Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Instruments
2.3. Analytical Methods
2.4. Batch Experiments
3. Results and Discussion
3.1. Simultaneous Degradation of E1, E2 and EE2 in UV/H2O2 System
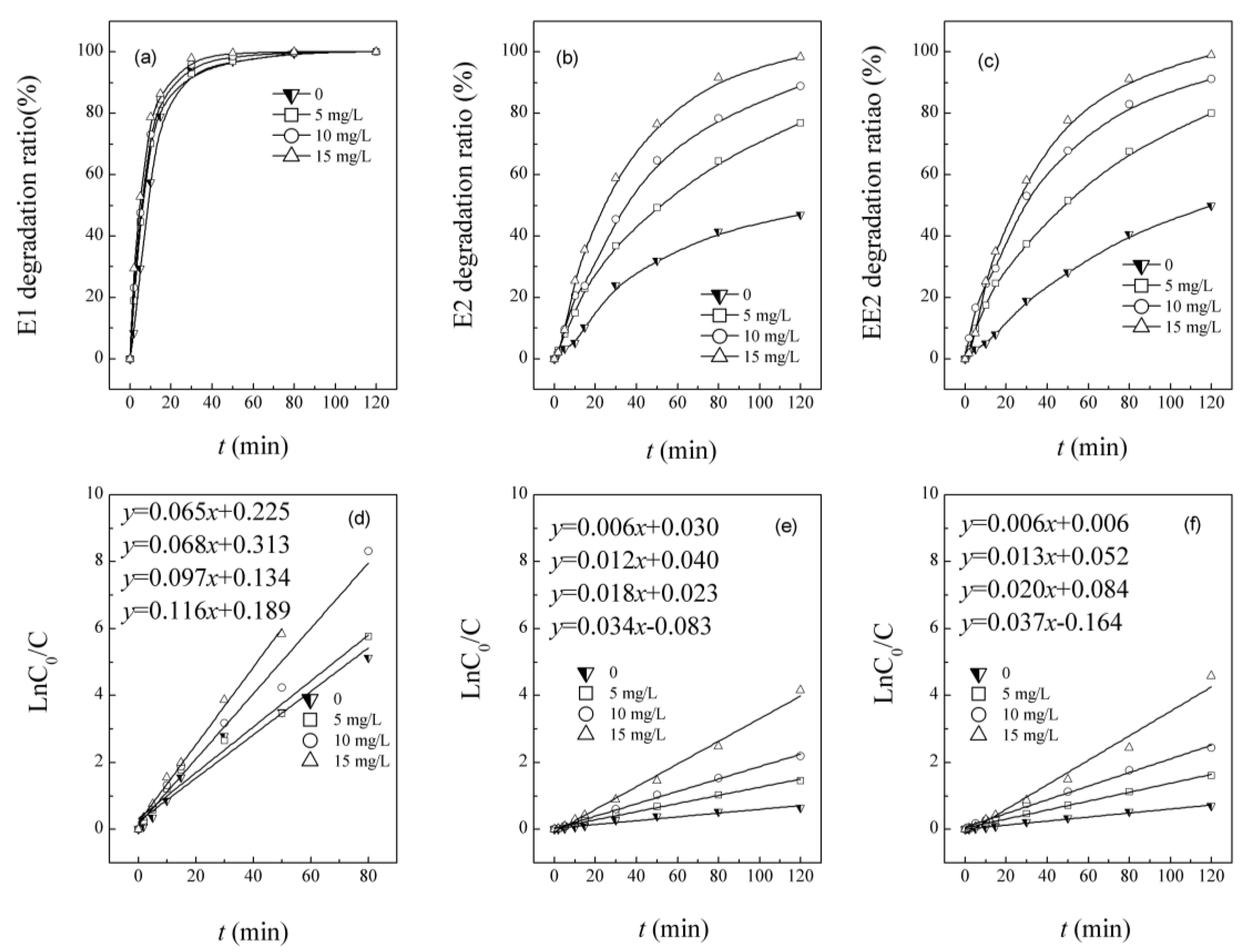
3.2. Competitive Influence of E2 and EE2 on E1 Degradation
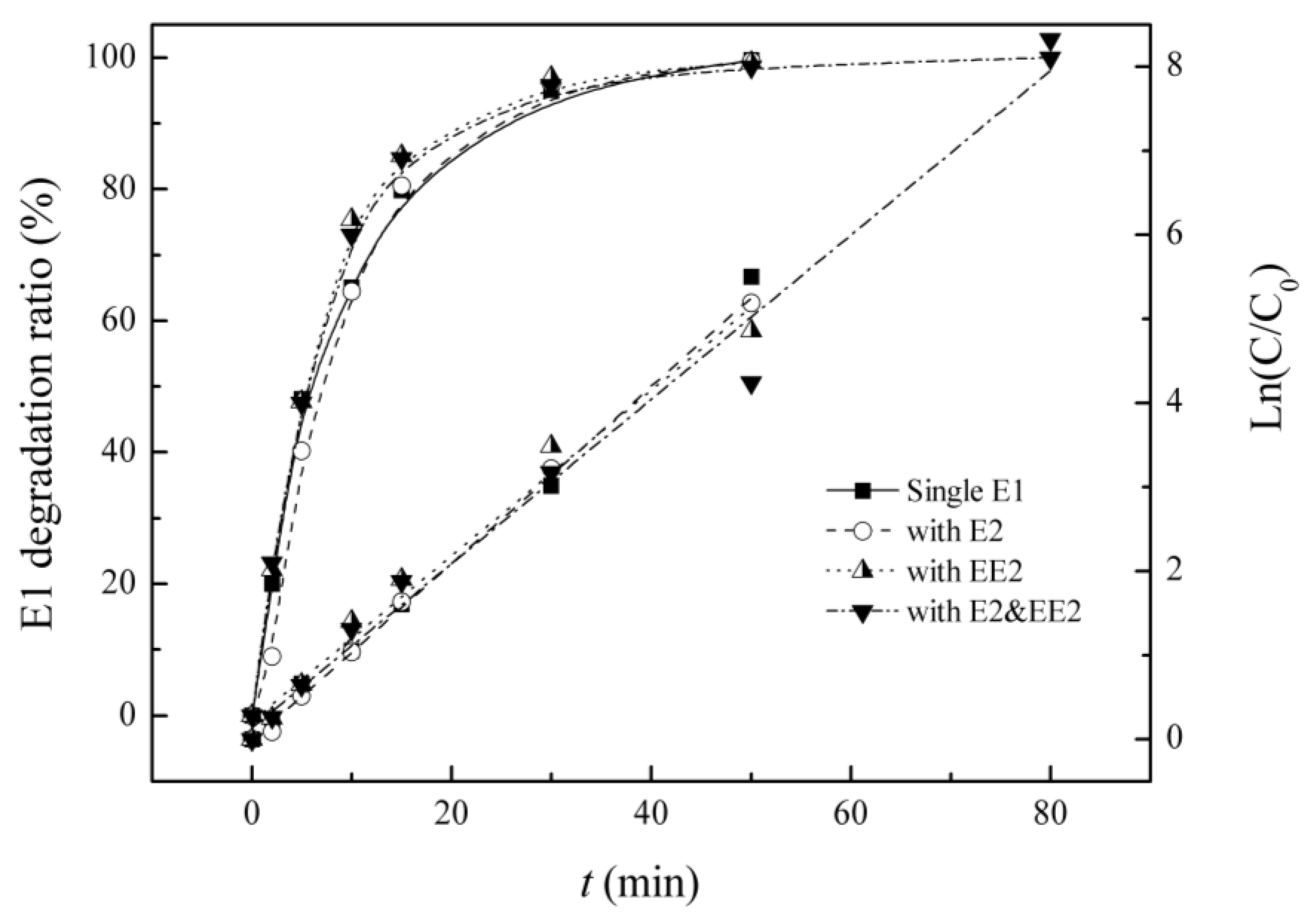
| Background Estrogens | Kinetic Equation | Reaction Rate Constant K/(min−1) | R2 | Half-lIfe/(min) |
|---|---|---|---|---|
| Blank | Ln(C0/C) = 0.107t + 0.001 | 0.107 | 0.996 | 6.4 |
| E2 | Ln(C0/C) = 0.106t − 0.015 | 0.106 | 0.999 | 6.5 |
| EE2 | Ln(C0/C) = 0.098t + 0.218 | 0.098 | 0.979 | 7.0 |
| E2&EE2 | Ln(C0/C) = 0.098t + 0.134 | 0.098 | 0.981 | 7.1 |
3.3. Competitive Influence of E1 and EE2 on E2 Degradation
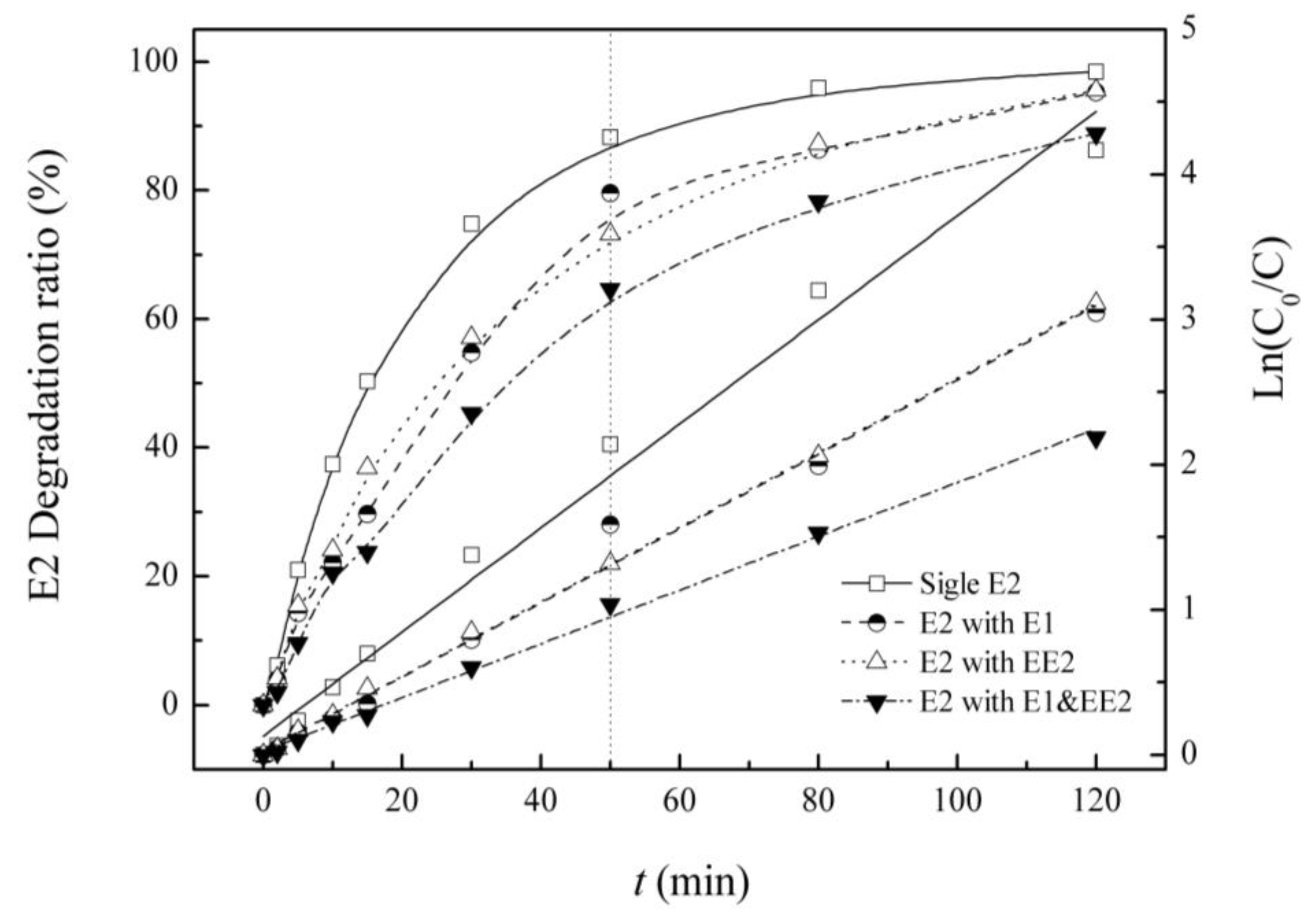
| Background Estrogens | Kinetic Equation | Reaction Rate Constant K/(min−1) | R2 | Half-Life/(min) |
|---|---|---|---|---|
| E2 | Ln(C0/C) = 0.036t + 0.129 | 0.036 | 0.985 | 19.3 |
| E1 | Ln(C0/C) = 0.026t + 0.025 | 0.026 | 0.988 | 27.0 |
| EE2 | Ln(C0/C) = 0.026t + 0.029 | 0.026 | 0.999 | 26.9 |
| E1&EE2 | Ln(C0/C) =0.018t + 0.023 | 0.018 | 0.996 | 37.4 |
3.4. Competitive Influence of E1 and E2 on EE2 Degradation
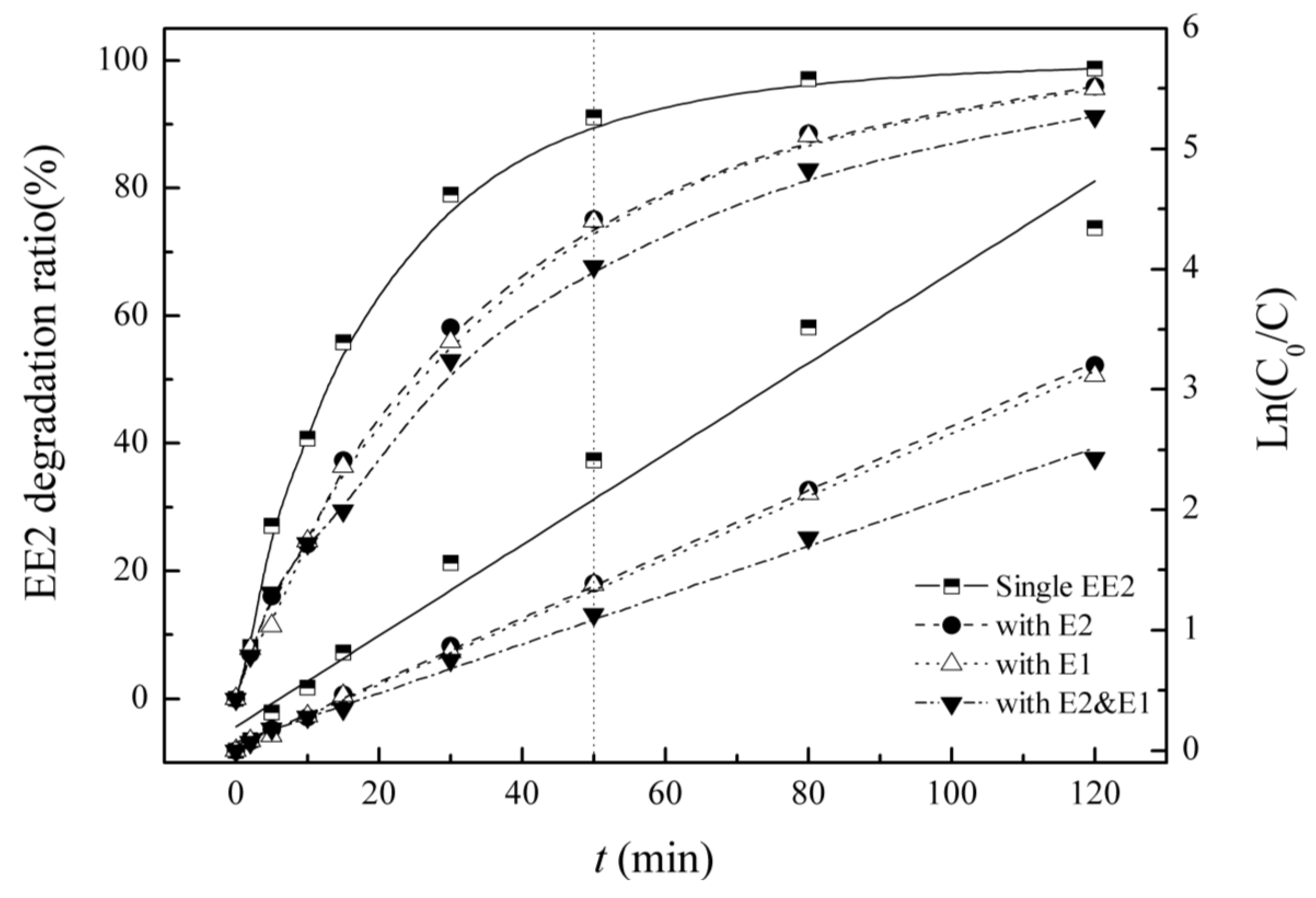
| Background Sterdidal Estrogens | Kinetic Equation | Reaction Rate Constant K/(min−1) | R2 | Half-Life/(min) |
|---|---|---|---|---|
| EE2 | Ln(C0/C) = 0.038t+ 0.199 | 0.038 | 0.973 | 18.4 |
| E2 | Ln(C0/C) = 0.026t + 0.037 | 0.026 | 0.999 | 26.1 |
| E1 | Ln(C0/C) = 0.026t + 0.029 | 0.026 | 0.999 | 26.6 |
| E2 & E1 | Ln(C0/C) = 0.020t + 0.067 | 0.020 | 0.995 | 33.9 |
3.5. Intermediate Product of Estrogens in UV/H2O2 System
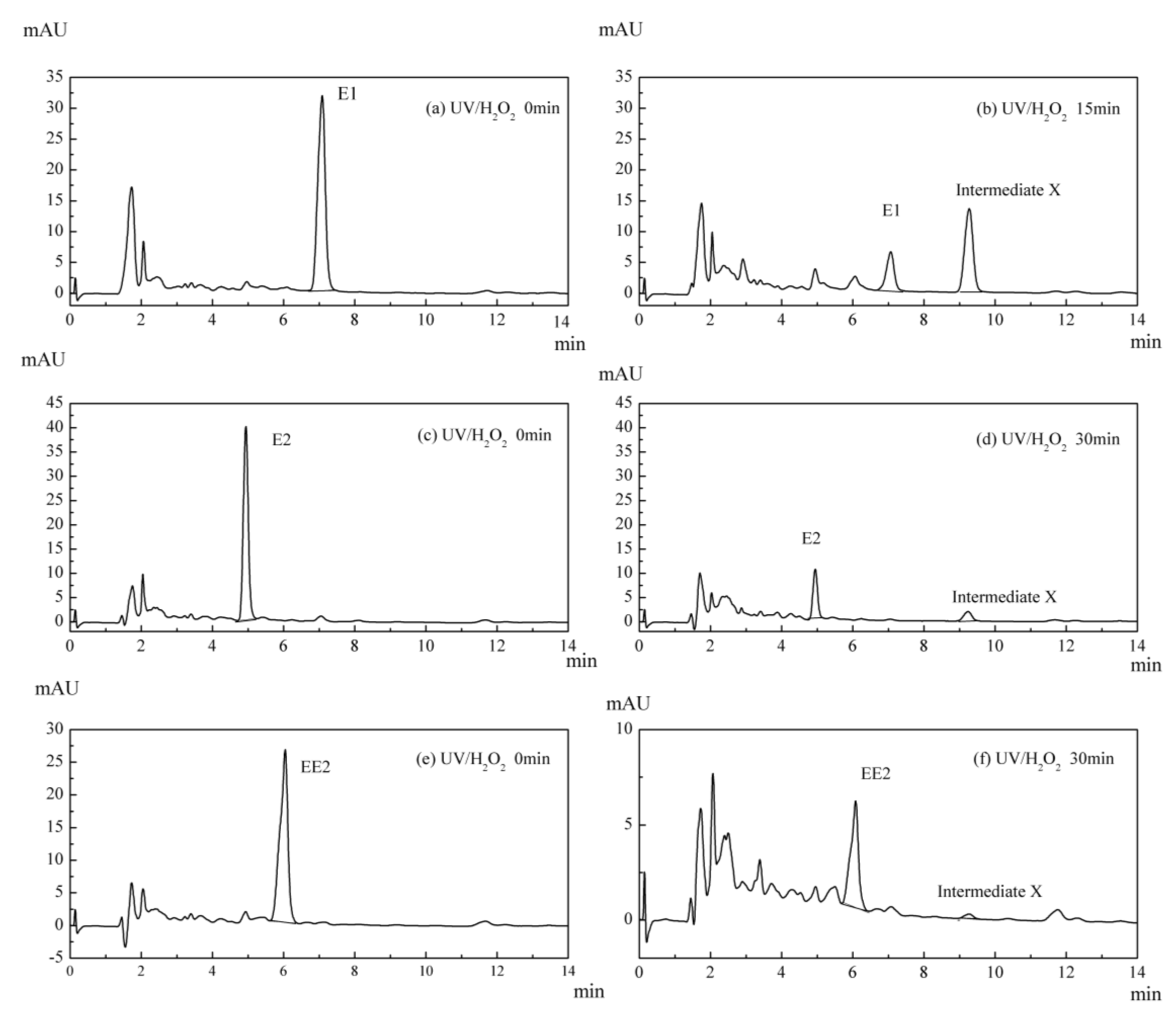
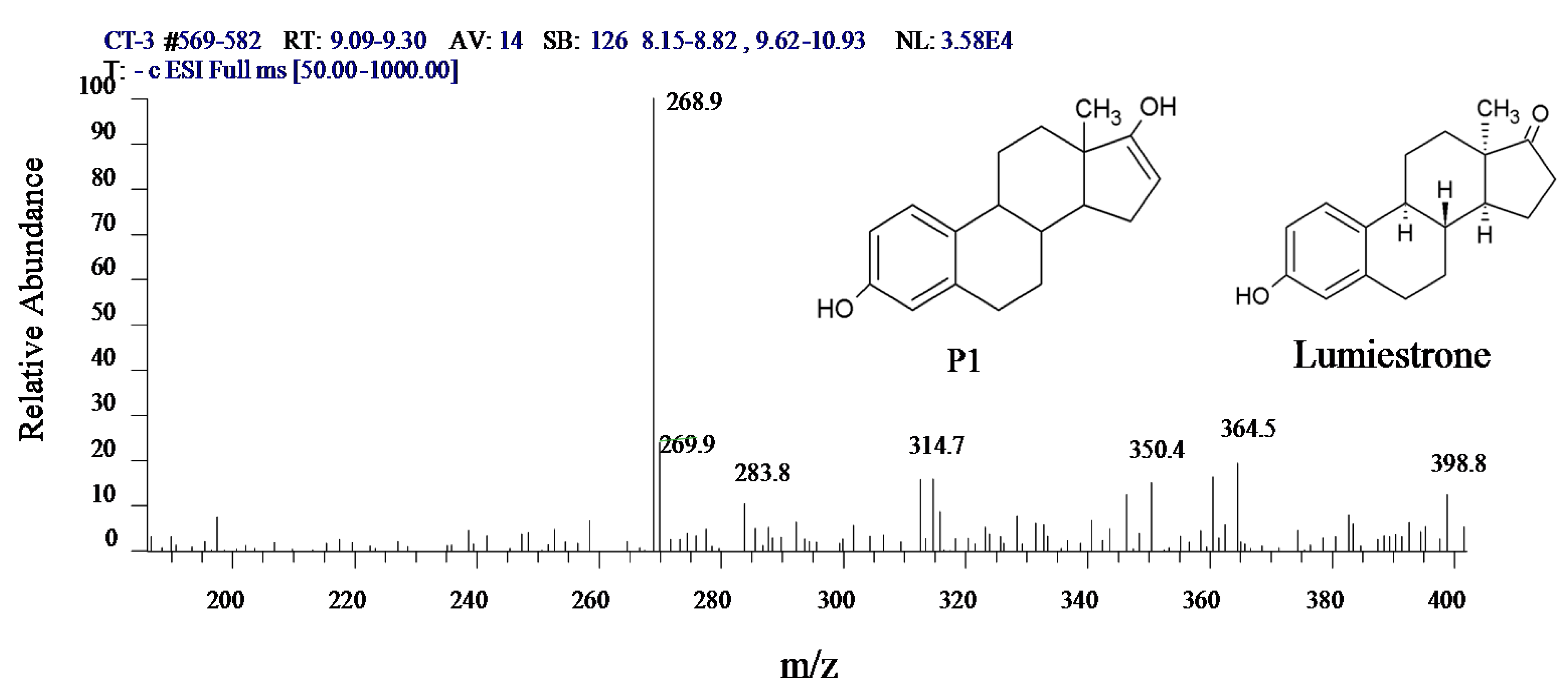
3.6. Variation in the Characteristics of Intermediate X in the UV/H2O2 System
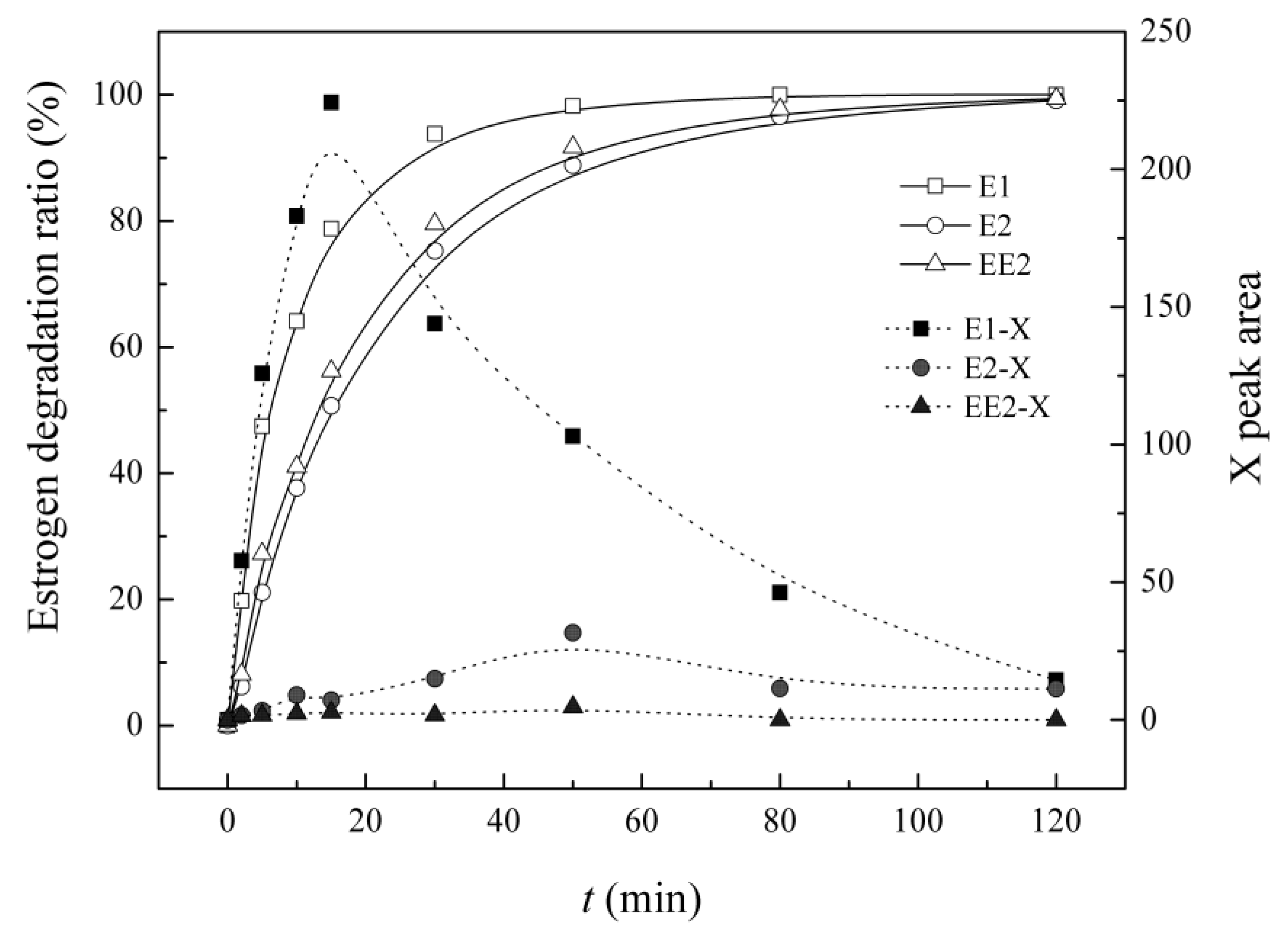

4. Conclusions
Acknowledgments
Abbreviations
| SEs | steroid estrogens |
| E1 | estrone |
| E2 | 17β-estradiol |
| EE2 | 17α-ethinyl estradiol |
| SPE | solid phase extraction |
Author Contributions
Conflicts of Interest
References
- Bastos, S.L.; Kamstra, J.H.; Cenijn, P.H.; van Rijt, L.S.; Hamers, T.; Legler, J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol. in Vitro 2013, 27, 1634–1643. [Google Scholar]
- Sun, Y.; Huang, H.; Sun, Y.; Wang, C.; Shi, X.L.; Hu, H.Y.; Kameya, T.; Fujie, K. Ecological risk of estrogenic endocrine disrupting chemicals in sewage plant effluent and reclaimed water. Environ. Pollut. 2013, 180, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Esteban, S.; Gorga, M.; Petrovic, M.; González-Alonso, S.; Barceló, D.; Valcárcel, Y. Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci. Total Environ. 2014, 466–467, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.M.; Dong, H.Y.; Zhu, B.Y.; Qu, J.H.; Nie, Y.F. A comparison of various rural wastewater treatment processes for the removal of endocrine-disrupting chemicals (EDCs). Chemosphere 2013, 92, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Song, W.T.; Lu, G.H.; Li, X.; Zhang, H.Z.; Qin, J. Characters of environmental estrogen pollution in Yangtze River (Nanjing section). Ecol. Environ. Sci. 2009, 18, 1615–1619. [Google Scholar]
- Li, Q.S.; Gao, N.Y.; Deng, Y.; Ma, X.Y.; Chu, W.H. Factors Affecting UV/H2O2 Oxidation of 17α-Ethynyestradiol in Water. Clean 2013, 41, 143–147. [Google Scholar]
- Jin, T.; Lü, X.; Zeng, Y.F.; Zhang, B.B.; Ma, K.; Jiang, P.; Tang, F. Comparison of the estrogenic activity of organic compounds in source water and finished water from the Yangtze River and Taihu Lake in certain areas of Jiangsu Provence. Environ. Sci. 2013, 34, 1351–1356. [Google Scholar]
- Li, B.G.; Cui, S.Y. Emission inventory and risk assessment of environmental estrogens in China. Urban Environ. Urban Ecol. 2011, 24, 24–28. [Google Scholar]
- Kuch, H.M.; Ballschmiter, K. Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC-(NCI)-MS in the picogram per liter range. Environ. Sci. Technol. 2001, 35, 3201–3206. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wen, T.Y.; Wang, G.S.; Cheng, H.W.; Lin, Y.H.; Lien, G.W. Determining estrogenic steroids in Taipei waters and removal in drinking water treatment using high-flow solid-phase extraction and liquid chromatography/tandem mass spectrometry. Sci. Total Environ. 2007, 378, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lin, D.H.; Mashayekhi, H.; Xing, B.S. Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nanomaterials. Environ. Sci. Technol. 2008, 42, 5480–5485. [Google Scholar] [CrossRef] [PubMed]
- Larcher, S.; Delbès, G.; Robaire, B.; Yargeau, V. Degradation of 17α-ethinylestradiol by ozonation-Identification of the by-products and assessment of their estrogenicity and toxicity. Environ. Int. 2012, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Rokhina, E.V.; Vattikonda, N.S.; Johnson, C.; Suri, R.P.S. Ozonation of a mixture of estrogens and progestins in aqueous solution: Interpretation of experimental results by computational methods. Chemosphere 2012, 89, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, T.; Iwasaki, S.; Kawashima, M.; Shinohara, O.; Abe, I. Adsorbability of estrone and 17β-estradiol in water onto activated carbon. Water Res. 2006, 40, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Zhou, J.L. Removal of estrone and 17β-estradiol from water by adsorption. Water Res. 2005, 39, 3991–4003. [Google Scholar] [CrossRef] [PubMed]
- Pholchan, P.; Jones, M.; Donnelly, T.; Sallis, P.J. Fate of estrogens during the biological treatment of synthetic wastewater in a nitrite-accumulating sequencing batch reactor. Environ. Sci. Technol. 2008, 42, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, J.H.; Liu, X.; Zhan, X.W.; Chen, Q.C. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China. Water Res. 2014, 58, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.Z.; Graham, N.; Gao, N.Y. The aqueous degradation of bisphenol A and steroid estrogens by ferrate. Water Res. 2008, 42, 109–120. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Pereira, R.; de Alda, M.L.; Joglar, J.; Daniel, L.A.; Barceló, D. Identification of new ozonation disinfection byproducts of 17β-estradiol and estrone in water. Chemosphere 2011, 84, 1535–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontistis, Z.; Xekoukoulotakis, N.P.; Hapeshi, E.; Venieri, D.; Fatta-Kassinos, D.; Mantzavinos, D. Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem. Eng. J. 2011, 178, 175–182. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.S.; Singhal, N.; Wang, L.Z.; Gao, W. Comparative photocatalytic degradation of estrone in water by ZnO and TiO2 under artificial UVA and solar irradiation. Chem. Eng. J. 2012, 213, 150–162. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.L. Direct photolysis of estrogens in aqueous solutions. Sci. Total Environ. 2004, 320, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Tang, K.; Song, Y.L.; Zhang, Z.H.; Li, Q.S.; Gao, N.Y. Competitive Degradation and Influences Analysis of Three Steriod Estrogens in Aqueous Solution by UV/H2O2 Process. Available online: http://www.researchgate.net/publication/279673219_Competitive_degradation_and_influences_analysis_of_three_steroid_estrogens_in_aqueous_solution_by_UVH2O2_process (accessed on 25 June 2015).
- Mazellier, P.; Méité, L.; Laat, J.D. Photodegradation of the steroid hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) in dilute aqueous solution. Chemosphere 2008, 73, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Puma, G.L.; Puddu, V.; Tsang, H.K.; Gora, A.; Toepfer, B. Photocatalytic oxidation of multicomponent mixtures of estrogens (estrone (E1), 17 β-estradiol (E2), 17α-ethynylestradiol (EE2) and estriol (E3)) under UVA andUVC radiation: Photon absorption, quantum yields and rate constants independent of photon absorption. Appl. Catal. B Environ. 2010, 99, 388–397. [Google Scholar]
- Caupos, E.; Mazellier, P.; Croue, J.P. Photodegradation of estrone enhanced by dissolved organic matter under simulated sunlight. Water Res. 2011, 45, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.L.; Heyne, B.; Blais, J.M.; Temussi, F.; Atkinson, S.K.; Pakdel, F.; Popesku, J.T.; Marlatt, V.L.; Scaiano, J.C.; Previtera, L.; et al. Lumiestrone is photochemically derived from estrone and may be released to the environment without detection. Front. Endocrinol. 2011, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whidbey, C.M.; Daumit, K.E.; Nguyen, T.H.; Ashworth, D.D.; Davis, J.C.; Latch, D.E. Photochemical induced changes of in vitro estrogenic activity of steroid hormones. Water Res. 2012, 46, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhang, C.; Deng, J.; Song, Y.; Li, Q.; Guo, Y.; Li, C. Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System. Int. J. Environ. Res. Public Health 2015, 12, 12016-12029. https://doi.org/10.3390/ijerph121012016
Ma X, Zhang C, Deng J, Song Y, Li Q, Guo Y, Li C. Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System. International Journal of Environmental Research and Public Health. 2015; 12(10):12016-12029. https://doi.org/10.3390/ijerph121012016
Chicago/Turabian StyleMa, Xiaoyan, Chao Zhang, Jing Deng, Yali Song, Qingsong Li, Yaping Guo, and Cong Li. 2015. "Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System" International Journal of Environmental Research and Public Health 12, no. 10: 12016-12029. https://doi.org/10.3390/ijerph121012016
APA StyleMa, X., Zhang, C., Deng, J., Song, Y., Li, Q., Guo, Y., & Li, C. (2015). Simultaneous Degradation of Estrone, 17β-Estradiol and 17α-Ethinyl Estradiol in an Aqueous UV/H2O2 System. International Journal of Environmental Research and Public Health, 12(10), 12016-12029. https://doi.org/10.3390/ijerph121012016






