Conversion Characteristics and Production Evaluation of Styrene/o-Xylene Mixtures Removed by DBD Pretreatment
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Set-Up
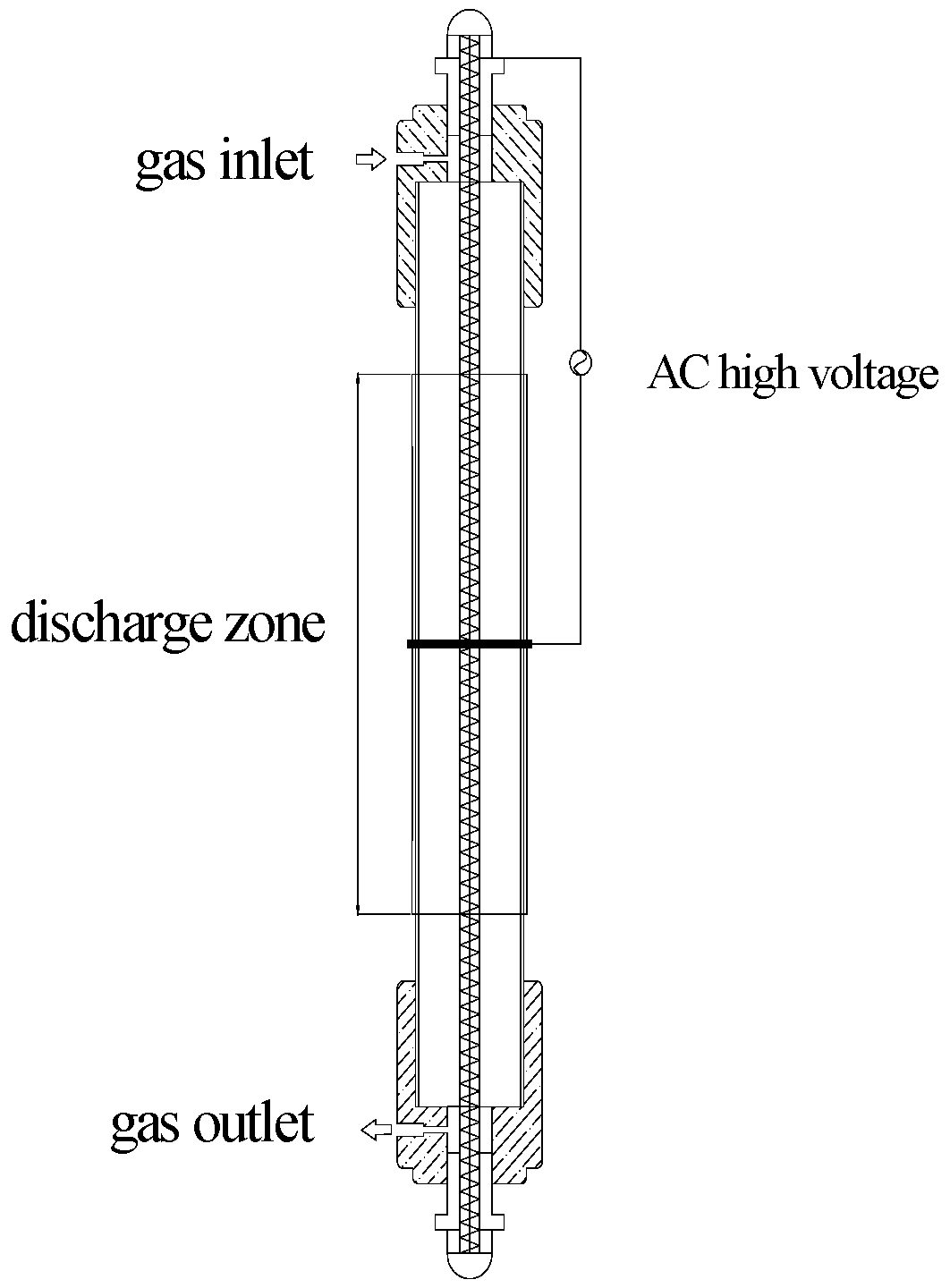
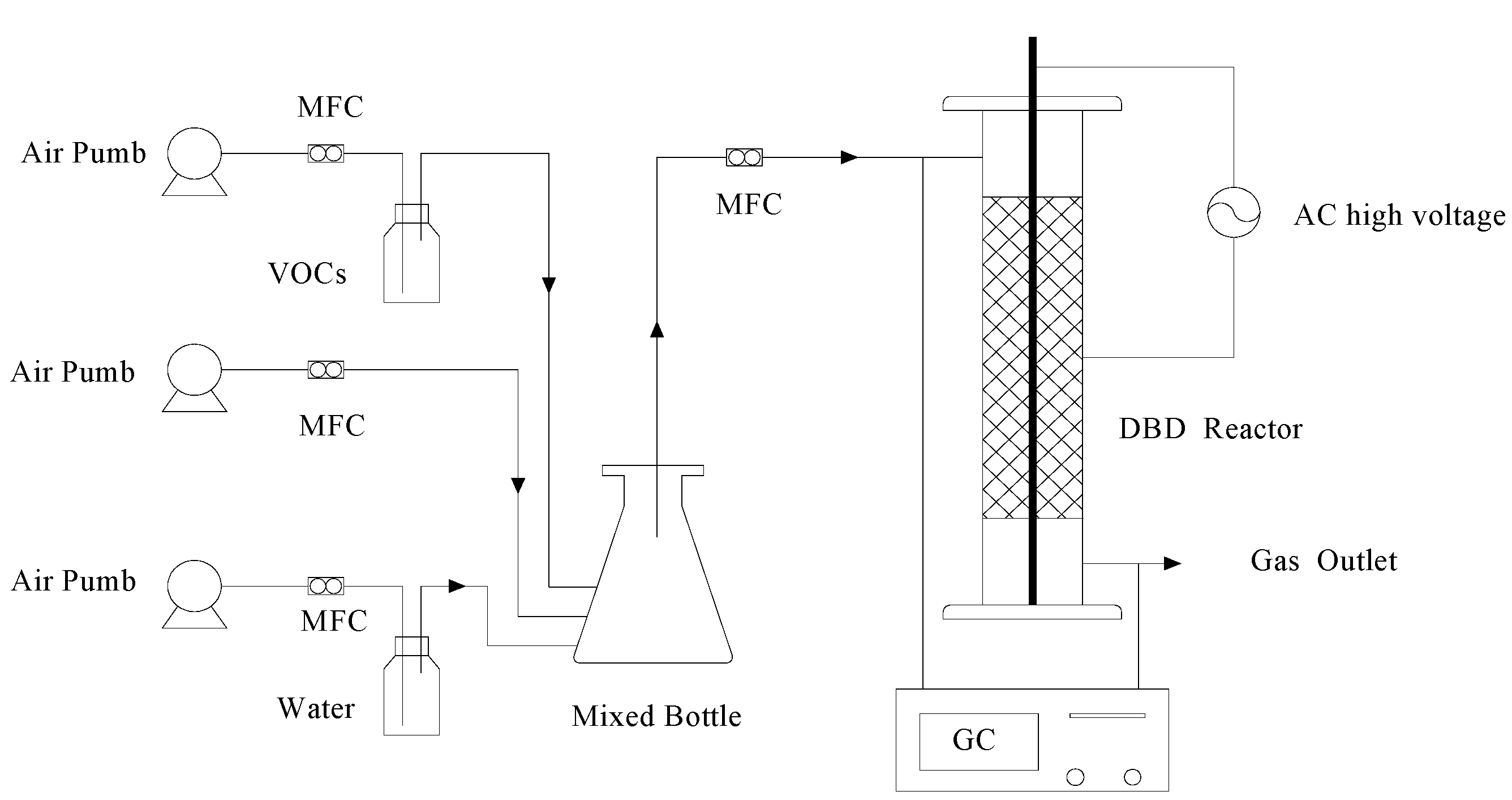
2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
3.1. Influence of the Initial Concentration on the Decomposition of the Mixture of VOCs

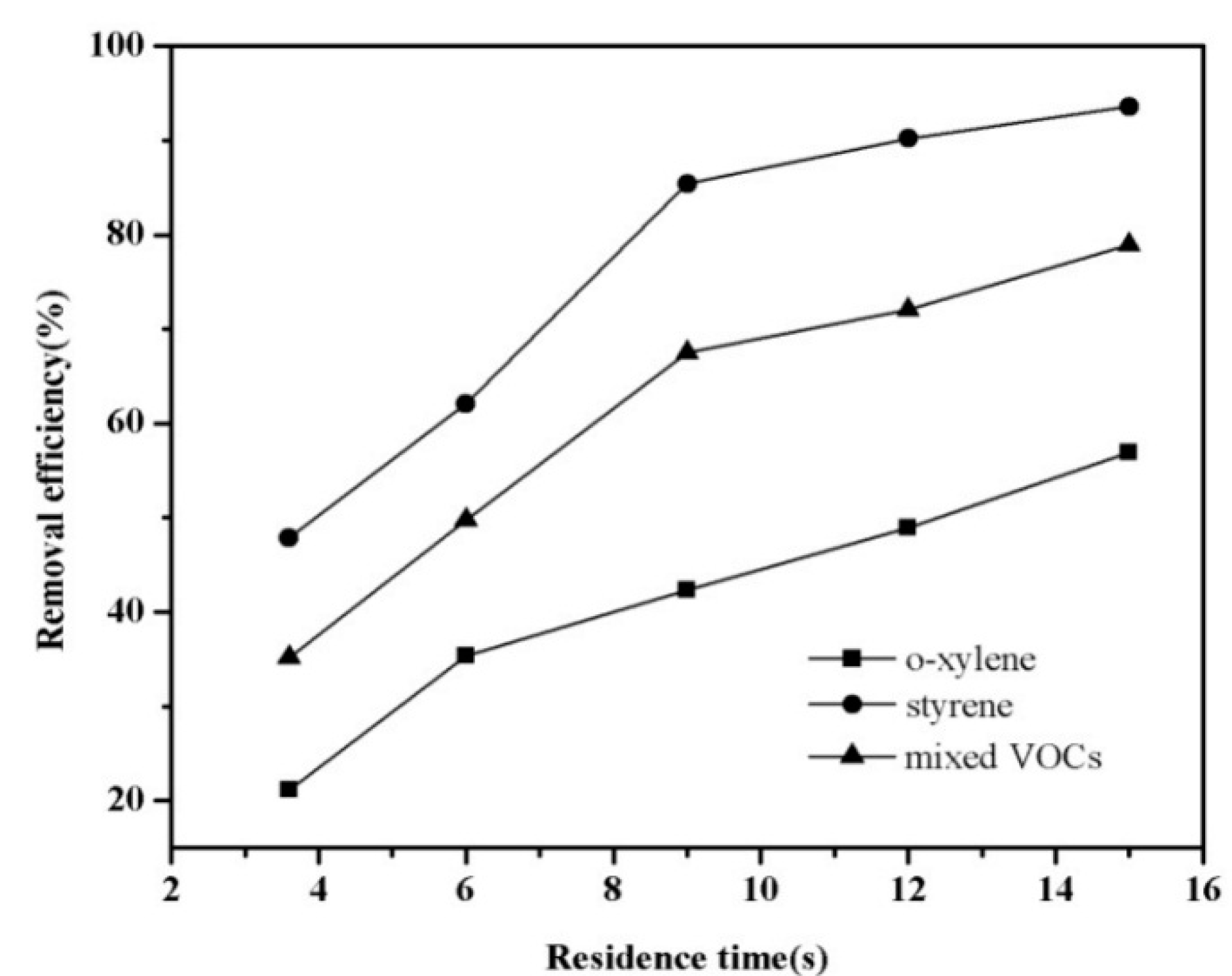
3.2. Influence of Residence Time on the Decomposition of the Mixture of VOCs
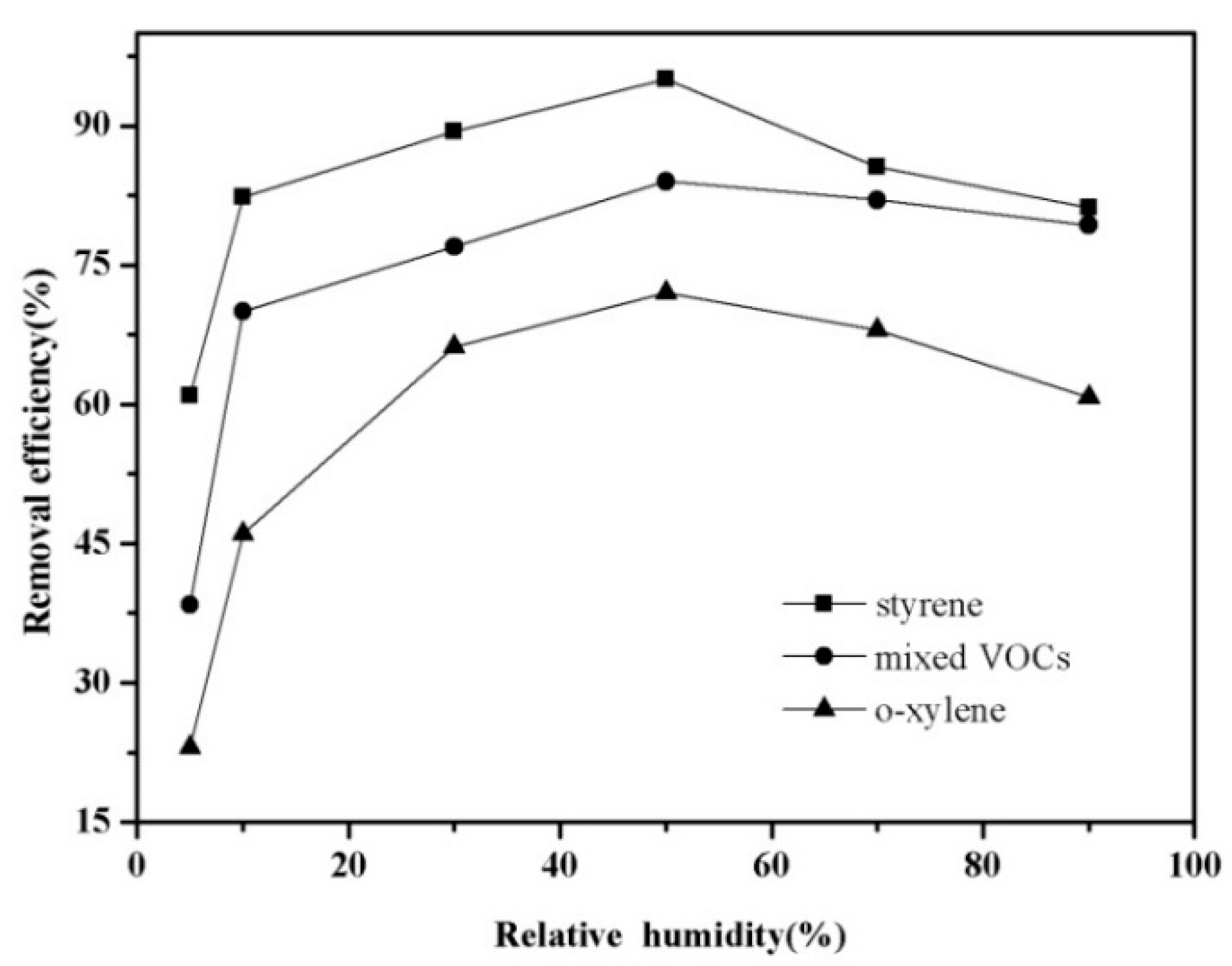
3.3. Influence of Relative Humidity on the Decomposition of the Mixture of VOCs
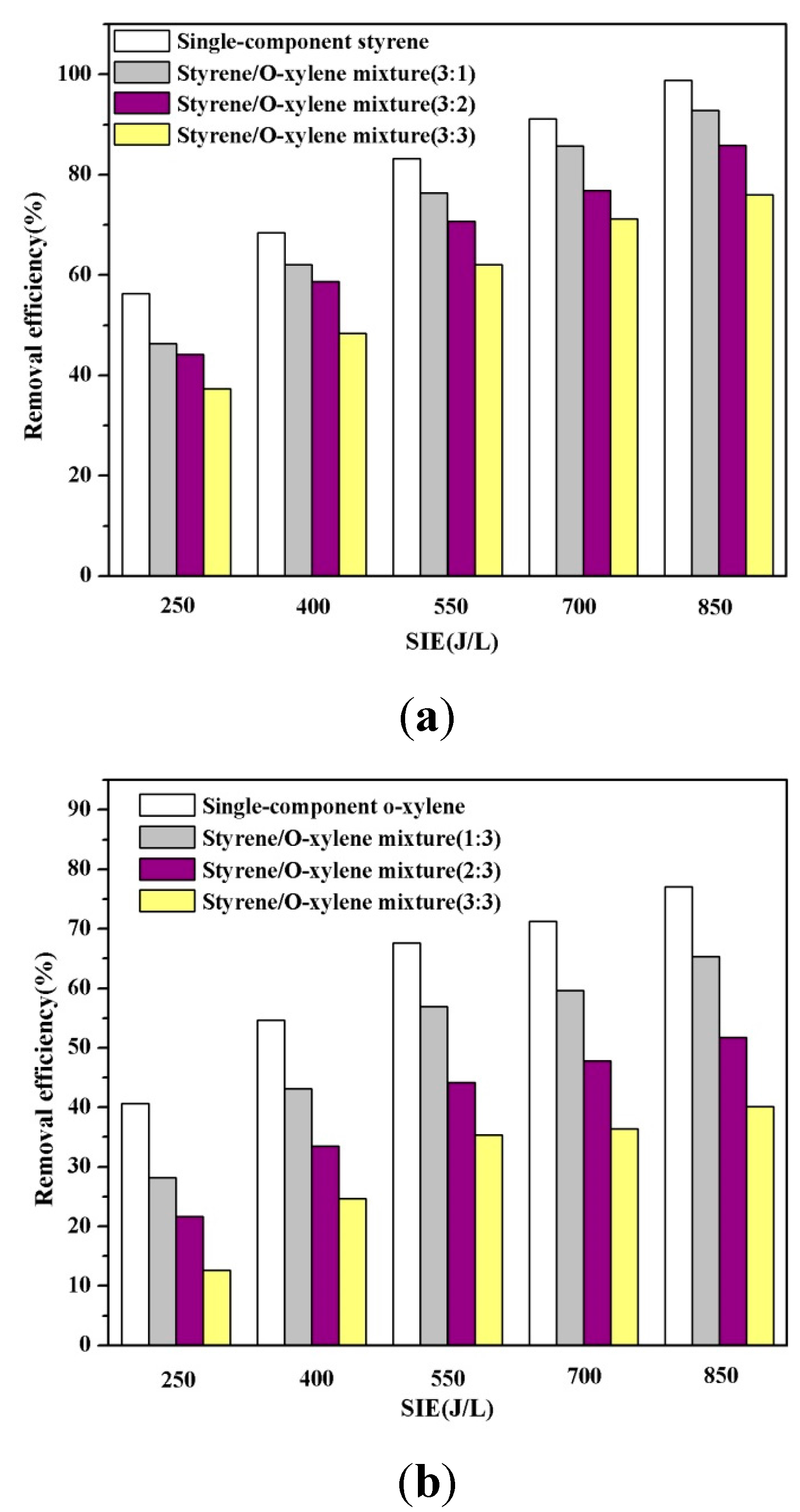
3.4. Interaction of Each Gaseous Compound in the Mixed VOCs
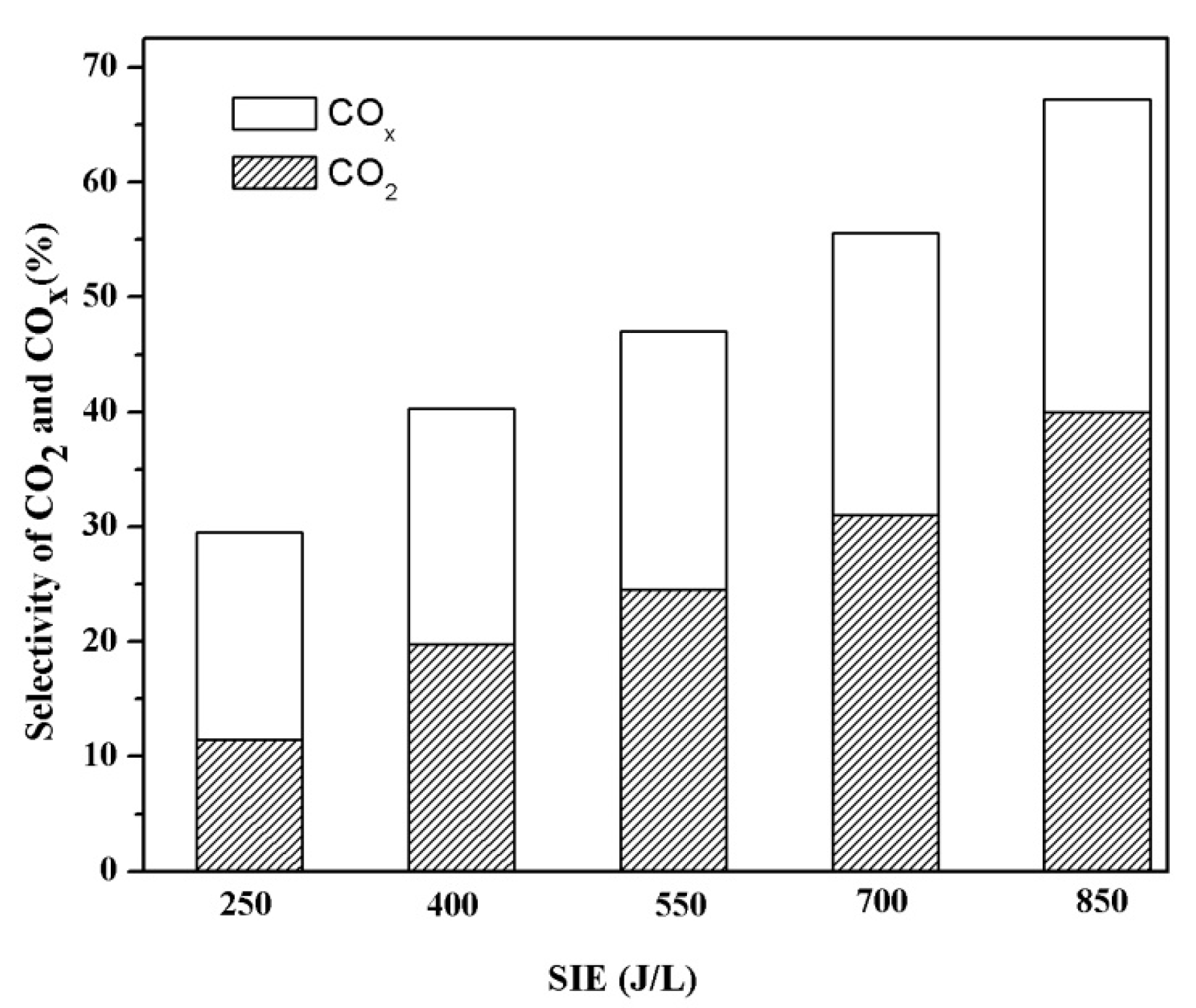
3.5. Generation of COX duringthe Decomposition of the Mixture of VOCs
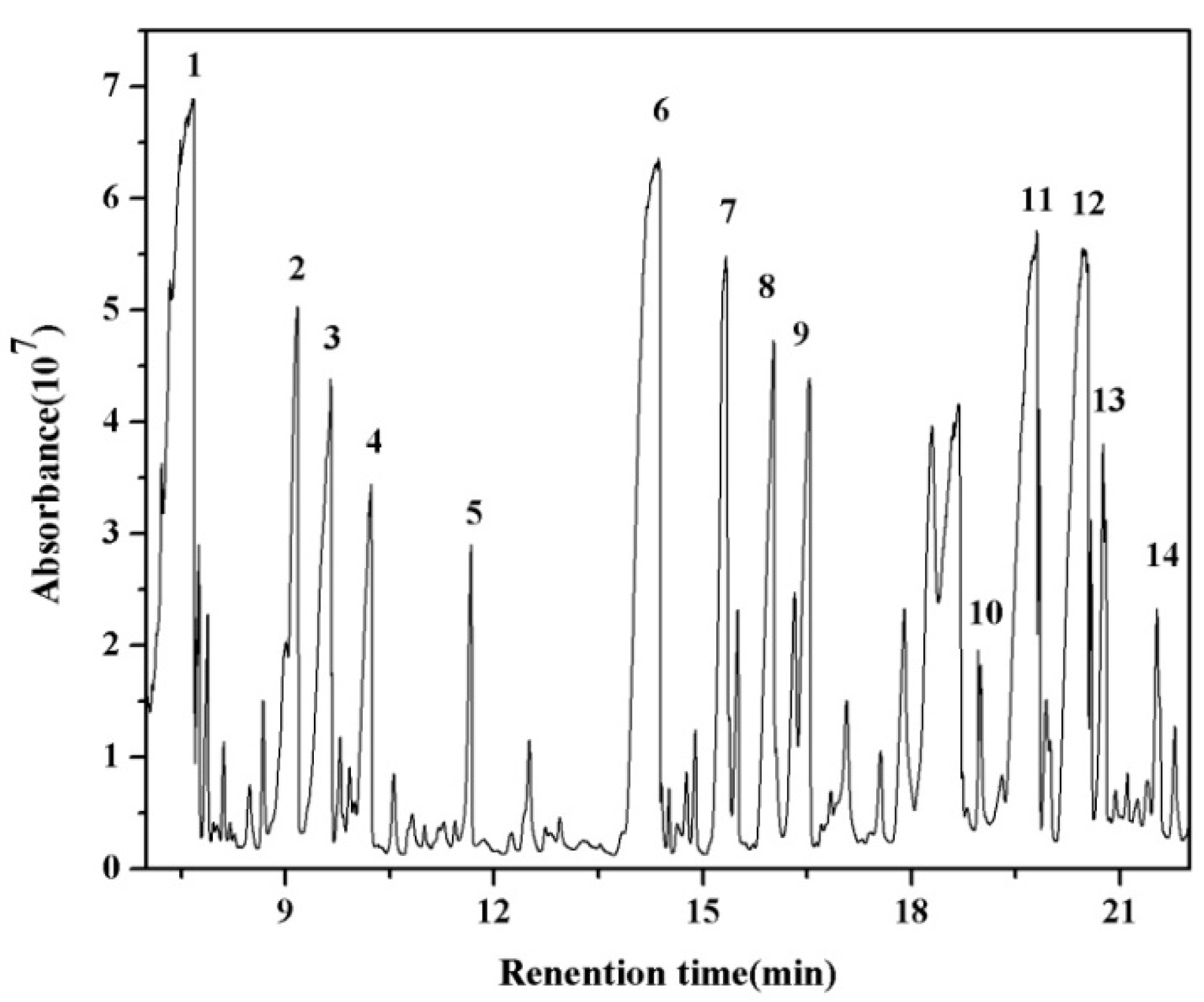
3.6. By-Products Analysis
| Peak No. | Byproducts | Peak No. | Byproducts |
|---|---|---|---|
| 1 |  | 2 | 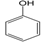 |
| 3 | 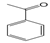 | 4 | 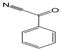 |
| 5 | 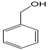 | 6 |  |
| 7 |  | 8 | 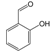 |
| 9 |  | 10 | 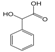 |
| 11 |  | 12 | 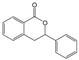 |
| 13 |  | 14 | 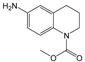 |
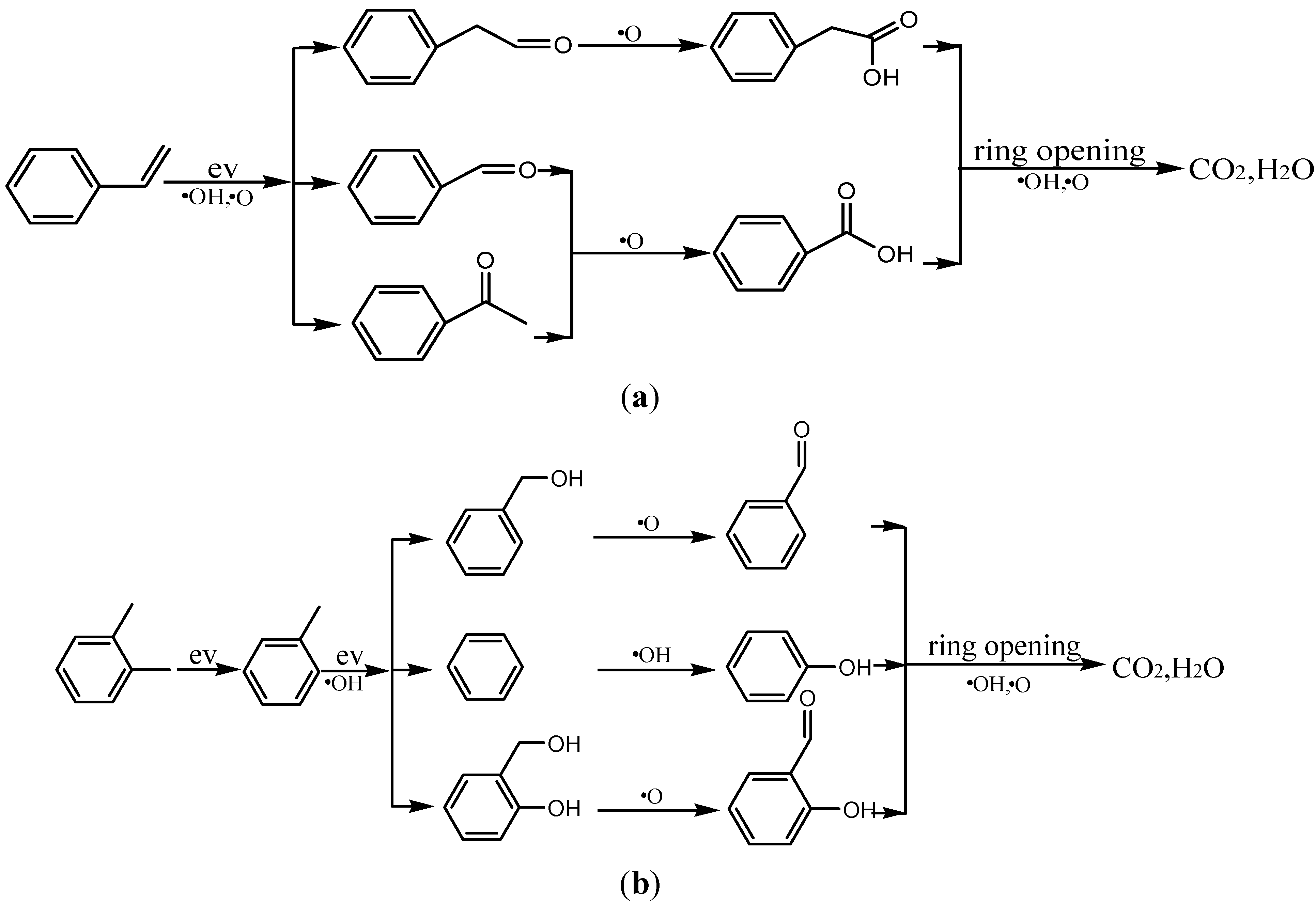
3.7. Formation of O3 duringthe Decomposition of the Mixture of VOCs
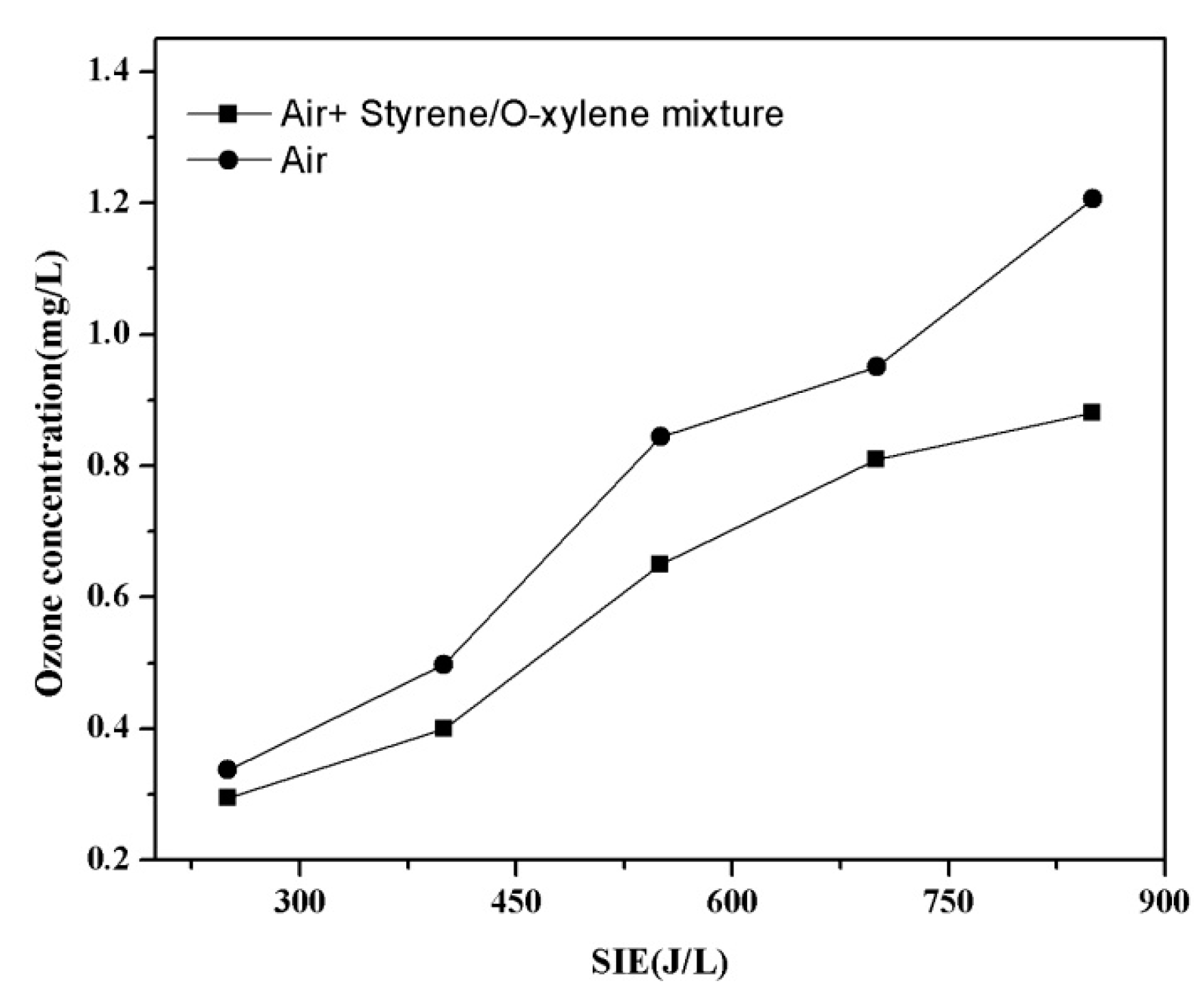
3.8. Biodegradability of Byproduct
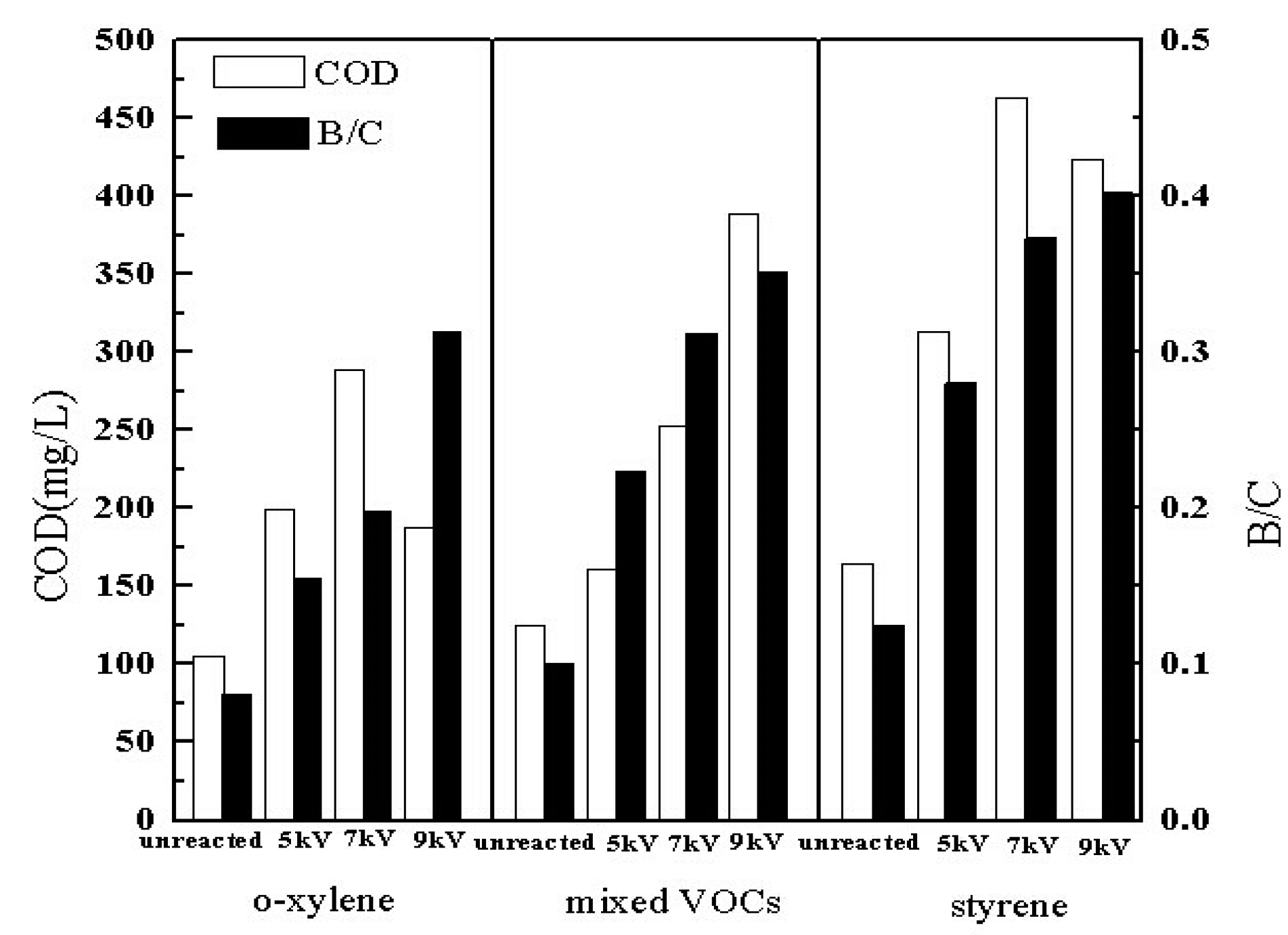
3.9. Biotoxicity of Byproducts
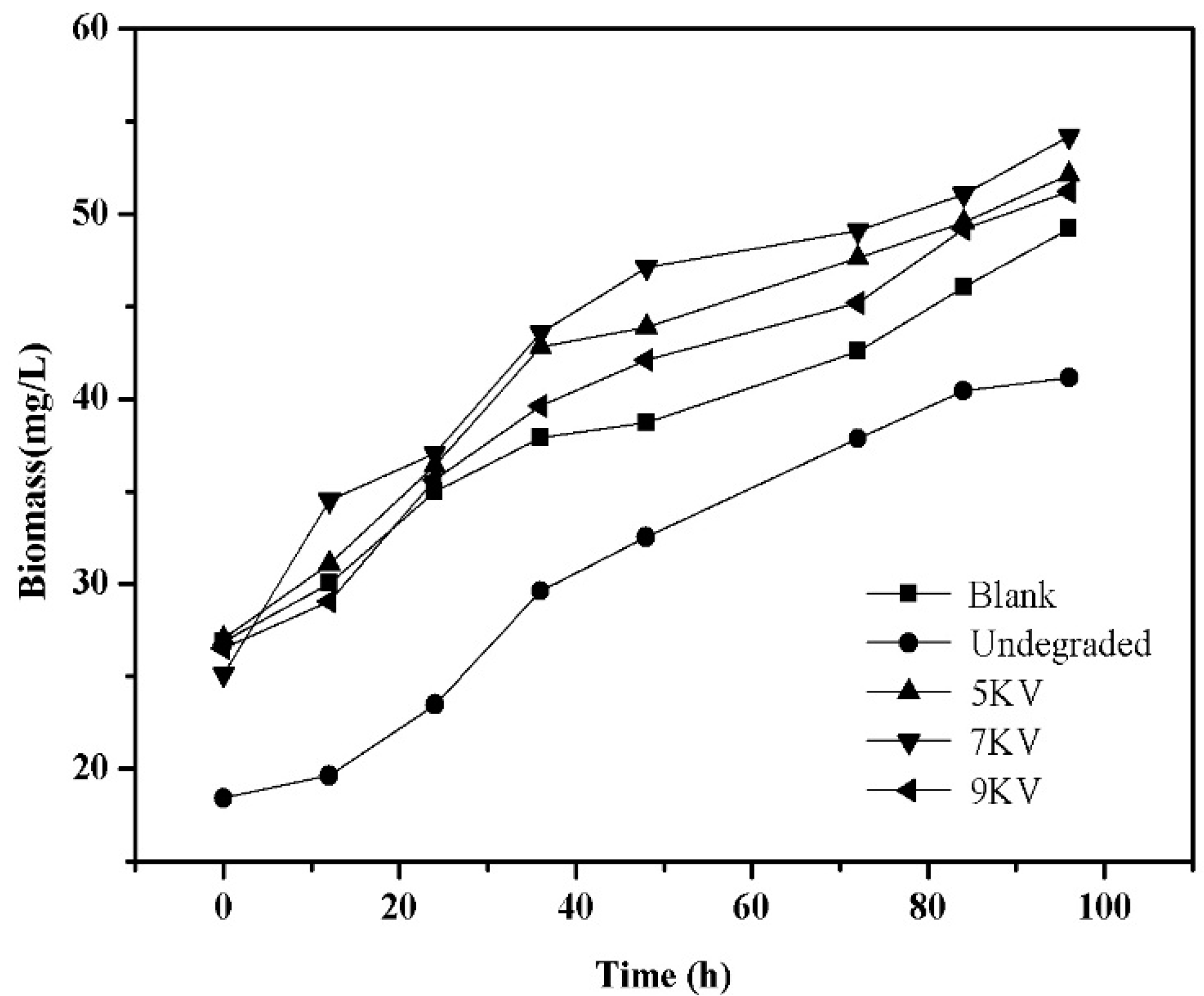
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dou, B.; Bin, F.; Wang, C.; Jia, Q.; Li, J. Discharge characteristics and abatement of volatile organic compounds using plasma reactor packed with ceramic Raschig rings. J. Electrostat. 2013, 71, 939–944. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Z.; Shi, J.; Liu, Z.; Shangguan, W. Post plasma-catalysis for VOCs degradation over different phase structure MnO2 catalysts. Chem. Eng. J. 2014, 241, 251–258. [Google Scholar] [CrossRef]
- Zhang, H.B.; Li, K.; Sun, T.H.; Jia, J.P.; Lou, Z.; Feng, L. Removal of styrene using dielectric barrier discharge plasmas combined with sol-gel prepared TiO2 coated γ-Al2O3. Chem. Eng. J. 2014, 241, 92–102. [Google Scholar] [CrossRef]
- Sun, S.; Bao, J.; Gao, C.; Ding, J.J. Photocatalytic degradation of gaseous o-xylene over M-TiO2 (M=Ag, Fe, Cu, Co) in different humidity levels under visible-light irradiation: Activity and kinetic study. Rare Met. 2011, 30, 147–152. [Google Scholar] [CrossRef]
- Liang, W.J.; Ma, L.; Liu, H.; Li, J. Toluene degradation by non-thermal plasma combined with a ferroelectric catalyst. Chemosphere 2013, 92, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Feng, F.; Ye, L.; Zhang, X.; Huang, Y.; Liu, Z.; Yan, K. Removal of dilute VOCs in air by post-plasma catalysis over Ag-based composite oxide catalysts. Catal. Today 2013, 211, 39–43. [Google Scholar] [CrossRef]
- Omri, I.; Bouallagui, H.; Aouidi, F.; Godon, J.J.; Hamdi, M. H2S gas biological removal efficiency and bacterial community diversity in biofilter treating wastewater odor. Bioresour. Technol. 2011, 102, 10202–10209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, R.; Tay, J.H. Simultaneous autotrophic biodegradation of H2S and NH3 in a biotrickling filter. Chemosphere 2009, 75, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tay, J.H. Operational characteristics of efficient removal of H2S and NH3 in a borizontal biotrickling filter using exhausted carbon. J. Hazard. Mater. 2010, 176, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Maestre, J.P.; Rovira, R.; Gamisans, X.; Kinney, K.A.; Kirisits, M.J.; Lafuente, J.; Gabriel, D. Characterization of the bacterial community in a biotrickling filter treating high loads of H2S by molecular biology tools. Water Sci. Technol. 2009, 59, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Jorio, H.; Bibeau, L.; Viel, G.; Heitz, M. Effects of gas flow rate and inlet concentration on xylene vapors biofiltration performance. Chem. Eng. J. 2000, 76, 209–221. [Google Scholar] [CrossRef]
- Moussavi, G.; Mohseni, M. Using UV pretreatment to enhance biofiltration of mixtures of aromatic VOCs. J. Hazard. Mater. 2007, 144, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.W.; Zhang, L.L.; Chen, J.M.; Yu, J.M.; Gao, Z.L.; Jiang, Y.F. Treatment of gaseous alpha-pinene by a combined system containing photo oxidation and aerobic biotrickling filtration. J. Hazard. Mater. 2011, 192, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Liu, W.; Cheng, Z.W.; Jiang, Y.F.; Cai, W.J.; Chen, J.M. Dichloromethane removal and microbial variations in a combination of UV pretreatment and biotrickling filtration. J. Hazard. Mater. 2014, 268, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, J.; Karvembu, R.; Subrahmanyam, C. The catalytic effect of MnOx and CoOx on the decomposition of nitrobenzene in a non-thermal plasma reactor. Chem. Eng. J. 2012, 180, 39–45. [Google Scholar] [CrossRef]
- Karuppiah, J.; Reddy, E.L.; Reddy, P.M.K.; Ramaraju, B.; Subrahmanyam, C. Catalytic nonthermal plasma reactor for the abatement of low concentrations of benzene. Int. J. Environ. Sci. Technol. 2013, 11, 311–318. [Google Scholar] [CrossRef]
- Chang, C.L.; Bai, H.; Lu, S. Destruction of styrene in an air stream by packed dielectric barrier discharge reactors. Plasma Chem. Plasma Process. 2005, 25, 641–657. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Morent, R.; De Geyter, N.; Leys, C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011, 195, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Xu, W.; Wu, R.; Ni, M.; Du, C.; Gao, X.; Luo, Z.; Cen, K. Non-Thermal plasmas for VOCs abatement. Plasma Chem. Plasma Process. 2014, 34, 1033–1065. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, W.; Yu, Z.; Li, S.; Zhu, J.; Yan, K. Comparison of styrene removal in air by positive and negative DC corona discharges. Int. J. Environ. Sci. Technol. 2013, 10, 1377–1382. [Google Scholar]
- Mok, Y.S.; Lee, S.B.; Oh, J.H.; Ra, K.S.; Sung, B.H. Abatement of trichloromethane by using nonthermal plasma reactors. Plasma Chem. Plasma Process. 2008, 28, 663–676. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Xia, Q.; Li, Z. Decomposition of toluene in a plasma catalysis system with NiO, MnO2, CeO2, Fe2O3, and CuO Catalysts. Plasma Chem. Plasma Process. 2013, 33, 1073–1082. [Google Scholar] [CrossRef]
- Hem, L.J.; Efraimsen, H. Assimilable organic carbon in molecular weight fractions of natural organic matter. Water Res. 2001, 35, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I.; Subrahmanyam, C.; Renken, A.; Kiwi-Minsker, L. Improved performance of non-thermal plasma reactor during decomposition of trichloroethylene: Optimization of the reactor geometry and introduction of catalytic electrode. Appl. Catal. B: Environ. 2007, 74, 270–277. [Google Scholar] [CrossRef]
- Chavadej, S.; Kiatubolpaiboon, W.; Rangsunvigit, P.; Sreethawong, T. A combined multistage corona discharge and catalytic system for gaseous benzene removal. J. Mol. Catal. A Chem. 2007, 263, 128–136. [Google Scholar]
- Hu, Z.; Zhang, J.; Li, S.; Xie, H. Impact of carbon source on nitrous oxide emission from anoxic/oxic biological nitrogen removal process and identification of its emission sources. Environ. Sci. Pollut. Res. Int. 2013, 20, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Kargi, F.; Uygur, A. Effect of carbon source on biological nutrient removal in a sequencing batch reactor. Bioresour. Technol. 2003, 89, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Chavadej, S.; Saktrakool, K.; Rangsunvigit, P.; Lobban, L.L.; Sreethawong, T. Oxidation of ethylene by a multistage corona discharge system in the absence and presence of Pt/TiO2. Chem. Eng. J. 2007, 132, 345–353. [Google Scholar]
- Chandrashekhar, B.; Pai, P.; Morone, A.; Sahu, N.; Pandey, R.A. Reduction of NOx in Fe-EDTA and Fe-NTA solutions by an enriched bacterial population. Bioresour. Technol. 2013, 130, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; De Haas, D.; Yuan, Z.; Lant, P. Nitrous oxide generation in full-scale biological nutrient removal wastewater treatment plants. Water Res. 2010, 44, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Li, D.; Wu, Y.; Li, J.; Li, G. Removal of four kinds of volatile organic compounds mixture in air using silent discharge reactor driven by bipolar pulsed power. J. Electrostat. 2009, 67, 547–553. [Google Scholar] [CrossRef]
- Karuppiah, J.; Sivachandiran, L.; Karvembu, R.; Subrahmanyam, C. Catalytic nonthermal plasma reactor for the abatement of low concentrations of isopropanol. Chem. Eng. J. 2010, 165, 194–199. [Google Scholar] [CrossRef]
- Einaga, H.; Teraoka, Y.; Ogat, A. Benzene oxidation with ozone over manganese oxide supported on zeolite catalysts. Catal. Today 2011, 164, 571–574. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Eloy, P.; Gaigneaux, E.M.; Parvulescu, V.I. Plasma-assisted catalysis for volatile organic compounds abatement. Appl. Catal. B Environ. 2005, 61, 12–20. [Google Scholar] [CrossRef]
- Guo, Y.F.; Ye, D.Q.; Tian, Y.F.; Chen, K.F. Humidity effect on toluene decomposition in a wire-plate dielectric barrier discharge reactor. Plasma Chem. Plasma Process. 2006, 26, 237–249. [Google Scholar] [CrossRef]
- Jiang, N.; Lu, N.; Shang, K.F.; Li, J.; Wu, Y. Innovative approach for benzene degradation using hybrid surface/packed-bed discharge plasmas. Environ. Sci. Technol. 2013, 47, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Han, B.W.; Hong, W.S.; Ryu, J.Y.; Kim, Y.J. A new combination system using biotrickling filtration and nonthermal plasma for the treatment of volatile organic compounds. Environ. Eng. Sci. 2009, 26, 1289–1297. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.Y.; Hu, H.Y.; Yao, Y. Advantages of combined UV photodegradation and biofiltration processes to treat gaseous chlorobenzene. J. Hazard. Mater. 2009, 171, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhu, R.; Mao, Y.; Chen, J.; Zhang, L. Conversion Characteristics and Production Evaluation of Styrene/o-Xylene Mixtures Removed by DBD Pretreatment. Int. J. Environ. Res. Public Health 2015, 12, 1334-1350. https://doi.org/10.3390/ijerph120201334
Jiang L, Zhu R, Mao Y, Chen J, Zhang L. Conversion Characteristics and Production Evaluation of Styrene/o-Xylene Mixtures Removed by DBD Pretreatment. International Journal of Environmental Research and Public Health. 2015; 12(2):1334-1350. https://doi.org/10.3390/ijerph120201334
Chicago/Turabian StyleJiang, Liying, Runye Zhu, Yubo Mao, Jianmeng Chen, and Liang Zhang. 2015. "Conversion Characteristics and Production Evaluation of Styrene/o-Xylene Mixtures Removed by DBD Pretreatment" International Journal of Environmental Research and Public Health 12, no. 2: 1334-1350. https://doi.org/10.3390/ijerph120201334
APA StyleJiang, L., Zhu, R., Mao, Y., Chen, J., & Zhang, L. (2015). Conversion Characteristics and Production Evaluation of Styrene/o-Xylene Mixtures Removed by DBD Pretreatment. International Journal of Environmental Research and Public Health, 12(2), 1334-1350. https://doi.org/10.3390/ijerph120201334





