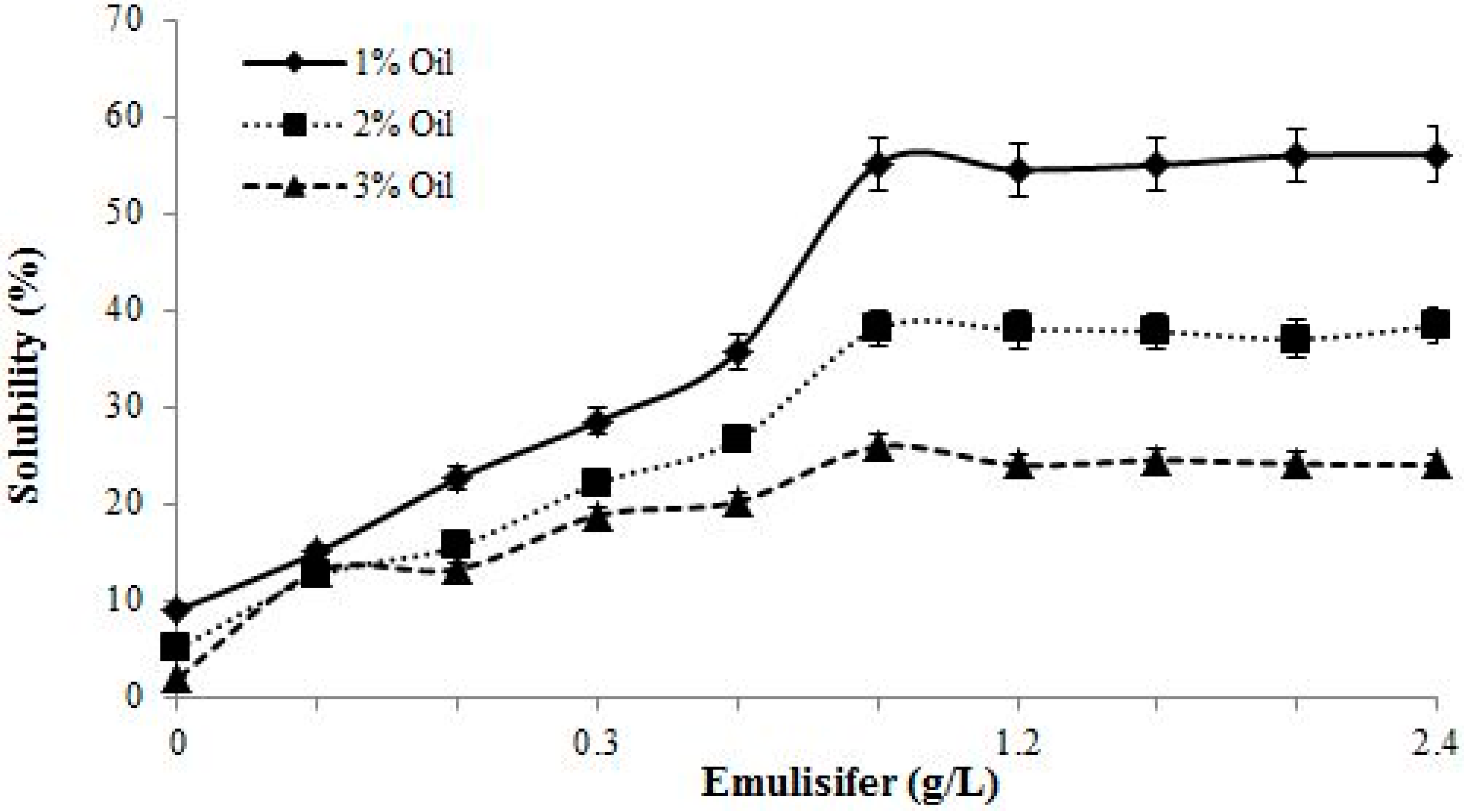
2.10. Lubricating Oil Solubilization Test
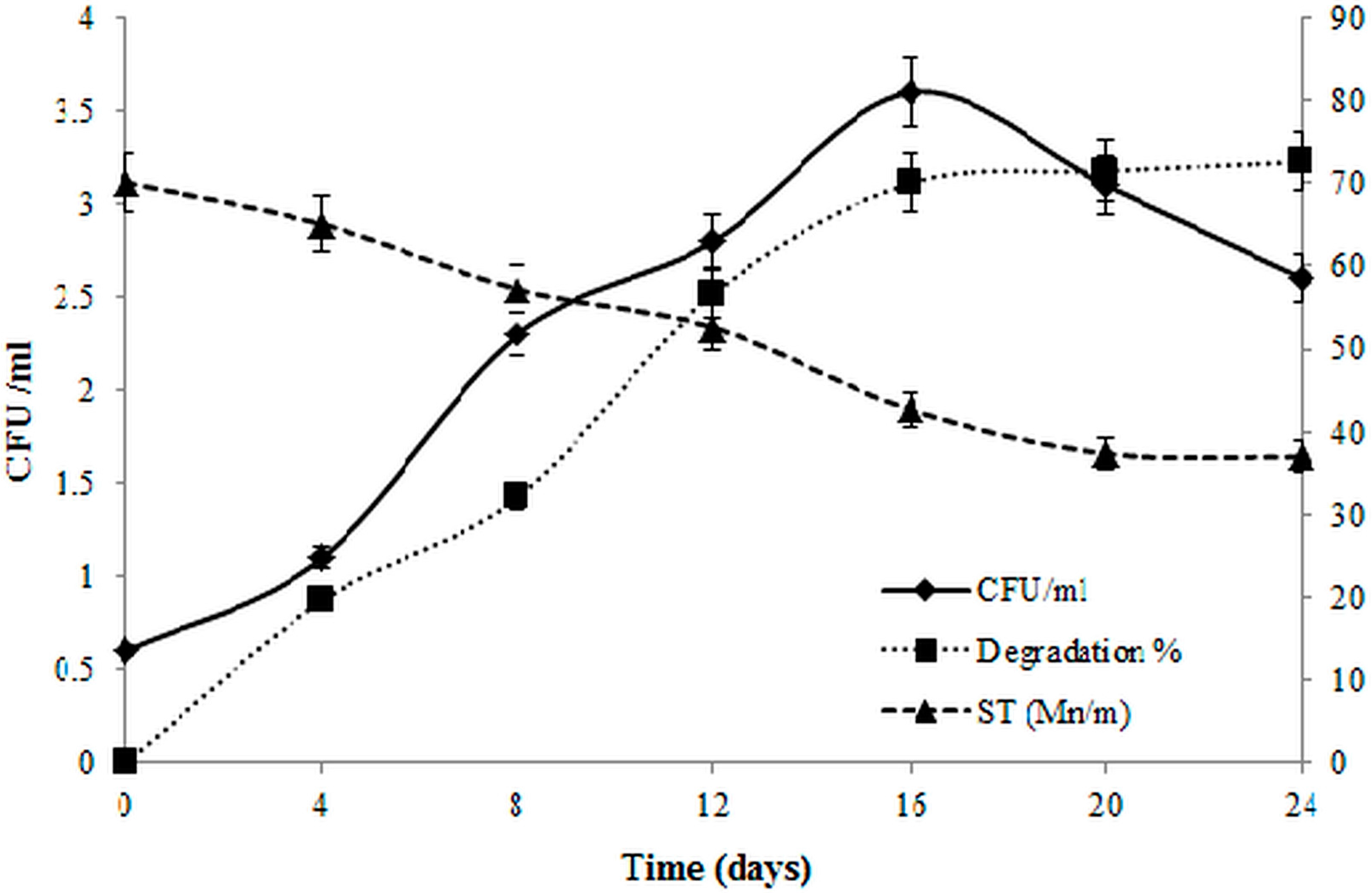
Lubricating Oil Degradation Assay
2.11. Statistical Analyses
3. Results and Discussion
3.1. Biosurfactant Activity Assay Tests
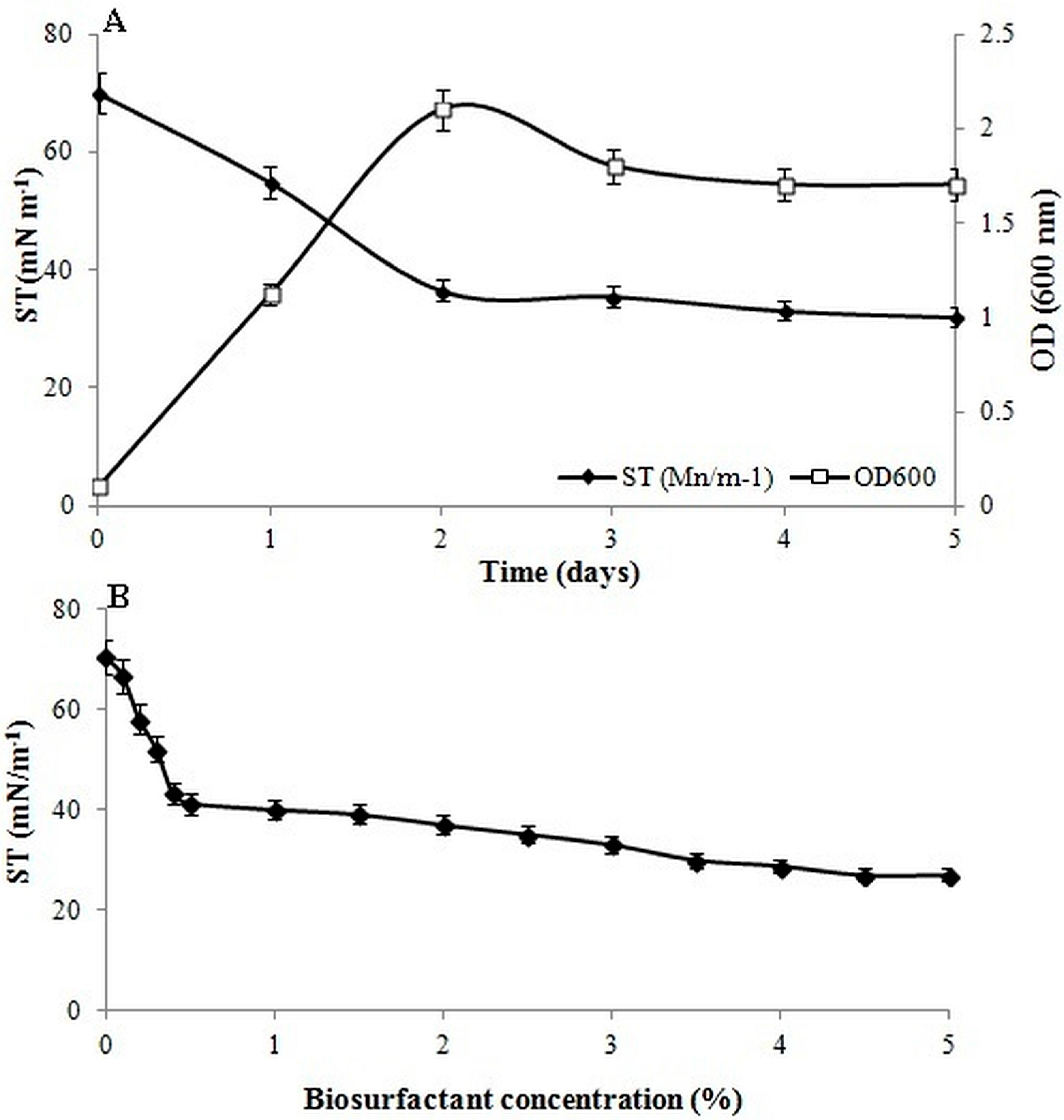
3.2. Critical Micelle Concentration
3.3. Fourier Transform Infrared Spectroscopy

3.4. Stability Studies

3.5. Solubilization Study
3.6. Biodegradation of Lubricating Oil
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bolliger, C. Bioremediation of a Heating Oil-Contaminated Aquifer-Quantification Of Processes By Chemical, Biological, and Stable Isotope Analyses. Available online: http://e-collection.library.ethz.ch/eserv/eth:23731/eth-23731-02.pdf (accessed on 24 June 2015).
- Ayed, B.H.; Jemil, N.; Maalej, H.; Bayoudh, A.; Hmidet, N.; Nasri, M. Enhancement of solubilization and biodegradation of diesel oil by biosurfactant from Bacillus amyloliquefaciens AN6. Int. Biodeter. Biodegr. 2015, 99, 8–14. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Salmah, I. Bio-enrichment of waste crude oil polluted soil: Amended with Bacillus 139SI and organic waste. Int. J. Environ. Sci. Dev. 2014, 4, 241–245. [Google Scholar]
- Ławniczak, Ł.; Marecik, R.; Łukasz, C. Contributions of biosurfactants to natural or induced bioremediation. Appl. Microbiol. Biotechnol. 2013, 97, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.S.; Baruah, R.; Borah, M.; Singh, A.K.; Deka Boruah, H.P.; Saikia, N.; Deka, M.; Dutta, N.; Chandra Bora, T. Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int. Biodeter. Biodegr. 2014, 94, 79–89. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Gudiña, E.J.; Costa, R.; Vitorino, R.; Teixeira, J.A.; Coutinho, J.A.P.; Rodrigues, L.R. Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel 2013, 111, 259–268. [Google Scholar] [CrossRef]
- Rufino, R.D.; de Luna, J.M.; de Campos Takaki, G.M.; Sarubbo, L.A. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014, 17, 34–38. [Google Scholar] [CrossRef]
- Al-Bahry, S.N.; Al-Wahaibi, Y.M.; Elshafie, A.E.; Al-Bemani, A.S.; Joshi, S.J.; Al-Makhmari, H.S.; Al-Sulaimani, H.S. Biosurfactant production by Bacillus subtilis b20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeter. Biodegr. 2013, 81, 141–146. [Google Scholar] [CrossRef]
- Al-Sulaimani, H.; Joshi, S.; Al-Wahaibi, Y.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A. Microbial biotechnology for enhancing oil recovery: Current developments and future prospects. Biotechnol. Bioinf. Bioeng. 2011, 1, 147–158. [Google Scholar]
- Healy, M.G.; Devine, C.M.; Murphy, R. Microbial production of biosurfactants. Resour. Conserv. Recy. 1996, 18, 41–57. [Google Scholar] [CrossRef]
- Neves, L.; de Oliveira, K.; Kobayashi, M.; Penna, T.; Converti, A. Biosurfactant production by cultivation of Bacillus atrophaeus ATCC 9372 in semidefined glucose/casein-based media. Appl. Biochem. Biotechnol. 2007, 137–140, 539–554. [Google Scholar] [PubMed]
- Ismail, S.; Dadrasnia, A. Biotechnological potential of Bacillus salmalaya 139SI: A novel strain for remediating water polluted with crude oil waste. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Rufino, R.; Sarubbo, L.; Neto, B.; Campos-Takaki, G. Experimental design for the production of tensio-active agent by Candida lipolytica. J. Int. Microbiol. Biotechnol. 2008, 35, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Rodriguez-Jasso, R.M. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef]
- Luna, J.M.; Rufino, R.D.; Sarubbo, L.A.; Campos-Takaki, G.M. Characterisation, surface properties and biological activity of a biosurfactant produced from industrial waste by Candida sphaerica UCP0995 for application in the petroleum industry. Colloid Surface. B 2013, 102, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, A.; Sabturani, N.; Radiman, S. Screening and optimization of biosurfactant production by the hydrocarbon-degrading bacteria. Sains Malays. 2013, 42, 615–623. [Google Scholar]
- Cunha, C.D.; do Rosário, M.; Rosado, A.S.; Leite, S.G.F. Serratia sp. Svgg16: A promising biosurfactant producer isolated from tropical soil during growth with ethanol-blended gasoline. Process Biochem. 2004, 39, 2277–2282. [Google Scholar] [CrossRef]
- Ruggeri, C.; Franzetti, A.; Bestetti, G.; Caredda, P.; La Colla, P.; Pintus, M.; Sergi, S.; Tamburini, E. Isolation and characterisation of surface active compound-producing bacteria from hydrocarbon-contaminated environments. Int. Biodeter. Biodegr. 2009, 63, 936–942. [Google Scholar] [CrossRef]
- Nitschke, M.; Pastore, G.M. Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresource Technol. 2006, 97, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Bozo-Hurtado, L.; Rocha, C.; Malavé, R.; Suárez, P. Biosurfactant production by marine bacterial isolates from the venezuelan atlantic front. B. Environ. Contam. Tox. 2012, 89, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, A.H.; Ye, R.Q.; Mu, B.Z. Effects of molecular structure on surfactant micellization activity. Acta Phys. Chim. Sin. 2011, 27, 1128–1134. [Google Scholar]
- Liu, Q.; Lin, J.; Wang, W.; Huang, H.; Li, S. Production of surfactin isomers by Bacillus subtilis BS-37and its applicability to enhanced oil recovery under laboratory conditions. Biochem. Eng. J. 2015, 93, 31–37. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Murty, V.R. Production of a lipopeptide biosurfactant by a novel Bacillus sp. And its applicability to enhanced oil recovery. ISRN Microb. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.F.; Teodoro-Martinez, D.S.; de Oliveira Barbosa, G.N.; Gontijo Vaz, B.; Serrano Silva, Í.; Garcia, J.S.; Tótola, M.R.; Eberlin, M.N.; Grossman, M.; Alves, O.L.; et al. Production and structural characterization of surfactin (c14/leu7) produced by Bacillus subtilis isolate lsfm-05 grown on raw glycerol from the biodiesel industry. Process Biochem. 2011, 46, 1951–1957. [Google Scholar] [CrossRef]
- Sousa, M.; Dantas, I.T.; Feitosa, F.X.; Alencar, A.E.V.; Soares, S.A.; Melo, V.M.M.; Gonçalves, L.R.B.; Sant’ana, H.B. Performance of a biosurfactant produced by Bacillus subtilis lami005 on the formation of oil / biosurfactant / water emulsion: Study of the phase behaviour of emulsified systems. Braz. J. Chem. Eng. 2014, 31, 613–623. [Google Scholar] [CrossRef]
- Moldes, A.B.; Paradelo, R.; Vecino, X.; Cruz, J.M.; GudiL, A.E.; Rodrigues, L.; Teixeira, J.A.; Domínguez, J.M.; Barral, M.T. Partial characterization of biosurfactant from Lactobacillus pentosus and comparison with sodium dodecyl sulphate for the bioremediation of hydrocarbon contaminated soil. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Joshi-Navare, K.; Prabhune, A. A biosurfactant-sophorolipid acts in synergy with antibiotics to enhance their efficiency. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-l.; Wu, Y.-t.; Qian, X.-p.; Meng, Q. Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J. Zhejiang Univ. Sci. B 2005, 6, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Burczyk, B. Biosurfactant production by Bacillus coagulans. J. Surfactants Deterg. 2003, 6, 61–64. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouze-Chaabouni, S.; Ghribi, D. Economic production of Bacillus subtilis SPB1 biosurfactant using local agro-industrial wastes and its application in enhancing solubility of diesel. J. Chem. Technol. Biot. 2013, 88, 779–787. [Google Scholar] [CrossRef]
- Joshi, S.; Bharucha, C.; Jha, S.; Yadav, S.; Nerurkar, A.; Desai, A.J. Biosurfactant production using molasses and whey under thermophilic conditions. Bioresource Technol. 2008, 99, 195–199. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadrasnia, A.; Ismail, S. Biosurfactant Production by Bacillus salmalaya for Lubricating Oil Solubilization and Biodegradation. Int. J. Environ. Res. Public Health 2015, 12, 9848-9863. https://doi.org/10.3390/ijerph120809848
Dadrasnia A, Ismail S. Biosurfactant Production by Bacillus salmalaya for Lubricating Oil Solubilization and Biodegradation. International Journal of Environmental Research and Public Health. 2015; 12(8):9848-9863. https://doi.org/10.3390/ijerph120809848
Chicago/Turabian StyleDadrasnia, Arezoo, and Salmah Ismail. 2015. "Biosurfactant Production by Bacillus salmalaya for Lubricating Oil Solubilization and Biodegradation" International Journal of Environmental Research and Public Health 12, no. 8: 9848-9863. https://doi.org/10.3390/ijerph120809848
APA StyleDadrasnia, A., & Ismail, S. (2015). Biosurfactant Production by Bacillus salmalaya for Lubricating Oil Solubilization and Biodegradation. International Journal of Environmental Research and Public Health, 12(8), 9848-9863. https://doi.org/10.3390/ijerph120809848






