Blood Cadmium Is Associated with Osteoporosis in Obese Males but Not in Non-Obese Males: The Korea National Health and Nutrition Examination Survey 2008–2011
Abstract
:1. Introduction
2. Material and Methods
2.1. Data source and Study Subjects
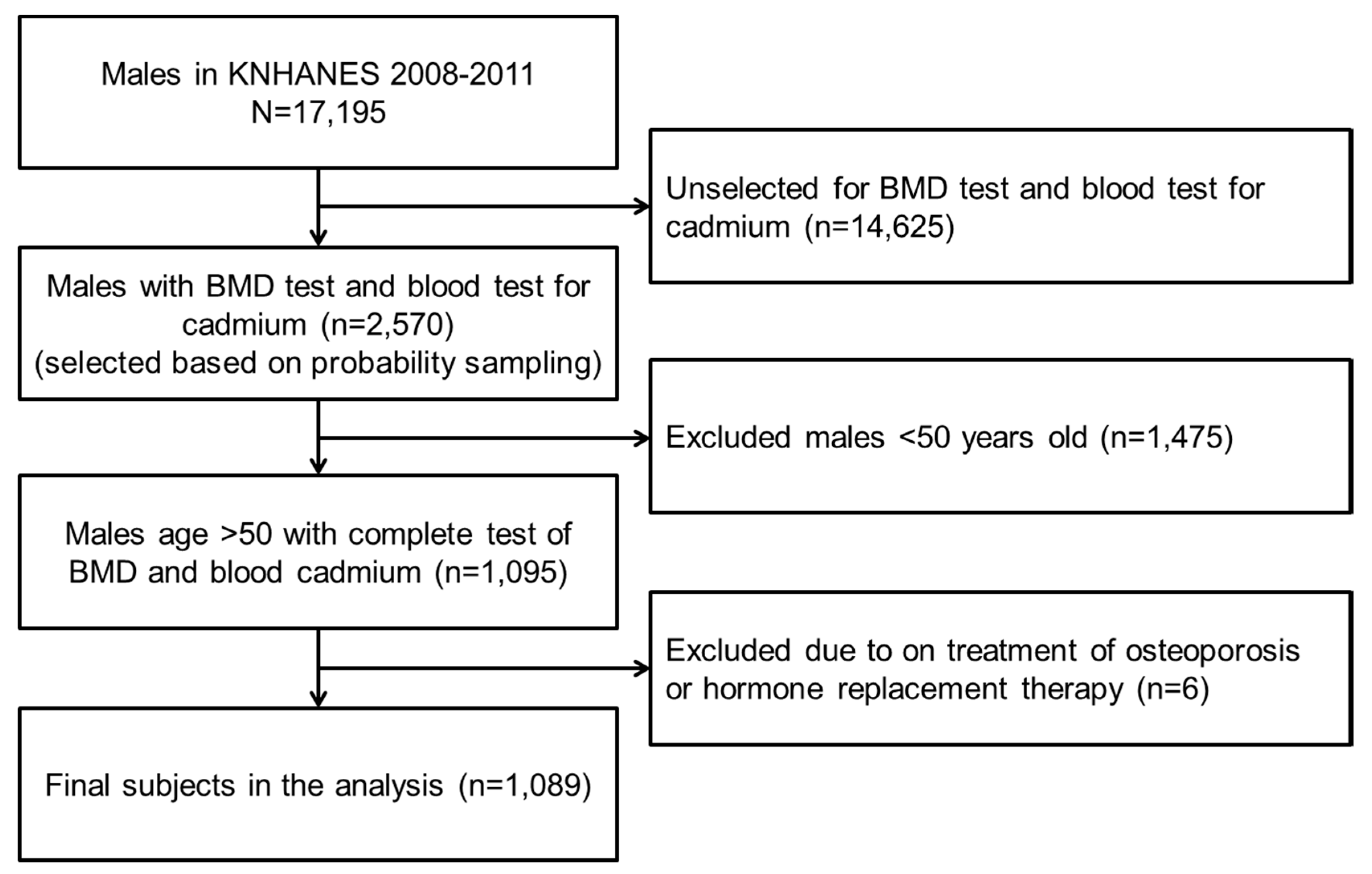
2.2. Measurement of BMD and Blood Cadmium
2.3. BMI and Other Potential Confounding Variables
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Subjects
| Variables | n | |||
| No. of subjects (weighted frequency) | 1089 (1,909,528) | |||
| Variables | Mean (SD (1)) | Min | Max | |
| Age (years) | 58.81 (7.47) | 50 | 87 | |
| Blood cadmium (μg/L) | 1.25 (0.66) | 0.02 | 6.04 | |
| Serum creatinine (mg/dL) | 0.96 (0.18) | 0.60 | 2.70 | |
| 25(OH)D (ng/mL) | 21.22 (7.49) | 4.85 | 47.01 | |
| Variables | No. of Subjects | Weighted Frequency | % (SE (2)) | |
| Osteoporosis (T-score < −2.5) | Total hip | 46 | 72,353 | 3.8 (0.03) |
| Femur neck | 229 | 368,429 | 19.3 (0.07) | |
| Lumbar spine | 46 | 76,254 | 4.0 (0.03) | |
| Any site | 243 | 394,525 | 20.7 (0.07) | |
| Obesity | BMI ≥25 kg/m2 | 368 | 682,304 | 35.7 (0.09) |
3.2. BMD and T-Score by Obesity
| BMI (1) | Frequency | Weighted Frequency | Total Hip | Femur Neck | Lumbar Spine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMD (2) | T-Score (3) | BMD | T-Score | BMD | T-Score | |||||||||
| Mean | SD (4) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| <25 | 721 | 1,227,224 | 0.91 | 0.12 | −0.96 | 0.97 | 0.74 | 0.11 | −1.76 | 1.06 | 0.92 | 0.14 | −0.72 | 1.21 |
| ≥25 | 368 | 682,304 | 0.99 | 0.12 | −0.29 | 0.97 | 0.80 | 0.11 | −1.14 | 1.11 | 0.98 | 0.14 | −0.17 | 1.24 |
3.3. Association between the Blood Cadmium and Bone Mineral Density
| Body Site | Variables | Model 1 (4) | Model 2 (5) | Model 3 (6) | |||
|---|---|---|---|---|---|---|---|
| Coefficient | p | Coefficient | p | Coefficient | p | ||
| Total hip | log Cd (2) (μg/L) | −0.025 | 0.01 | −0.021 | 0.01 | −0.012 | 0.13 |
| Age (years) | −0.005 | <0.01 | −0.004 | <0.01 | |||
| BMI (3) (kg/m2) | 0.017 | <0.01 | |||||
| Serum creatinine (mg/dL) | 0.006 | 0.75 | |||||
| Serum 25(OH)D (ng/mL) | 0.001 | 0.06 | |||||
| Current smoking | −0.004 | 0.66 | |||||
| Alcohol drinking | 0.006 | 0.55 | |||||
| Physical activity | 0.008 | 0.38 | |||||
| Femur neck | log Cd (μg/L) | −0.014 | 0.07 | −0.009 | 0.21 | −0.001 | 0.88 |
| Age (years) | −0.005 | <0.01 | −0.005 | <0.01 | |||
| BMI (kg/m2) | 0.013 | <0.01 | |||||
| Serum creatinine (mg/dL) | −0.022 | 0.26 | |||||
| Serum 25(OH)D (ng/mL) | 0.001 | 0.07 | |||||
| Current smoking | −0.009 | 0.27 | |||||
| Alcohol drinking | 0.006 | 0.56 | |||||
| Physical activity | 0.003 | 0.74 | |||||
| Lumbar spine | log Cd (μg/L) | −0.011 | 0.23 | −0.010 | 0.29 | −0.001 | 0.92 |
| Age (years) | −0.001 | 0.02 | −0.001 | 0.06 | |||
| BMI (kg/m2) | 0.012 | <0.01 | |||||
| Serum creatinine (mg/dL) | 0.090 | <0.01 | |||||
| Serum 25(OH)D (ng/mL) | 0.001 | 0.82 | |||||
| Current smoking | −0.012 | 0.28 | |||||
| Alcohol drinking | 0.002 | 0.89 | |||||
| Physical activity | 0.003 | 0.81 | |||||
3.4. Association between the Blood Cadmium Level and Osteoporosis by Obesity Status
4. Discussion
| Strata | Variables | Prevalence of Osteoporosis | Model 1 (4) | Model 2 (7) | Model 3 (8) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Weighted Frequency | Prevalence (%) | OR (5) | 95% CI (6) | p-for-Trend | OR | 95% CI | p-for-Trend | OR | 95% CI | p-for-Trend | ||
| BMI (2) <25 kg/m2 | Cd (μg/L)3) | ||||||||||||
| <1.00 | 78/281 | 126,359/487,263 | 25.9 | Ref | 0.98 | Ref | 0.93 | Ref | 0.22 | ||||
| 1.00~1.50 | 61/227 | 90,736/373,098 | 24.3 | 0.92 | 0.59 1.42 | 0.84 | 0.53 1.33 | 0.83 | 0.51 1.36 | ||||
| >1.50 | 59/213 | 96,126/366,862 | 26.2 | 1.01 | 0.64 1.60 | 0.99 | 0.61 1.61 | 0.72 | 0.42 1.23 | ||||
| Age (years) | 1.10 | 1.08 1.13 | 1.10 | 1.08 1.13 | |||||||||
| BMI (kg/m2) | 0.71 | 0.64 0.79 | |||||||||||
| Serum creatinine (mg/dL) | 1.48 | 0.54 4.06 | |||||||||||
| Vitamin D deficiency | 0.82 | 0.54 1.24 | |||||||||||
| Current smoking | 1.47 | 0.92 2.36 | |||||||||||
| Alcohol drinking | 1.13 | 0.62 2.08 | |||||||||||
| Physical activity | 1.11 | 0.65 1.90 | |||||||||||
| BMI ≥25 kg/m2 | Cd (μg/L) | ||||||||||||
| <1.00 | 15 /167 | 17,180/301,348 | 5.7 | Ref | <0.01 | Ref | <0.01 | Ref | 0.01 | ||||
| 1.00~1.50 | 12 /119 | 29,225/226,105 | 12.9 | 2.46 | 1.01 6.01 | 2.21 | 0.85 5.73 | 2.36 | 0.92 6.08 | ||||
| >1.50 | 18 / 82 | 34,900/154,850 | 22.5 | 4.81 | 2.07 11.19 | 4.25 | 1.82 9.91 | 5.71 | 1.99 16.38 | ||||
| Age (years) | 1.08 | 1.03 1.14 | 1.08 | 1.03 1.14 | |||||||||
| BMI (kg/m2) | 1.26 | 1.01 1.58 | |||||||||||
| Serum creatinine (mg/dL) | 0.56 | 0.07 4.35 | |||||||||||
| Vitamin D deficiency | 1.01 | 0.45 2.25 | |||||||||||
| Current smoking | 0.64 | 0.25 1.62 | |||||||||||
| Alcohol drinking | 0.58 | 0.20 1.70 | |||||||||||
| Physical activity | 0.65 | 0.26 1.64 | |||||||||||
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Radak, T.L. Caloric restriction and calcium’s effect on bone metabolism and body composition in overweight and obese premenopausal women. Nutr. Rev. 2004, 62, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Relationships among body mass, its components, and bone. Bone 2002, 31, 547–555. [Google Scholar] [CrossRef]
- Frost, H.M.; Ferretti, J.L.; Jee, W.S. Perspectives: Some roles of mechanical usage, muscle strength, and the mechanostat in skeletal physiology, disease, and research. Calcif. Tissue. Int. 1998, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, M.E.; Yesil, Y.; Ozturk, Z.A.; Cinar, E.; Kizilarslanoglu, C.; Halil, M.; Ulger, Z.; Yesil, N.K.; Cankurtaran, M.; Ariogul, S. The association between homocysteine (HCY) and serum natural antioxidants in elderly bone mineral densitometry (BMD). Arch. Gerontol. Geriatr. 2012, 55, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Engstrom, A.; Michaelsson, K.; Suwazono, Y.; Wolk, A.; Vahter, M.; Akesson, A. Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J. Bone Miner. Res. 2011, 26, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.D.; Michaelsson, K.; Julin, B.; Wolk, A.; Akesson, A. Dietary cadmium exposure and fracture incidence among men: A population-based prospective cohort study. J. Bone Miner. Res. 2011, 26, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Trzcinka-Ochocka, M.; Jakubowski, M.; Szymczak, W.; Janasik, B.; Brodzka, R. The effects of low environmental cadmium exposure on bone density. Environ. Res. 2010, 110, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.H. Cadmium osteotoxicity in experimental animals: Mechanisms and relationship to human exposures. Toxicol. Appl. Pharmacol. 2009, 238, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Coonse, K.G.; Coonts, A.J.; Morrison, E.V.; Heggland, S.J. Cadmium induces apoptosis in the human osteoblast-like cell line Saos-2. J. Toxicol. Environ. Health A 2007, 70, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, M.M.; Rogalska, J.; Kupraszewicz, E. The involvement of oxidative stress in the mechanisms of damaging cadmium action in bone tissue: A study in a rat model of moderate and relatively high human exposure. Toxicol. Appl. Pharmacol. 2011, 250, 327–335. [Google Scholar] [CrossRef] [PubMed]
- KCDC. Quality Control and Development of Standardization Manual for the Medical Laboratory Tests in the Nation-wide Health Survey of 2008 Year; Korea Center for Disease Control and Prevention: Osong, Korea, 2009. [Google Scholar]
- KCDC. Quality Control and Establishment of Reference Range for the Clinical Laboratory Tests in the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-3), 2009; Korea Center for Disease Control and Prevention: Osong, Korea, 2010. [Google Scholar]
- KCDC. Quality Control and Examination of Measurement Traceability for the Clinical Laboratory Tests in the Nation-wide Health Survey of the year of 2010; Korea Center for Disease Control and Prevention: Osong, Korea, 2011. [Google Scholar]
- KCDC. Quality Control of the Clinical Laboratory for the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-2, 2011); Korea Center for Disease Control and Prevention: Osong, Korea, 2012. [Google Scholar]
- Follin, S.L.; Black, J.N.; McDermott, M.T. Lack of diagnosis and treatment of osteoporosis in men and women after hip fracture. Pharmacotherapy 2003, 23, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Adachi, J.D.; Saag, K.G. Bridging the osteoporosis quality chasm. J. Bone Miner. Res. 2009, 24, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Leder, B.Z.; LeBlanc, K.M.; Schoenfeld, D.A.; Eastell, R.; Finkelstein, J.S. Differential effects of androgens and estrogens on bone turnover in normal men. J. Clin. Endocrinol. Metable 2003, 88, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mellstrom, D.; Vandenput, L.; Mallmin, H.; Holmberg, A.H.; Lorentzon, M.; Oden, A.; Johansson, H.; Orwoll, E.S.; Labrie, F.; Karlsson, M.K.; et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J. Bone Miner. Res. 2008, 23, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129.
- Wu, X.P.; Liao, E.Y.; Huang, G.; Dai, R.C.; Zhang, H. A comparison study of the reference curves of bone mineral density at different skeletal sites in native Chinese, Japanese, and American Caucasian women. Calcif. Tissue Int. 2003, 73, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, U.D.; Pham, H.N.; Nguyen, N.D.; Nguyen, T.V. Reference ranges for bone mineral density and prevalence of osteoporosis in Vietnamese men and women. BMC Musculoskelet. Disord. 2011, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Bae, S.H.; Lee, S.H.; Lee, J.; Lee, D.R. New reference data on bone mineral density and the prevalence of osteoporosis in Korean adults aged 50 years or older: The Korea National Health and Nutrition Examination Survey 2008–2010. J. Korean Med. Sci. 2014, 29, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, G.; Shi, Y.; Weng, S.; Jin, T.; Kong, Q.; Nordberg, G.F. Influence of environmental cadmium exposure on forearm bone density. J. Bone Miner. Res. 2003, 18, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Gielen, E.; Boonen, S.; Vanderschueren, D.; Sinnesael, M.; Verstuyf, A.; Claessens, F.; Milisen, K.; Verschueren, S. Calcium and vitamin D supplementation in men. J. Osteoporos. 2011, 2011, 875249. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, G.; Gu, S.; Jin, T.; Shao, C. Effects of cadmium on osteoblasts and osteoclasts in vitro. Environ. Toxicol. Pharmacol. 2009, 28, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Tsuritani, I.; Noborisaka, Y.; Suzuki, H.; Ishizaki, M.; Yamada, Y. Urinary cadmium excretion is correlated with calcaneal bone mass in Japanese women living in an urban area. Environ. Res. 2003, 91, 63–70. [Google Scholar] [CrossRef]
- Nawrot, T.; Geusens, P.; Nulens, T.S.; Nemery, B. Occupational cadmium exposure and calcium excretion, bone density, and osteoporosis in men. J. Bone Miner. Res. 2010, 25, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Rogenfelt, A.; Elinder, C.G.; Nogawa, K.; Kjellstrom, T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand. J. Work Environ. Health 1983, 9, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24, 1–51. [Google Scholar] [PubMed]
- Zhang, L.L.; Lu, L.; Pan, Y.J.; Ding, C.G.; Xu, D.Y.; Huang, C.F.; Pan, X.F.; Zheng, W. Baseline blood levels of manganese, lead, cadmium, copper, and zinc in residents of Beijing suburb. Environ. Res. 2015, 140, 10–17. [Google Scholar] [CrossRef] [PubMed]
- CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. (February, 2015); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015. [Google Scholar]
- Guney, E.; Kisakol, G.; Ozgen, G.; Yilmaz, C.; Yilmaz, R.; Kabalak, T. Effect of weight loss on bone metabolism: Comparison of vertical banded gastroplasty and medical intervention. Obes. Surg. 2003, 13, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Shah, K.; Banks, M.R.; Sinacore, D.R.; Klein, S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: A one-year randomized controlled trial. J. Clin. Endocrinol. Metable 2008, 93, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Liu, Y.J.; Liu, P.Y.; Hamilton, J.; Recker, R.R.; Deng, H.W. Relationship of obesity with osteoporosis. J. Clin. Endocrinol. Metable 2007, 92, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metable 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Greco, E.A.; Fornari, R.; Donini, L.M.; Lenzi, A. Is obesity in women protective against osteoporosis? Diabetes Metab. Syndr. Obes. 2011, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.-J.; Han, S.-H. Blood Cadmium Is Associated with Osteoporosis in Obese Males but Not in Non-Obese Males: The Korea National Health and Nutrition Examination Survey 2008–2011. Int. J. Environ. Res. Public Health 2015, 12, 12144-12157. https://doi.org/10.3390/ijerph121012144
Choi W-J, Han S-H. Blood Cadmium Is Associated with Osteoporosis in Obese Males but Not in Non-Obese Males: The Korea National Health and Nutrition Examination Survey 2008–2011. International Journal of Environmental Research and Public Health. 2015; 12(10):12144-12157. https://doi.org/10.3390/ijerph121012144
Chicago/Turabian StyleChoi, Won-Jun, and Sang-Hwan Han. 2015. "Blood Cadmium Is Associated with Osteoporosis in Obese Males but Not in Non-Obese Males: The Korea National Health and Nutrition Examination Survey 2008–2011" International Journal of Environmental Research and Public Health 12, no. 10: 12144-12157. https://doi.org/10.3390/ijerph121012144
APA StyleChoi, W.-J., & Han, S.-H. (2015). Blood Cadmium Is Associated with Osteoporosis in Obese Males but Not in Non-Obese Males: The Korea National Health and Nutrition Examination Survey 2008–2011. International Journal of Environmental Research and Public Health, 12(10), 12144-12157. https://doi.org/10.3390/ijerph121012144





