Difference in Pro-Inflammatory Cytokine Responses Induced in THP1 Cells by Particulate Matter Collected on Days with and without ASIAN Dust Storms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Airborne Particles
2.2. Cell Line Culture
2.3. Cytokine Quantification
2.4. Measurement of Metal Elements
2.5. Statistical Analyses
3. Results
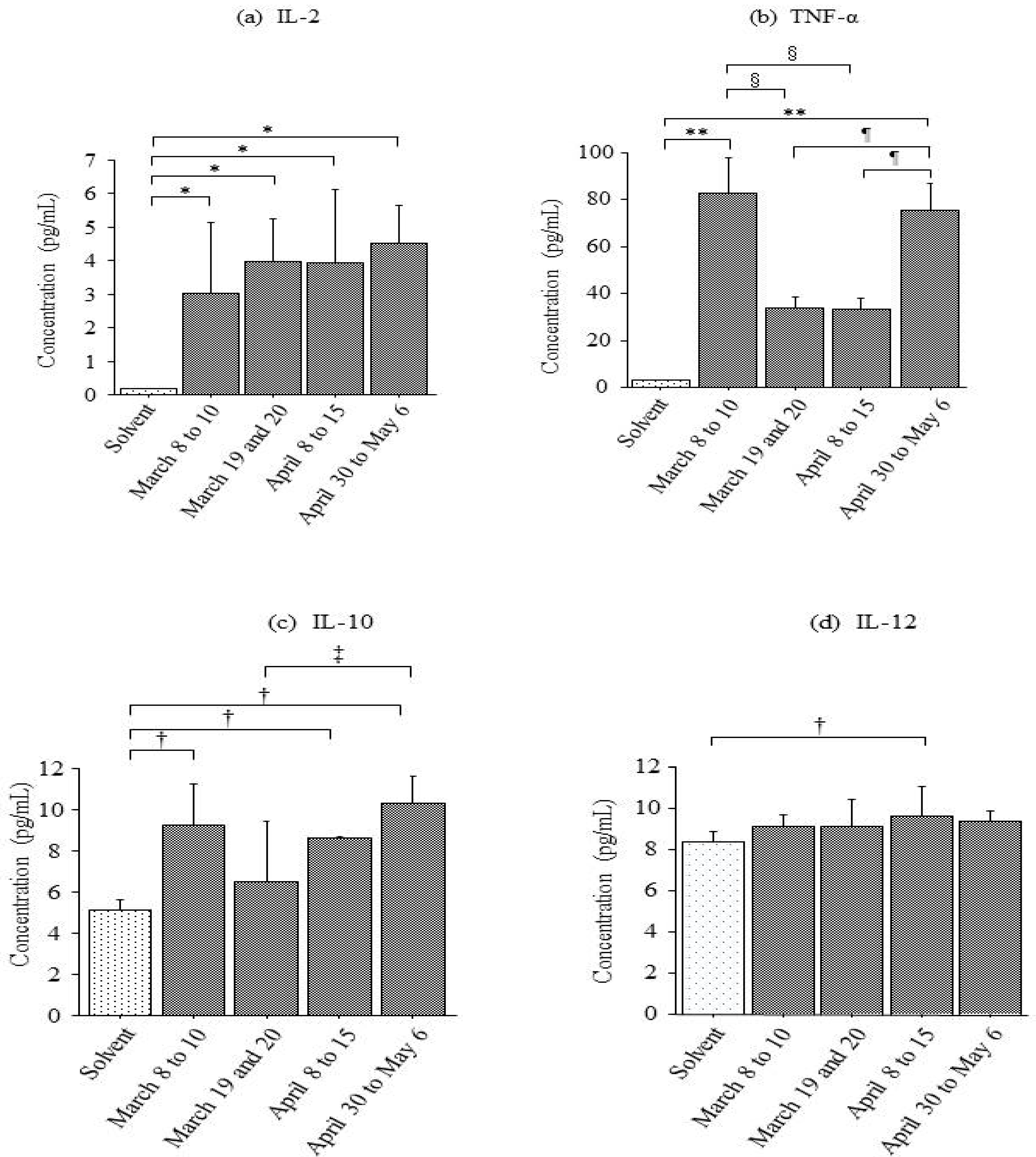
3.1. Production of Pro-Inflammatory Cytokines Caused by Airborne Particles
3.2. Concentration of Metal Elements
| Metals | 8 to 10 March | 19 and 20 March | 8 to 15 April | 30 April to 6 May |
|---|---|---|---|---|
| Al | 22.4 | 14.8 | 23.8 | 27.4 |
| As | ND | ND | ND | ND |
| Ba | 0.18 | 0.10 | 0.16 | 0.11 |
| Ca | 44.0 | 31.2 | 44.0 | 28.1 |
| Cd | ND | ND | ND | ND |
| Cr | ND | ND | ND | ND |
| Cu | ND | 0.07 | 0.11 | 0.13 |
| Fe | 22.3 | 16.8 | 15.4 | 21.5 |
| Mg | 16.8 | 14.4 | 16.0 | 13.7 |
| Mn | 0.56 | 0.40 | 0.48 | 0.49 |
| Ni | 0.15 | 0.11 | 0.12 | 0.13 |
| Pb | 0.12 | 0.06 | 0.08 | 0.06 |
| Si | 108.0 | 72.0 | 116.0 | 133.0 |
| Sr | 0.22 | 0.16 | 0.16 | 0.15 |
| Ti | 0.92 | 0.52 | 0.76 | 0.91 |
| Zn | 0.64 | 0.60 | 0.48 | 0.49 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADS | Asian dust storm | IL | interleukin |
| TNF | tumor necrosis factor | PM10 | particulate matter smaller than 10 μm |
| PM2.5 | particulate matter smaller than 2.5μm | PM0.5 | particulate matter smaller than 0.5μm |
| SD | standard deviation |
References
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Madaniyazi, L.; Guo, Y.; Yu, W.; Tong, S. Projecting future air pollution-related mortality under a changing climate: Progress, uncertainties and research needs. Environ. Int. 2015, 75, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.H.; et al. Natural cause mortality and long-term exposure to particle components: An analysis of 19 European cohorts within the Multi-Center ESCAPE Project. Environ. Health Perspect. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Goudie, A.S. Desert dust and human health disorders. Environ. Int. 2014, 63, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ramanathan, V.; Li, F.; Kim, D. Dust plumes over the Pacific, Indian, and Atlantic Oceans: Climatology and radiative impact. J. Geophys. Res. 2007, 112. [Google Scholar] [CrossRef]
- Tanaka, T.Y.; Chiba, M. A numerical study of the concentrations of dust source regions to the global dust budget. Glob. Planet. Chang. 2006, 52, 88–104. [Google Scholar] [CrossRef]
- Zaady, E.; Offer, Z.Y.; Shachak, M. The content and contributions of deposited aeolian organic matter in a dry land ecosystem of the Negev Desert, Israel. Atmos. Environ. 2001, 35, 769–776. [Google Scholar] [CrossRef]
- Zosky, G.R.; Boylen, C.E.; Wong, R.S.; Smirk, M.N.; Gutiérrez, L.; Woodward, R.C.; Siah, W.S.; Devine, B.; Maley, F.; Cook, A. Variability and consistency in lung inflammatory responses to particles with a geogenic origin. Respirology 2014, 19, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Sheen, P.C.; Chen, E.R.; Liu, Y.K.; Wu, T.N.; Yang, C.Y. Effects of Asian dust storm events on daily mortality in Taipei, Taiwan. Environ. Res. 2004, 95, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Levy, J.K.; Lin, Z. The effect of sandstorms and air pollution on cause-specific hospital admissions in Taipei, Taiwan. Occup. Environ. Med. 2008, 65, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Chuang, K.J.; Chen, W.J.; Chang, W.T.; Lee, C.T.; Peng, C.M. Increasing cardiopulmonary emergency visits by long-range transported Asian dust storms in Taiwan. Environ. Res. 2008, 106, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Hioki, T.; Nakanishi, S.; Mukai, H.; Murano, K. Analysis of long-range transported and local air pollution with trace metal concentration ratio and lead isotope ratio in precipitation. J. Jpn. Soc. Atmos. Environ. 2008, 43, 100–111. [Google Scholar]
- Wuebbles, D.J.; Lei, H.; Lin, J. Intercontinental transport of aerosols and photochemical oxidants from Asia and its consequences. Environ. Pollut. 2007, 150, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Schikowski, T.; Adam, M.; Buschka, A.; Carsin, A.E.; Jacquemin, B.; Marcon, A.; Sanchez, M.; Vierkötter, A.; Al-Kanaani, Z.; et al. Cross-sectional associations between air pollution and chronic bronchitis: An ESCAPE meta-analysis across five cohorts. Thorax 2014, 69, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Pan, X.C.; Kim, S.Y.; Park, K.; Park, E.J.; Jin, X.; Yi, S.M.; Kim, Y.H.; Park, C.H.; Song, S.; et al. Asian Dust Storm and pulmonary function of school children in Seoul. Sci. Total Environ. 2010, 408, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Roemer, W.; Hoek, G.; Brunekreef, B.; Haluszka, J.; Kalandidi, A.; Pekkanen, J. Daily variations in air pollution and respiratory health in a multicentre study: The PEACE project. Pollution Effects on Asthmatic Children in Europe. Eur. Respir. J. 1998, 12, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.T.; Dales, R.; Krewski, D.; Vincent, R.; Dann, T.; Brook, J.R. Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am. J. Epidemiol. 1995, 142, 15–22. [Google Scholar] [PubMed]
- De Kok, T.M.C.M.; Driece, H.A.L.; Hogervorst, J.G.F.; Briedé, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef] [PubMed]
- De Kok, T.M.; Hogervorst, J.G.; Briedé, J.J.; van Herwijnen, M.H.; Maas, L.M.; Moonen, E.J.; Driece, H.A.; Kleinjans, J.C. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ. Mol. Mutagen. 2005, 46, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.J.; Roberts, K.T.; Jones, N.; Harrison, R.M.; Ayres, J.G.; Hussain, S.; Walters, S. Effects of daily variation in outdoor particulates and ambient acid species in normal and asthmatic children. Thorax 2002, 57, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yang, C.Y. Effects of Asian dust storm events on daily hospital admissions for cardiovascular disease in Taipei, Taiwan. J. Toxicol. Environ. Health A 2005, 68, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.W.; Cheng, W.L. The impact of air quality on respiratory admissions during Asian dust storm periods. Int. J. Environ. Health. Res. 2008, 18, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chen, Y.S.; Chiu, H.F.; Goggins, W.B. Effects of Asian dust storm events on daily stroke admissions in Taipei, Taiwan. Environ. Res. 2005, 99, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Tsai, S.S.; Chang, C.C.; Ho, S.C. Effects of Asian dust storm events on daily admissions for asthma in Taipei, Taiwan. Inhal. Toxicol. 2005, 17, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Cheng, M.H.; Chen, C.C. Effects of Asian dust storm events on hospital admissions for congestive heart failure in Taipei, Taiwan. J. Toxicol. Environ. Health A 2009, 72, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Noma, H.; Kurai, J.; Sano, H.; Saito, R.; Abe, S.; Kimura, Y.; Aiba, S.; Oshimura, M.; Yamasaki, A.; et al. Decreased pulmonary function in school children in western Japan after exposures to Asian desert dusts and its association with interleukin-8. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bilos, C.; Colombo, J.C.; Skorupka, C.N.; Rogriguez Presa, M.J. Sources, distribution and variability of airborne trace metals in La Plata City area, Argentina. Environ. Pollut. 2001, 111, 149–158. [Google Scholar] [CrossRef]
- Sweet, C.W.; Vermette, S.J.; Landsberger, S. Sources of toxic trace elements in urban air in Illinois. Environ. Sci. Technol. 1993, 27, 2502–2510. [Google Scholar] [CrossRef]
- Sullivan, R.; Woods, I. Using emission factors to characterise heavy metal emissions from sewage sludge incinerators in Australia. Atmos. Environ. 2000, 34, 4571–4577. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Selzle, K.; Pöschl, U. Hazardous components and health effects of atmospheric aerosol particles: Reactive oxygen species, soot, polycyclic aromatic compounds and allergenic proteins. Free Radic. Res. 2012, 46, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Hetland, R.B.; Cassee, F.R.; Låg, M.; Refsnes, M.; Dybing, E.; Schwarze, P.E. Cytokine release from alveolar macrophages exposed to ambient particulate matter: Heterogeneity in relation to size, city and season. Part. Fibre Toxicol. 2005, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, W.E.; Chow, J.C.; Claiborn, C.; Fusheng, W.; Engelbrecht, J.; Watson, J.G. Monitoring of particulate matter outdoors. Chemosphere 2002, 49, 1009–1043. [Google Scholar] [CrossRef]
- Gong, H.J., Jr.; Linn, W.S.; Sioutas, C.; Terrell, S.L.; Clark, K.W.; Anderson, K.R.; Terrell, L.L. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient fine particles in Los Angeles. Inhal. Toxicol. 2003, 15, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, N.; Nordenhäll, C.; Salvi, S.S.; Mudway, I.; Soderberg, M.; Blomberg, A.; Helleday, R.; Levin, J.O.; Holgate, S.T.; Kelly, F.J.; et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur. Respir. J. 2004, 23, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Shadie, A.M.; Bucknall, M.P.; Rutlidge, H.; Garthwaite, L.; Herbert, C.; Halliburton, B.; Parsons, K.S.; Wark, P.A. Differential injurious effects of ambient and traffic-derived particulate matter on airway epithelial cells. Respirology 2015, 20, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, P.E.; Øvrevik, J.; Låg, M.; Refsnes, M.; Nafstad, P.; Hetland, R.B.; Dybing, E. Particulate matter properties and health effects: Consistency of epidemiological and toxicological studies. Hum. Exp. Toxicol. 2006, 25, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Iosifidis, T.; Perks, K.; Ditcham, W.G.; Devadason, S.G.; Siah, W.S.; Devine, B.; Maley, F.; Cook, A. The concentration of iron in real-world geogenic PM10 is associated with increased inflammation and deficits in lung function in mice. PLoS ONE 2014, 9, e90609. [Google Scholar] [CrossRef] [PubMed]
- Mossman, B.T.; Borm, P.J.; Castranova, V.; Costa, D.L.; Donaldson, K.; Kleeberger, S.R. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Part. Fibre Toxicol. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Loxham, M.; Morgan-Walsh, R.J.; Cooper, M.J.; Blume, C.; Swindle, E.J.; Dennison, P.W.; Howarth, P.H.; Cassee, F.R.; Teagle, D.A.; Palmer, M.R.; et al. The effects on bronchial epithelial mucociliary cultures of corase, fine, and ultrafine particulate matter from an underground railway station. Toxicol. Sci. 2015, 145, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dergham, M.; Lepers, C.; Verdin, A.; Billet, S.; Cazier, F.; Courcot, D.; Shirali, P.; Garçon, G. Prooxidant and proinflammatory potency of air pollution particulate matter (PM2.5–0.3) produced in rural, urban, or industrial surroundings in human bronchial epithelial cells (BEAS-2B). Chem. Res. Toxicol. 2012, 25, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Baulig, A.; Singh, S.; Marchand, A.; Schins, R.; Barouki, R.; Garlatti, M.; Marano, F.; Baeza-Squiban, A. Role of Paris PM2.5 components in the pro-inflammatory response induced in airway epithelial cells. Toxicology 2009, 261, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Bonvallot, V.; Baeza-Squiban, A.; Baulig, A.; Brulant, S.; Boland, S.; Muzeau, F.; Barouki, R.; Marano, F. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am. J. Respir. Cell Mol. Biol. 2001, 25, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Schins, R.P.F.; Lightbody, J.H.; Borm, P.J.A.; Shi, T.; Donaldson, K.; Stone, V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol. Appl. Pharmacol. 2004, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kurai, J.; Tomita, K.; Sano, H.; Abe, S.; Saito, R.; Minato, S.; Igishi, T.; Burioka, N.; Sako, T.; et al. Effects on asthma and induction of interleukin-8 caused by Asian dust particles collected in western Japan. J. Asthma 2014, 51, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Kurosaki, Y.; Otani, S.; Yoshida, A.; Sugimoto, N.; Kurozawa, Y. Atmospheric transport route determines components of Asian dust and health effects in Japan. Atmos. Environ. 2012, 49, 94–102. [Google Scholar] [CrossRef]
- Mitschik, S.; Schierl, R.; Nowak, D.; Jörres, R.A. Effects of particulate matter on cytokine production in vitro: A comparative analysis of published studies. Inhal. Toxicol. 2008, 20, 399–414. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Kurai, J.; Sano, H.; Yamasaki, A.; Shimizu, E. Difference in Pro-Inflammatory Cytokine Responses Induced in THP1 Cells by Particulate Matter Collected on Days with and without ASIAN Dust Storms. Int. J. Environ. Res. Public Health 2015, 12, 7725-7737. https://doi.org/10.3390/ijerph120707725
Watanabe M, Kurai J, Sano H, Yamasaki A, Shimizu E. Difference in Pro-Inflammatory Cytokine Responses Induced in THP1 Cells by Particulate Matter Collected on Days with and without ASIAN Dust Storms. International Journal of Environmental Research and Public Health. 2015; 12(7):7725-7737. https://doi.org/10.3390/ijerph120707725
Chicago/Turabian StyleWatanabe, Masanari, Jun Kurai, Hiroyuki Sano, Akira Yamasaki, and Eiji Shimizu. 2015. "Difference in Pro-Inflammatory Cytokine Responses Induced in THP1 Cells by Particulate Matter Collected on Days with and without ASIAN Dust Storms" International Journal of Environmental Research and Public Health 12, no. 7: 7725-7737. https://doi.org/10.3390/ijerph120707725
APA StyleWatanabe, M., Kurai, J., Sano, H., Yamasaki, A., & Shimizu, E. (2015). Difference in Pro-Inflammatory Cytokine Responses Induced in THP1 Cells by Particulate Matter Collected on Days with and without ASIAN Dust Storms. International Journal of Environmental Research and Public Health, 12(7), 7725-7737. https://doi.org/10.3390/ijerph120707725





