Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
| Variable | Non-Smokers (N = 27) | Smokers (N = 34) |
|---|---|---|
| Age (years) | 47.3 ± 11.9 | 47.3 ± 10.5 |
| Education (years) | 16.5 ± 2.1 | 14.9 ± 2.1 * |
| Male (%) | 85 | 87 |
| Caucasian (%) | 63 | 71 |
| Body mass index | 24.8 ± 3.0 | 27.1 ± 4.5 * |
| Beck Depression Inventory | 3 ± 3 | 5 ± 4 |
| STAI-trait | 30 ± 10 | 34 ± 9 |
| 1-yr average drinks/month | 15 ± 14 | 23 ± 20 |
| Lifetime average drinks/month | 18 ± 12 | 25 ± 14 * |
| FTND | NA | 5 ± 2 |
| Cigarettes/day | NA | 18 ± 7 |
| Total lifetime years of smoking | NA | 30 ± 12 |
| Pack years | NA | 25 ± 14 |
| Interval from last cigarette to scan (min) | NA | 29 ± 18 |
2.2. Psychiatric, Medical, and Substance/Alcohol Consumption Assessment
2.3. Magnetic Resonance Data Acquisition and Processing
2.4. Statistical Analyses
| Region | Subregion (Bilateral) |
|---|---|
| BREOS | Caudal anterior cingulate cortex |
| Rostral anterior cingulate cortex | |
| Insula | |
| Caudal middle frontal gyrus | |
| Rostral middle frontal gyrus | |
| Lateral orbitofrontal cortex | |
| Medial orbitofrontal cortex | |
| Superior frontal gyrus | |
| AD | Isthmus of cingulate |
| Posterior cingulate | |
| Inferior parietal lobule | |
| Superior parietal lobule | |
| Precuneus | |
| Supramarginal gyrus | |
| Non-BREOS/AD | Cuneus |
| Frontal pole | |
| Paracentral | |
| Pars opercularis | |
| Pars orbitalis | |
| Pars triangularis | |
| Post central gyrus | |
| Precentral gyrus | |
| Superior temporal gyrus |
3. Results
3.1. Participant Characteristics
3.2. Comparisons of Smokers and Non-Smokers on Regional Cortical Perfusion
3.2.1. BREOS
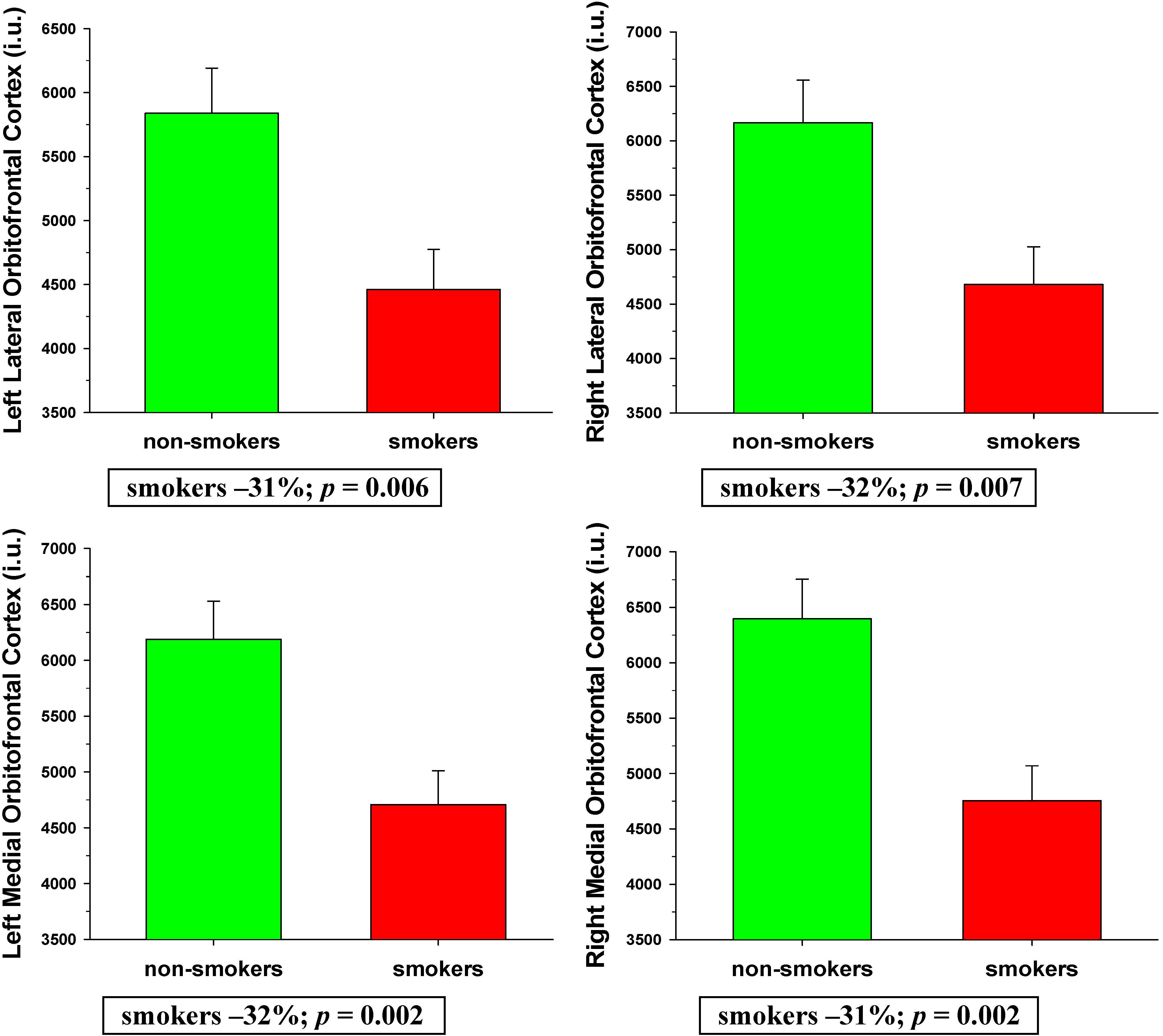
3.2.2. AD Regions
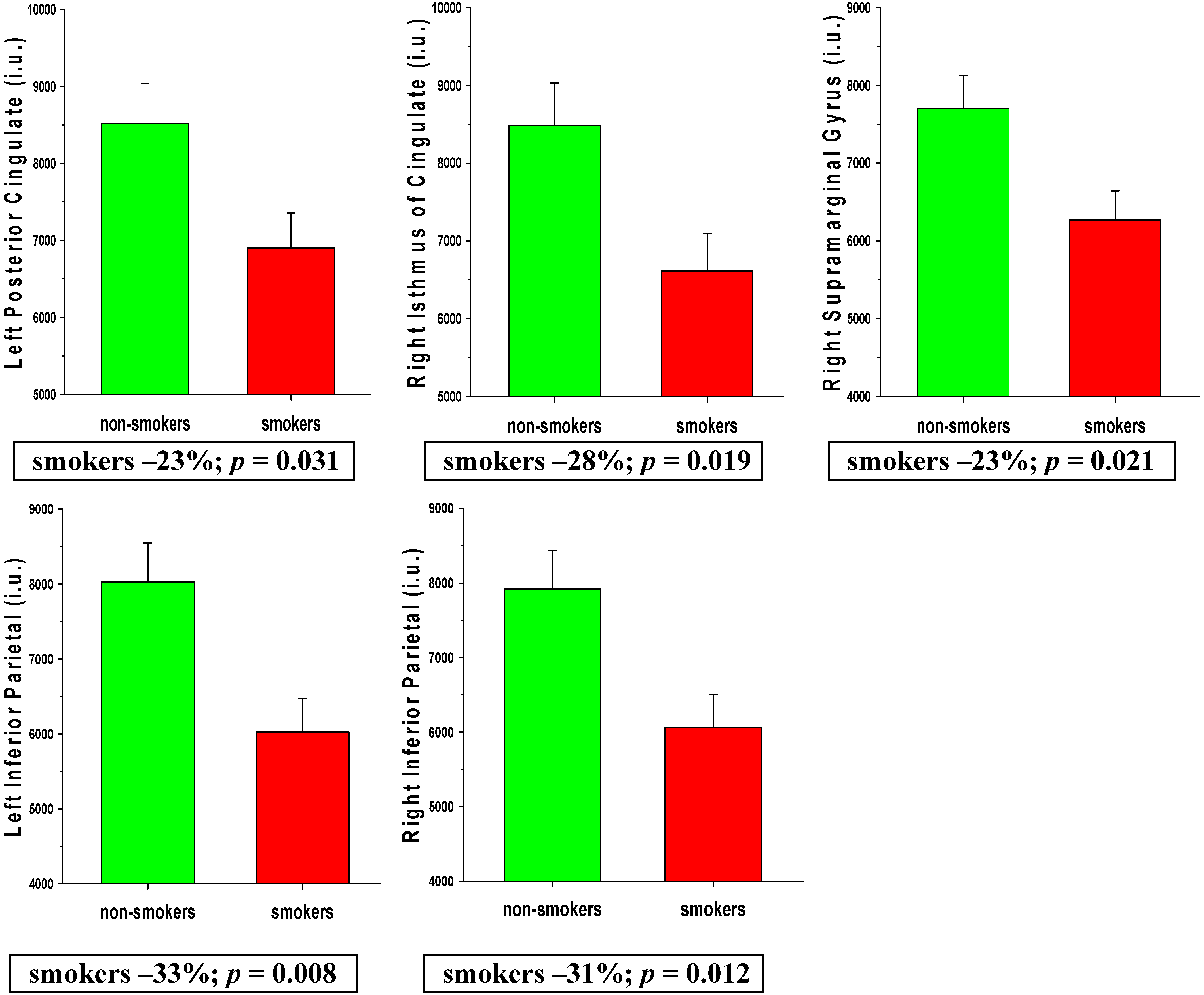
3.2.3. Non-BREOS/AD Regions
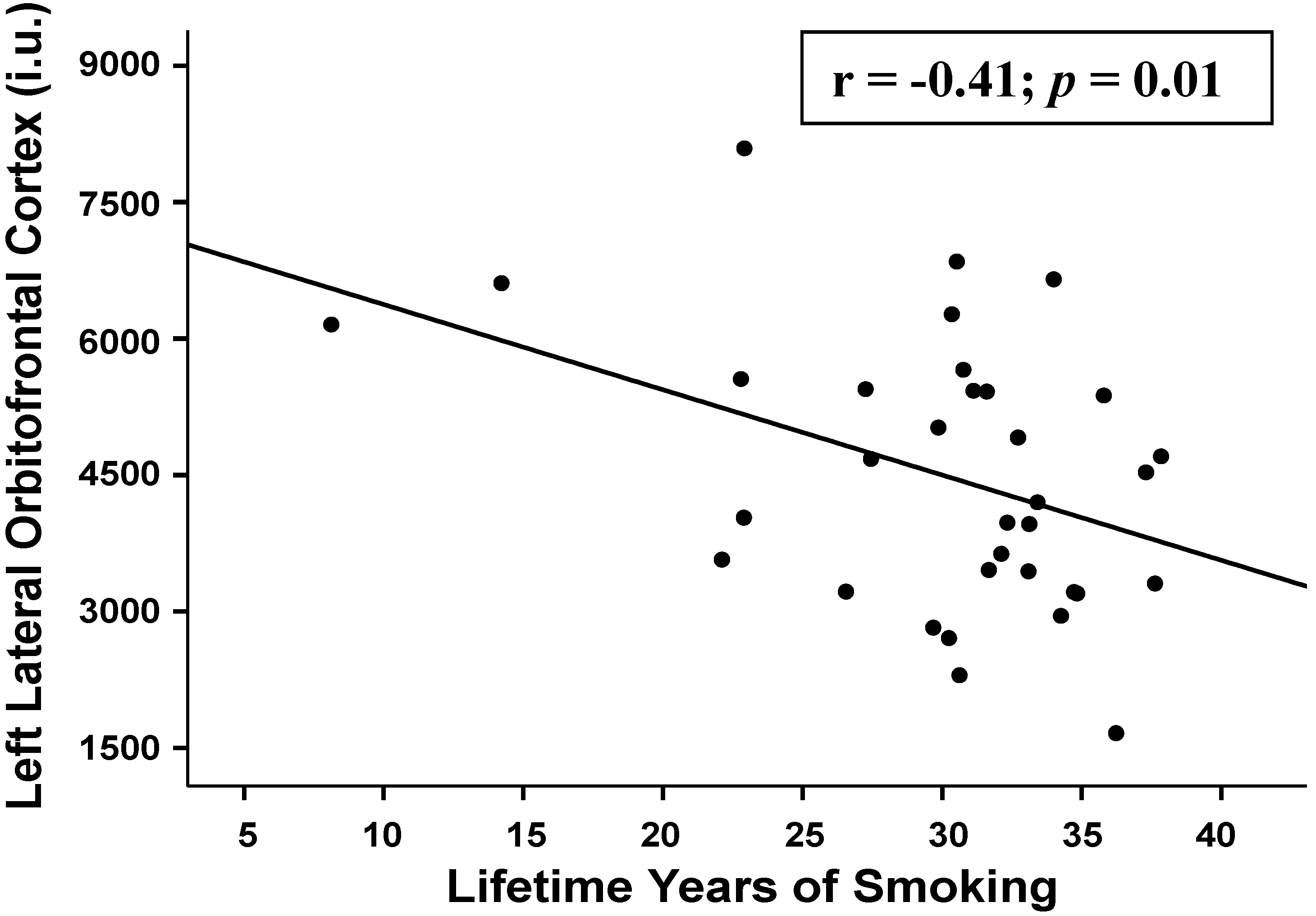
3.2.4. Associations between Regional Perfusion and Smoking Severity Measures
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Durazzo, T.C.; Meyerhoff, D.J.; Nixon, S.J. Chronic cigarette smoking: Implications for neurocognition and brain neurobiology. Int. J. Environ. Res. Public Health 2010, 7, 3760–3791. [Google Scholar] [CrossRef] [PubMed]
- Azizian, A.; Monterosso, J.; O’Neill, J.; London, E.D. Magnetic resonance imaging studies of cigarette smoking. In Nicotine Psychopharm; Springer: Berlin, Heidelberg, Germany, 2009; Volume 192, pp. 113–143. [Google Scholar]
- Sharma, A.; Brody, A. In vivo brain imaging of human exposure to nicotine and tobacco. In Nicotine Psychopharm; Springer: Berlin, Heidelberg, Germany, 2009; Volume 192, pp. 145–171. [Google Scholar]
- Durazzo, T.C.; Meyerhoff, D.J.; Nixon, S.J. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depen. 2013, 133, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mon, A.; Pennington, D.; Abe, C.; Gazdzinski, S.; Meyerhoff, D.J. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict. Boil. 2014, 19, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Kűhn, S.; Romanowski, A.; Schilling, C.; Mobascher, A.; Warbrick, T.; Winterer, G.; Gallinat, J. Brain grey matter deficits in smokers: Focus on the cerebellum. Brain Struct. Funct. 2012, 217, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Kűhn, S.; Schubert, F.; Gallinat, J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol. Psychiat. 2010, 68, 1061–1065. [Google Scholar] [CrossRef]
- Gallinat, J.; Meisenzahl, E.; Jacobsen, L.K.; Kalus, P.; Bierbrauer, J.; Kienast, T.; Witthaus, H.; Leopold, K.; Seifert, F.; Schubert, F.; et al. Smoking and structural brain deficits: A volumetric MR investigation. Eur. J. Neurosci. 2006, 24, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Garrido, G.J.; Lautenschlager, N.T.; Hulse, G.K.; Jamrozik, K.; Flicker, L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am. J. Geriat. Psychiat. 2008, 16, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.R.; Wetherill, R.R.; Jagannathan, K.; Johnson, B.; Mumma, J.; Hager, N.; Rao, H.; Childress, A.R. The effects of chronic cigarette smoking on gray matter volume: Influence of sex. PloS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Karama, S.; Ducharme, S.; Corley, J.; Chouinard-Decorte, F.; Starr, J.M.; Wardlaw, J.M.; Bastin, M.E.; Deary, I.J. Cigarette smoking and thinning of the brain’s cortex. Mol. Psychiatr. 2015, 20, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Gallinat, J.; Lang, U.E.; Jacobsen, L.K.; Bajbouj, M.; Kalus, P.; von Haebler, D.; Seifert, F.; Schubert, F. Abnormal hippocampal neurochemistry in smokers: Evidence from proton magnetic resonance spectroscopy at 3 tesla. J. Clin. Psychopharm. 2007, 27, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Schubert, F.; Seifert, F.; Bajbouj, M.; Gallinat, J. Proton MRS at 3 tesla reveals altered neurochemistry in smokers. Available online: http://cds.ismrm.org/ismrm-2006/files/00909.pdf (accessed on 28 May 2015).
- O’Neill, J.; Tobias, M.C.; Hudkins, M.; Oh, E.Y.; Hellemann, G.S.; Nurmi, E.L.; London, E.D. Thalamic glutamate decreases with cigarette smoking. Psychopharmacology 2014, 231, 2717–2724. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Meyerhoff, D.J.; Mon, A.; Abe, C.; Gazdzinski, S.; Murray, D.E. Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain n-acetylaspartate and glutamate levels. Biol. Psychiat. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Salmeron, B.J.; Ross, T.J.; Geng, X.; Yang, Y.; Stein, E.A. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage 2011, 54, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Weiland, B.J.; Sabbineni, A.; Calhoun, V.D.; Welsh, R.C.; Hutchison, K.E. Reduced executive and default network functional connectivity in cigarette smokers. Hum. Brain Mapp. 2014, 36, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wu, G.; Zhu, L.; Lei, H. Altered brain functional networks in heavy smokers. Addict. Biol. 2014, 20, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mon, A.; Gazdzinski, S.; Meyerhoff, D.J. Chronic cigarette smoking in alcohol dependence: Associations with cortical thickness and n-acetylaspartate levels in the extended brain reward system. Addict. Boil. 2013, 18, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Tosun, D.; Buckley, S.; Gazdzinski, S.; Mon, A.; Fryer, S.L.; Meyerhoff, D.J. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: Relationships to relapse and extended abstinence. Alcohol. Clin. Exp. Res. 2011, 35, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. 2012, 52, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mattsson, N.; Weiner, M.W. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 2014, 10, 122–145. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kobayashi, S.; Yamaguchi, S.; Kitani, M.; Tsunematsu, T. Effect of smoking on regional cerebral blood flow in the normal aged volunteers. Gerontology 1988, 34, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.L.; Meyer, J.S.; Shaw, T.G.; Mortel, K.F.; Hardenberg, J.P.; Zaid, R.R. Cigarette smoking decreases cerebral blood flow suggesting increased risk for stroke. JAMA 1983, 250, 2796–2800. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.L.; Meyer, J.S.; Judd, B.W.; Mortel, K.F. Abstention from cigarette smoking improves cerebral perfusion among elderly chronic smokers. JAMA 1985, 253, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Rourke, S.B.; Dupont, R.M.; Grant, I.; Lehr, P.P.; Lamoureux, G.; Halpern, S.; Yeung, D.W. Reduction in cortical IMP-SPET tracer uptake with recent cigarette consumption in a young group of healthy males. San Diego HIV neurobehavioral research center. Eur. J. Nucl. Med. 1997, 24, 422–427. [Google Scholar] [PubMed]
- Durazzo, T.C.; Mattsson, N.; Weiner, M.W. Interaction of cigarette smoking history with apoe genotype and age on amyloid level, glucose metabolism, and neurocognition in cognitively normal elders. Nicotine Tob. Res. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wolk, D.A.; Reddin, J.S.; Korczykowski, M.; Martinez, P.M.; Musiek, E.S.; Newberg, A.B.; Julin, P.; Arnold, S.E.; Greenberg, J.H.; et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 2011, 77, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Jueptner, M.; Weiller, C. Review: Does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 1995, 2, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Vital signs: Current cigarette smoking among adults aged ≥ 18 years—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1135–1140. [Google Scholar]
- Durazzo, T.C.; Meyerhoff, D.J.; Nixon, S.J. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depen. 2012, 122, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.; Meyerhoff, D.J.; Nixon, S.J. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depen. 2013, 133, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Leigh, J.S.; Williams, D.S.; Koretsky, A.P. Perfusion imaging. Magnet. Reson. Med. 1992, 23, 37–45. [Google Scholar] [CrossRef]
- Tosun, D.; Mojabi, P.; Weiner, M.W.; Schuff, N. Joint analysis of structural and perfusion MRI for cognitive assessment and classification of Alzheimer’s disease and normal aging. Neuroimage 2010, 52, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; Van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Fischl, B.; Van der Kouwe, A.; Destrieux, C.; Halgren, E.; Segonne, F.; Salat, D.H.; Busa, E.; Seidman, L.J.; Goldstein, J.; Kennedy, D.; et al. Automatically parcellating the human cerebral cortex. Cereb. cortex 2004, 14, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.E.; Durazzo, T.C.; Mon, A.; Schmidt, T.; Meyerhoff, D.J. Brain perfusion in polysubstance users: Relationship to substance and tobacco use, cognition, and self-regulation. Drug Alcohol Depen. 2015, 150, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sankoh, A.J.; Huque, M.F.; Dubey, S.D. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997, 16, 2529–2542. [Google Scholar] [CrossRef]
- Durazzo, T.; Insel, P.S.; Weiner, M.W. The Alzheimer’s disease neuroimaging initiative, a greater regional brain atrophy rate in healthy elders with a history of cigarette smoking. Alzheimers Dement. 2012, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tosun, D.; Rosen, H.; Miller, B.L.; Weiner, M.W.; Schuff, N. MRI patterns of atrophy and hypoperfusion associations across brain regions in frontotemporal dementia. Neuroimage 2012, 59, 2098–2109. [Google Scholar] [CrossRef] [PubMed]
- Tosun, D.; Schuff, N.; Weiner, M. An integrated multimodality MR brain imaging study: Gray matter tissue loss mediates the association between cerebral hypoperfusion and Alzheimer’s disease. In Proceedings of Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009.
- Rolls, E.T.; Grabenhorst, F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog. Neurobiol. 2008, 86, 216–244. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Moallem, N.R.; Ray, L.A. Dimensions of impulsivity among heavy drinkers, smokers, and heavy drinking smokers: Singular and combined effects. Addict. Behav. 2012, 37, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Bernow, N.; Kruck, B.; Pfeifer, P.; Lieb, K.; Tuscher, O.; Fehr, C. Impulsiveness and venturesomeness in german smokers. Nicotine Tob. Res. 2011, 13, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Paperwalla, K.N.; Levin, T.T.; Weiner, J.; Saravay, S.M. Smoking and depression. Med. Clin. N. Am. 2004, 88, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Odum, A.L.; Madden, G.J. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology 1999, 146, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Nestor, L.; McCabe, E.; Jones, J.; Clancy, L.; Garavan, H. Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage 2011, 56, 2258–2275. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. Memory and executive function in aging and ad: Multiple factors that cause decline and reserve factors that compensate. Neuron 2004, 44, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Whishaw, I.Q. Fundamentals of Human Neuropsychology, 6th ed.; Worth Publishers: New York, NY, USA, 2009; pp. 3–4. [Google Scholar]
- Addicott, M.; Froeliger, B.; Kozink, R.; Van Wert, D.; Westman, E.; Rose, J.; McClernon, F. Nicotine and non-nicotine smoking factors differentially modulate craving, withdrawal and cerebral blood flow as measured with arterial spin labeling. Neuropsychopharmacology 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Yokoi, T. Interindividual variability in nicotine metabolism: C-oxidation and glucuronidation. Drug Metab. Pharmacok. 2005, 20, 227–235. [Google Scholar] [CrossRef]
- Hukkanen, J.; Jacob, P., 3rd; Benowitz, N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef] [PubMed]
- Gazdzinski, S.; Durazzo, T.C.; Jahng, G.H.; Ezekiel, F.; Meyerhoff, D.J. Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion—a preliminary Magnetic resonance study. Alcohol. Clin. Exp. Res. 2006, 30, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Mon, A.; Durazzo, T.C.; Gazdzinski, S.; Meyerhoff, D.J. The impact of chronic cigarette smoking on Recovery from Cortical Gray Matter Perfusion Deficits in Alcohol Dependence: Longitudinal Arterial Spin Labeling MRI. Alcohol. Clin. Exp. Res. 2009, 33, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Gdovinova, Z. Blood flow velocity in the middle cerebral artery in heavy alcohol drinkers. Alcohol 2001, 36, 346–348. [Google Scholar] [CrossRef]
- Terborg, C.; Bramer, S.; Weiller, C.; Rother, J. Short-term effect of cigarette smoking on co(2)-induced vasomotor reactivity in man: A study with near-infrared spectroscopy and tanscranial doppler sonography. J. Neurol. Sci. 2002, 205, 15–20. [Google Scholar] [CrossRef]
- Terborg, C.; Birkner, T.; Schack, B.; Witte, O.W. Acute effects of cigarette smoking on cerebral oxygenation and hemodynamics: A combined study with near-infrared spectroscopy and transcranial doppler sonography. J. Neurol. Sci. 2002, 205, 71–75. [Google Scholar] [CrossRef]
- Wang, J.; Fernandez-Seara, M.A.; Wang, S.; St Lawrence, K.S. When perfusion meets diffusion: In vivo measurement of water permeability in human brain. J. Cerebr. Blood F. Met. 2006, 27, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durazzo, T.C.; Meyerhoff, D.J.; Murray, D.E. Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults. Int. J. Environ. Res. Public Health 2015, 12, 8198-8213. https://doi.org/10.3390/ijerph120708198
Durazzo TC, Meyerhoff DJ, Murray DE. Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults. International Journal of Environmental Research and Public Health. 2015; 12(7):8198-8213. https://doi.org/10.3390/ijerph120708198
Chicago/Turabian StyleDurazzo, Timothy C., Dieter J. Meyerhoff, and Donna E. Murray. 2015. "Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults" International Journal of Environmental Research and Public Health 12, no. 7: 8198-8213. https://doi.org/10.3390/ijerph120708198
APA StyleDurazzo, T. C., Meyerhoff, D. J., & Murray, D. E. (2015). Comparison of Regional Brain Perfusion Levels in Chronically Smoking and Non-Smoking Adults. International Journal of Environmental Research and Public Health, 12(7), 8198-8213. https://doi.org/10.3390/ijerph120708198





