Increasing Incidence of Canine Leptospirosis in Switzerland
Abstract
:1. Introduction
2. Experimental Section
2.1. Data Collection
2.2. Clinical and Laboratory Characterization
2.3. Data and Statistical Analysis
3. Results and Discussion
3.1. Results
3.1.1. Dogs: Signalment
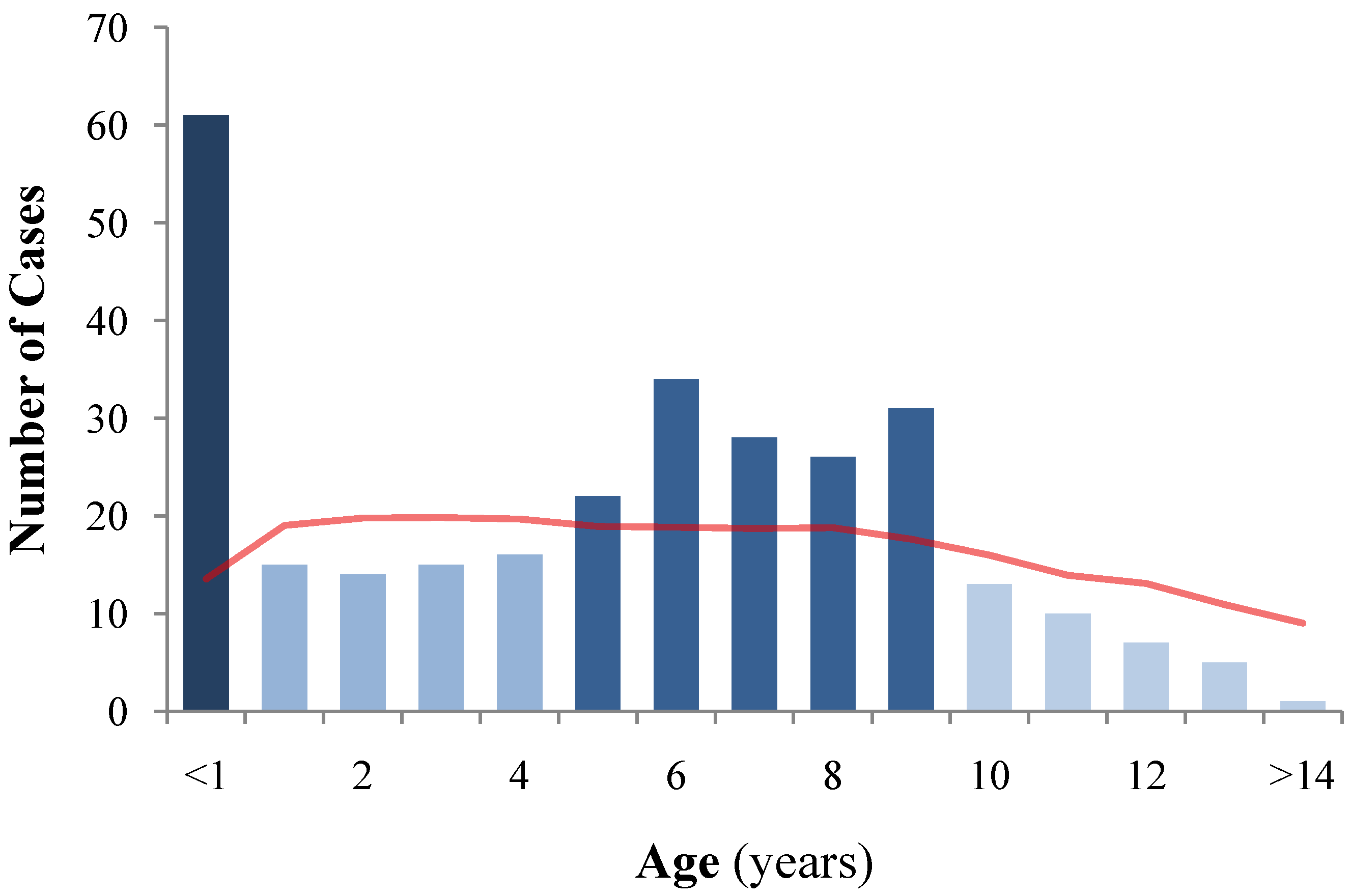
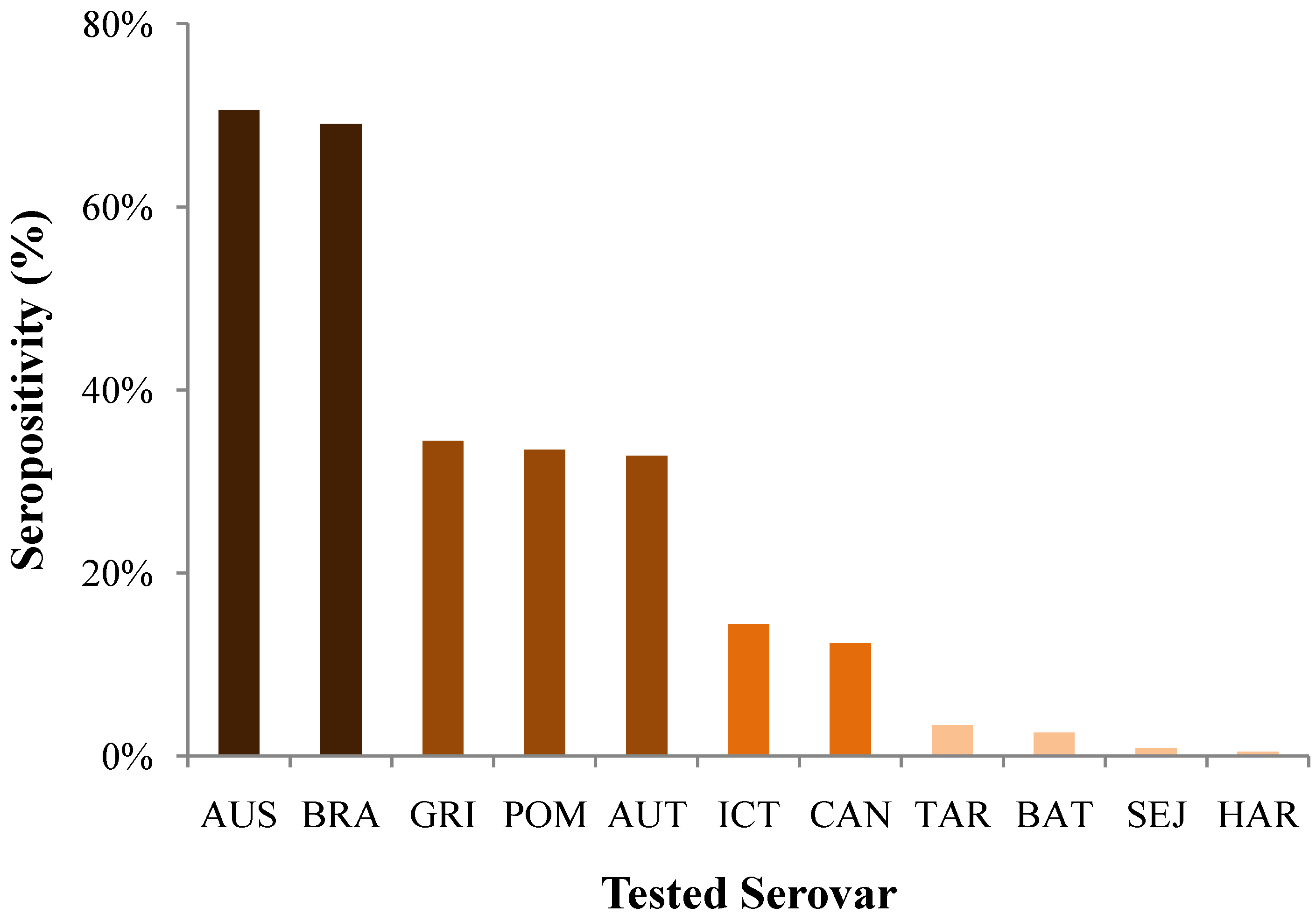
3.1.2. Disease: Diagnosis, Organ Manifestations, and Outcome
| Organ Involvement | N affected/N total | % affected | OR for negative outcome | 95% CI | P |
|---|---|---|---|---|---|
| Renal | 255/256 | 99.6% | n/a | n/a | n/a |
| Serum creatinine at presentation | 1.0019 | 1.0011–1.0027 | <0.001 | ||
| Pulmonary | 194/253 | 76.7% | 3.6 | 1.8–7.2 | <0.001 |
| Hepatic | 66/254 | 26.0% | 16.3 | 7.7–34.5 | <0.001 |
| Hemorrhagic | 38/209 | 18.2% | 7.9 | 3.4–18.4 | <0.001 |
3.1.3. Geographic Distribution of the Cases
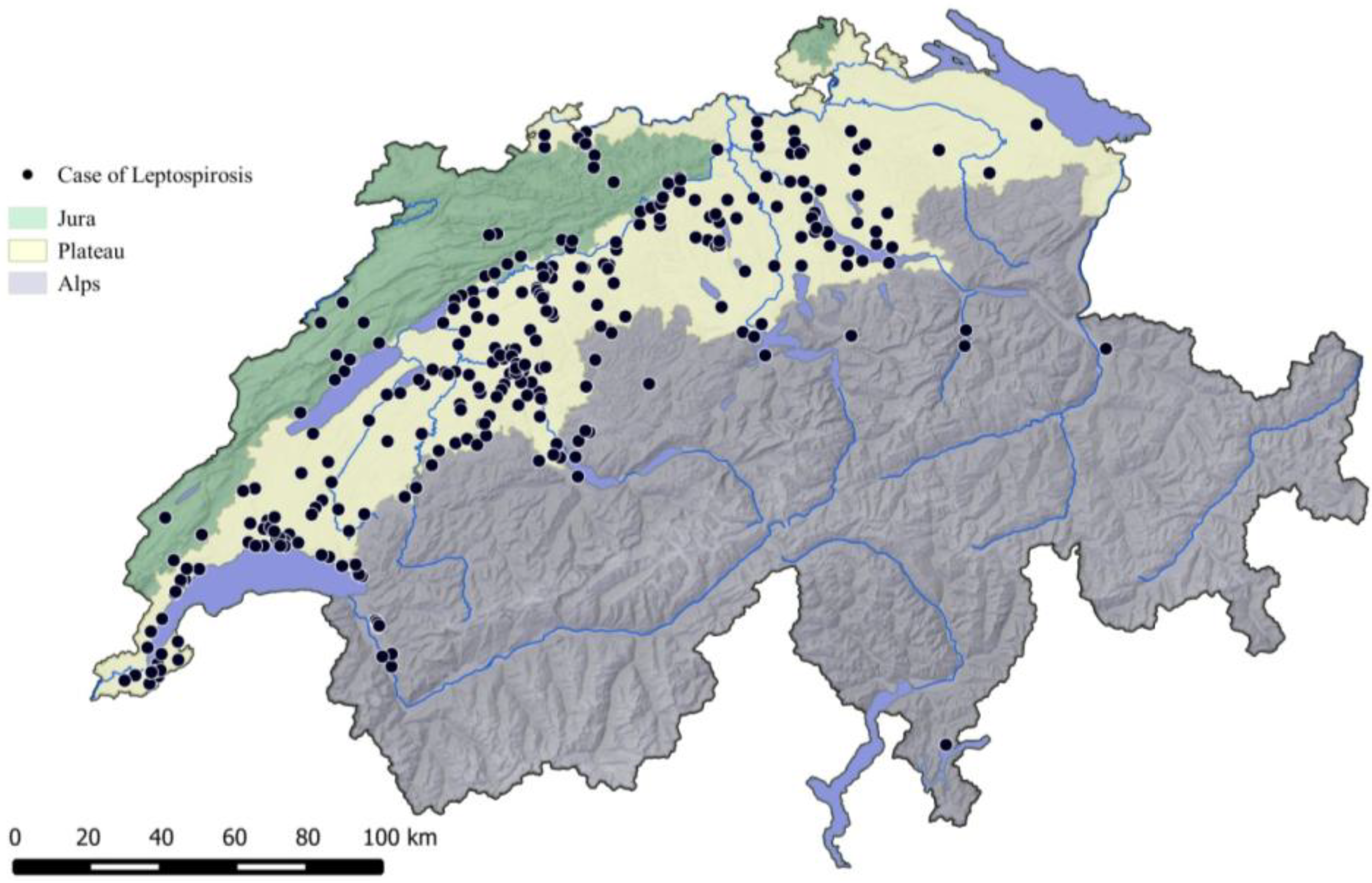
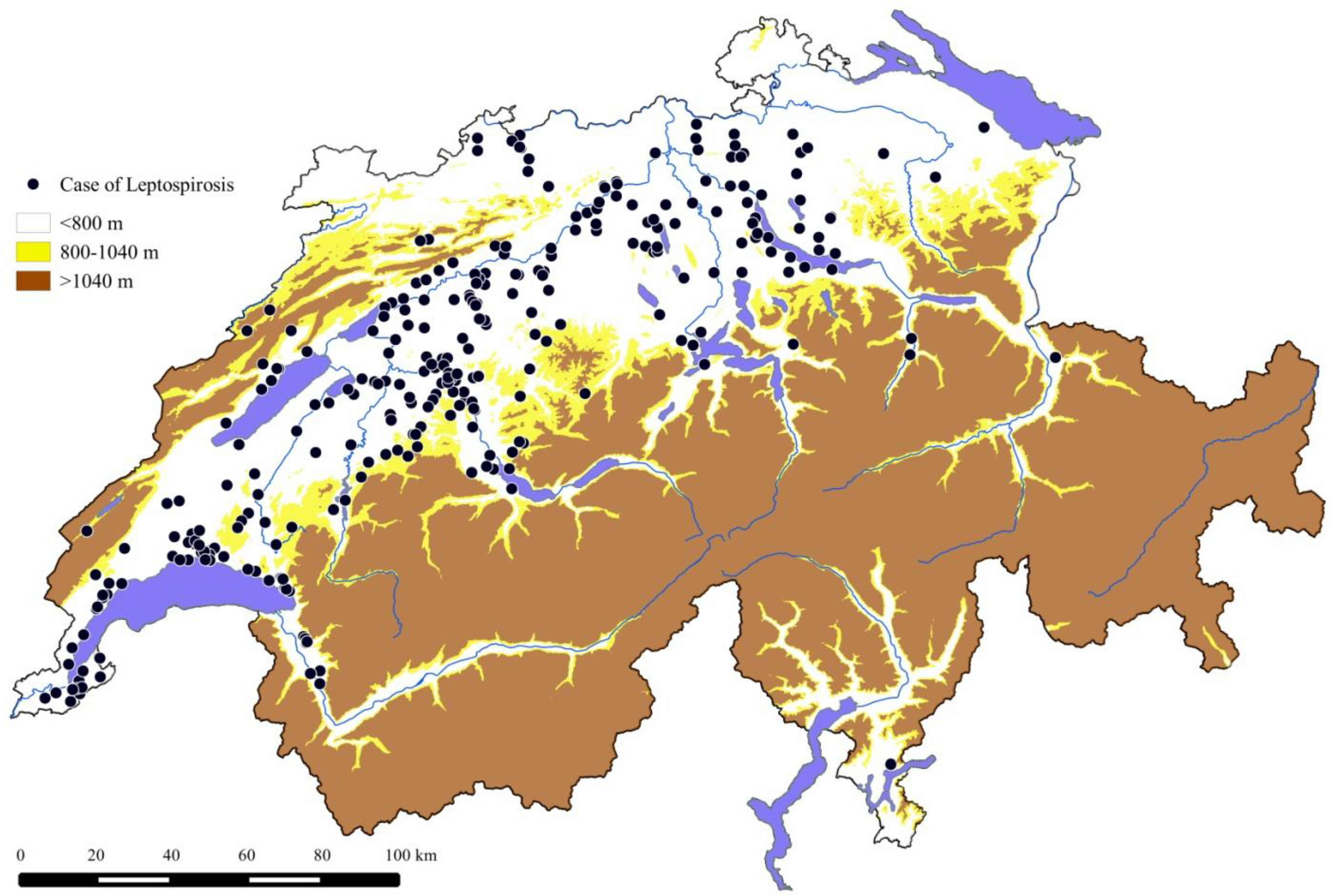
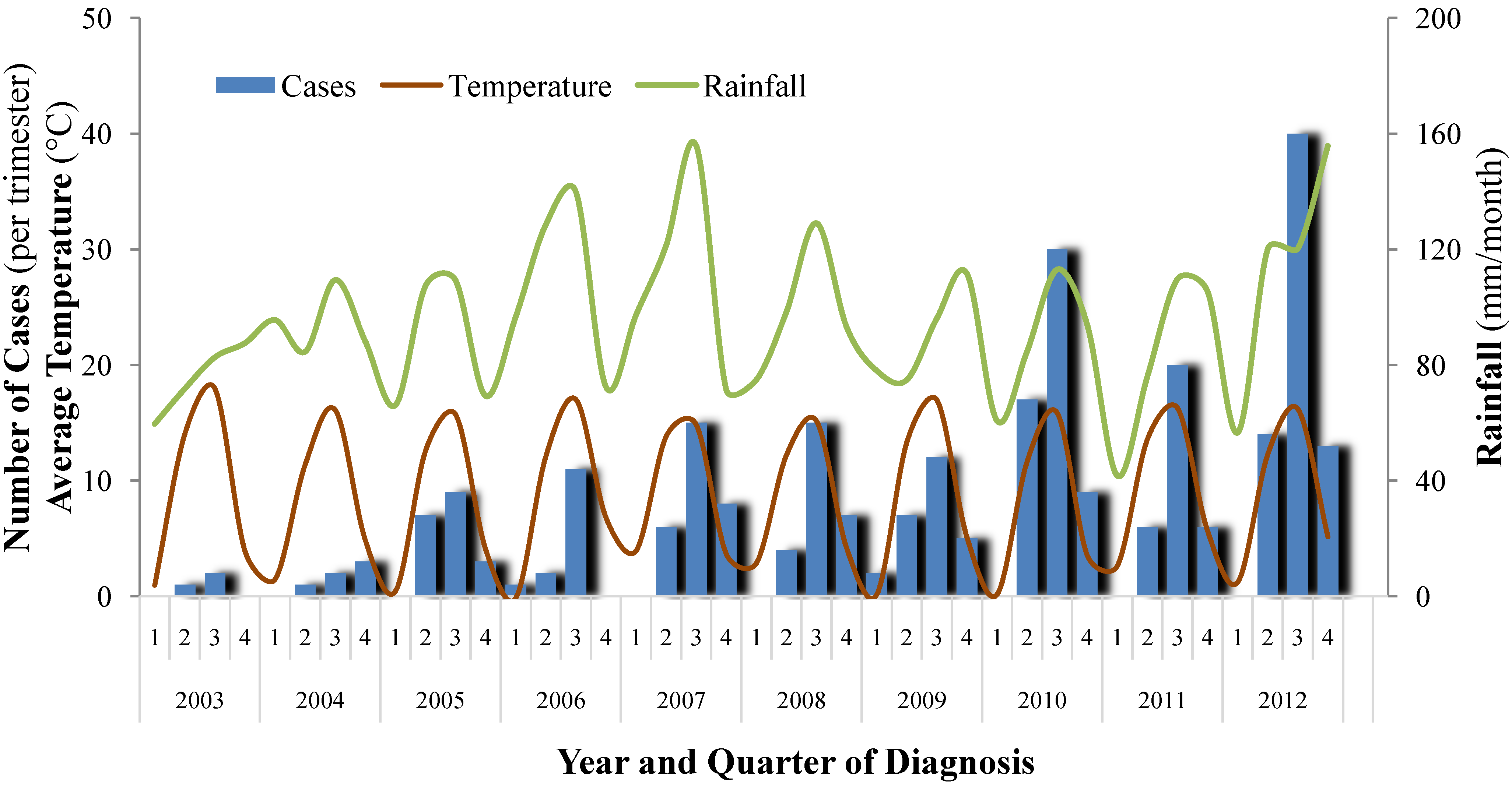
3.1.4. Incidence and Trend over the Years
| Canton | Cases | Annual Incidence of Diagnosis | Canine Population | Human Population | Altitude | Temperature | Rainfall | |
|---|---|---|---|---|---|---|---|---|
| n | Average | Peak | n × 1000 | n × 1000 | Average (m) | °C | mm/year | |
| n/100,000 dogs/y | ||||||||
| Most affected cantons (>5 cases/10y) | ||||||||
| BE | 90 | 13.6 | 27.1 | 66.4 | 963 | 1198 | 6.1 | 115.6 |
| VD | 59 | 9.8 | 21.7 | 60.0 | 672 | 827 | 8.5 | 100.9 |
| ZH | 32 | 5.5 | 17.3 | 57.7 | 1308 | 533 | 9.5 | 97.5 |
| AG | 25 | 6.4 | 28.1 | 39.1 | 582 | 476 | 9.7 | 88.3 |
| FR | 19 | 8.8 | 18.6 | 21.5 | 263 | 856 | 8.2 | 96.9 |
| GE | 14 | 4.9 | 13.9 | 28.7 | 438 | 419 | 10.9 | 73.9 |
| SO | 13 | 6.1 | 14.0 | 21.4 | 250 | 630 | 9.1 | 95.8 |
| BL | 9 | 5.0 | 22.0 | 18.1 | 269 | 521 | 9.7 | 87.3 |
| NE | 9 | 7.3 | 24.4 | 12.3 | 170 | 919 | 7.8 | 106.7 |
| LU | 8 | 3.9 | 24.4 | 20.5 | 364 | 777 | 8.3 | 113.7 |
| Median (IQR) | 6.2 | 21.9 | 25.1 | 401 | 704 | 8.8 | 97.2 | |
| (5.1–8.4) | (17.6–24.4) | (20.7–53.1) | (265–649) | (524–849) | (8.2–9.6) | (90.2–105.3) | ||
| Least affected cantons (<5 cases/10y) | ||||||||
| GL | 2 | 7.9 | 79.0 | 2.6 | 38 | 1589 | 3.9 | 145.5 |
| SG | 2 | 0.7 | 3.7 | 27.1 | 466 | 1000 | 7.5 | 121.2 |
| SZ | 2 | 2.7 | 13.6 | 7.3 | 141 | 1082 | 6.6 | 147.8 |
| TG | 2 | 1.1 | 5.7 | 17.5 | 238 | 495 | 9.5 | 83.8 |
| GR | 1 | 0.7 | 7.5 | 13.4 | 189 | 2021 | 1.9 | 95.1 |
| NW | 1 | 6.2 | 61.5 | 1.6 | 40 | 1077 | 6.3 | 135.4 |
| TI | 1 | 0.4 | 3.8 | 26.4 | 329 | 1412 | 6.3 | 138.4 |
| VS | 1 | 0.5 | 4.5 | 22.2 | 299 | 2140 | 1.7 | 109.2 |
| ZG | 1 | 2.3 | 23.3 | 4.3 | 109 | 651 | 8.6 | 117.3 |
| AI | 0 | 0.0 | 0.0 | 0.9 | 16 | 1126 | 7.2 | 136.1 |
| AR | 0 | 0.0 | 0.0 | 4.5 | 53 | 935 | 7.8 | 126.0 |
| BS | 0 | 0.0 | 0.0 | 5.0 | 185 | 522 | 10.9 | 71.6 |
| JU | 0 | 0.0 | 0.0 | 8.3 | 70 | 690 | 8.7 | 101.3 |
| OW | 0 | 0.0 | 0.0 | 1.8 | 34 | 1329 | 5.4 | 141.0 |
| SH | 0 | 0.0 | 0.0 | 4.7 | 75 | 538 | 9.4 | 75.9 |
| UR | 0 | 0.0 | 0.0 | 1.6 | 35 | 1896 | 2.4 | 134.3 |
| Median (IQR) | 0.4 | 3.7 | 4.9 | 92 | 1080 | 6.9 | 123.6 | |
| (0.0–1.4) | (0.0–9.0) | (2.4–14.4) | (40–201) | (680–1456) | (5.0–8.6) | (99.7–136.7) | ||
| P | <0.001 | 0.004 | <0.001 | <0.001 | 0.02 | 0.03 | 0.04 | |
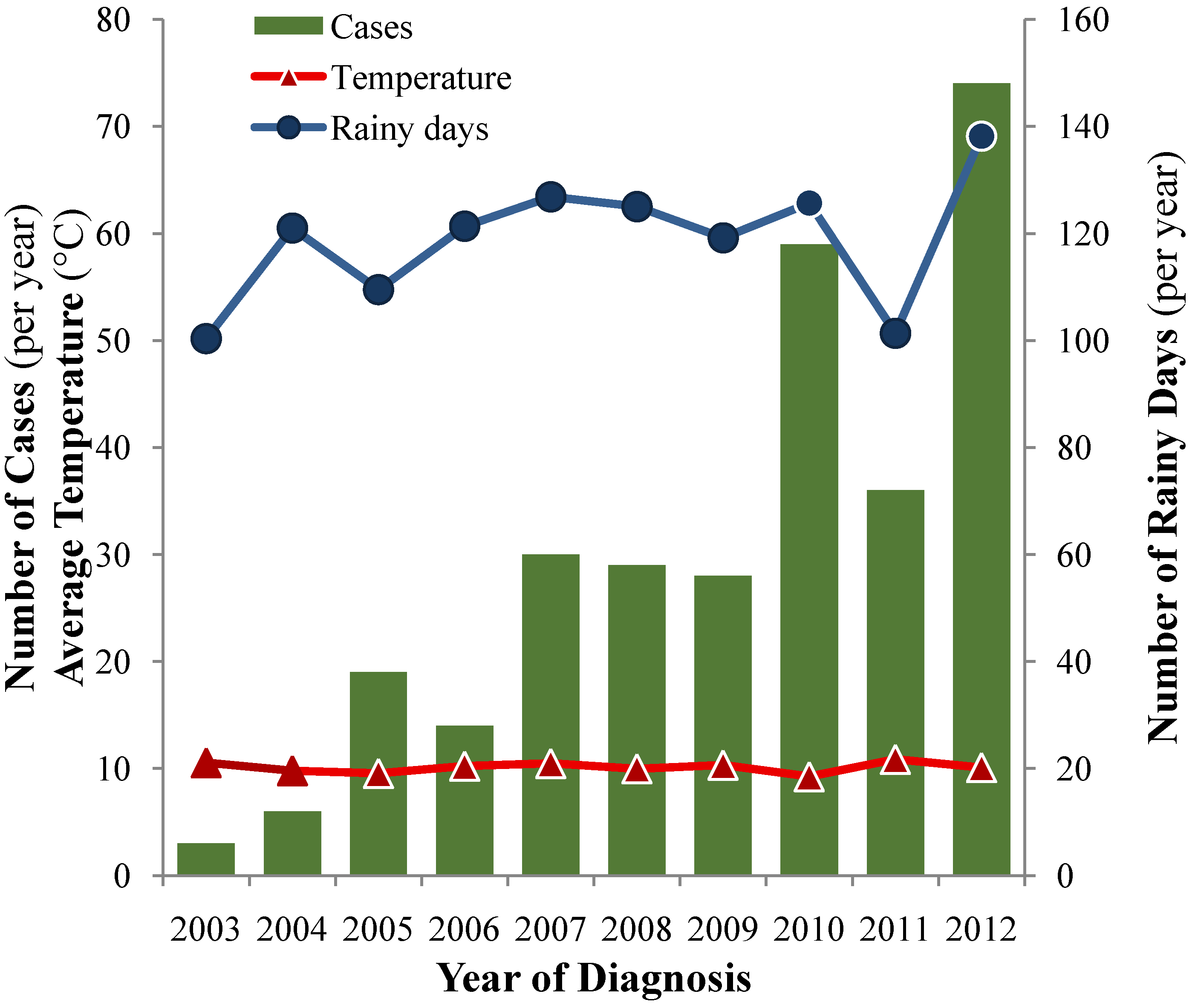
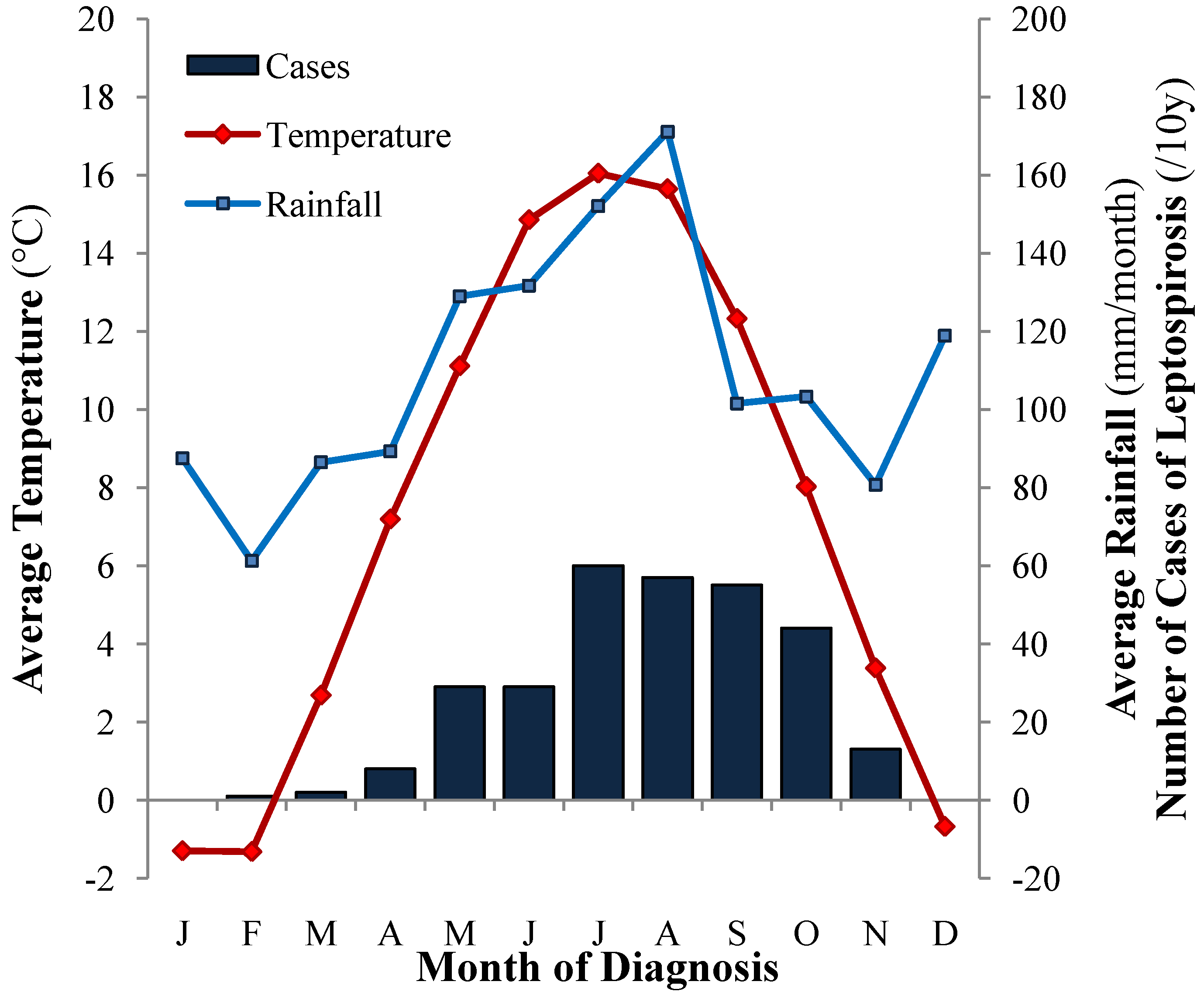
3.1.5. Seasonality and Climatic Factors
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Levine, M.; Guptill-Yoran, C.; Johnson, A.J.; von Kamecke, P.; Moore, G.E. Regional and temporal variations of Leptospira seropositivity in dogs in the United States, 2000–2010. J. Vet. Intern. Med. 2014, 28, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Monahan, A.M.; Miller, I.S.; Nally, J.E. Leptospirosis: Risks during recreational activities. J. Appl. Microbiol. 2009, 107, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Wallner, C.; Allerberger, F.; Schmoll, F.; Seeber, K.; Wagner, J.; Valentin, T.; Zollner-Schwetz, I.; Flick, H.; Krause, R. Autochthonous leptospirosis in South-East Austria, 2004-2012. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Schoneberg, I.; Frank, C.; Alpers, K.; Schneider, T.; Stark, K. Leptospirosis in Germany, 1962–2003. Emerg. Infect. Dis. 2005, 11, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Coello, M.; Vado-Solis, I.; Cardenas-Marrufo, M.F.; Rodriguez-Buenfil, J.C.; Ortega-Pacheco, A. Serological survey of canine leptospirosis in the tropics of Yucatan Mexico using two different tests. Acta Trop. 2008, 106, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Calderon, A.; Rodriguez, V.; Mattar, S.; Arrieta, G. Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop. Anim. Health Prod. 2014, 46, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vivas, C.M.; Thiry, D.; Rodriguez, V.; Calderon, A.; Arrieta, G.; Mattar, S.; Cuello, M.; Levett, P.N.; Falconar, A.K. Molecular serovar characterization of Leptospira isolates from animals and water in Colombia. Biomedica 2013, 33, 179–184. [Google Scholar] [PubMed]
- Gautam, R.; Wu, C.C.; Guptill, L.F.; Potter, A.; Moore, G.E. Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the united states, 2000–2007. J. Am. Vet. Med. Assoc. 2010, 237, 293–298. [Google Scholar] [PubMed]
- Chapola, E.G.; dos Santos, M.; Bessa, T.A.; de Oliveira, M.L. Human and canine leptospirosis: Serological data of Sao Paulo city, Brazil, 2000 to 2003. Rev. Cubana Med. Trop. 2005, 57, 61–62. [Google Scholar] [PubMed]
- Harkin, K.R.; Roshto, Y.M.; Sullivan, J.T. Clinical application of a polymerase chain reaction assay for diagnosis of leptospirosis in dogs. J. Am. Vet. Med. Assoc. 2003, 222, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Gay, N.; Soupe-Gilbert, M.E.; Goarant, C. Though not reservoirs, dogs might transmit Leptospira in New Caledonia. Int. J. Environ. Res. Public Health 2014, 11, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, J.R.; Krupta-Dyachenko, I.; Rettinger, A.L.; Dyachenko, V.; Stamm, I.; Kopp, P.A.; Straubinger, R.K.; Hartmann, K. Prevalence of Leptospira urinary shedding in healthy dogs from Southern Germany. J. Vet. Intern. Med. 2013, 28, 711–744. [Google Scholar]
- Utzinger, J.; Becker, S.L.; Knopp, S.; Blum, J.; Neumayr, A.L.; Keiser, J.; Hatz, C.F. Neglected tropical diseases: Diagnosis, clinical management, treatment and control. Swiss Med. Wkly. 2012, 142, 1–24. [Google Scholar]
- Francey, T. Clinical features and epidemiology of presumptive canine leptospirosis in Western Switzerland, 2003–2005. J. Vet. Intern. Med. 2006, 20, 1530–1531. [Google Scholar]
- Witmer, T.; Francey, T.; Schweighauser, A. Leptospirosis in dogs. A retrospective study of epidemiology, clinic, diagnosis, and spread of leptospirosis in Switzerland. Master thesis, University of Bern, Bern, Switzerland, 2012. [Google Scholar]
- Barmettler, R.; Schweighauser, A.; Bigler, S.; Grooters, A.M.; Francey, T. Assessment of exposure to Leptospira serovars in veterinary staff and dog owners in contact with infected dogs. J. Am. Vet. Med. Assoc. 2011, 238, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.; Penna, B.; Lilenbaum, W. The dog in the transmission of human leptospirosis under tropical conditions: Victim or villain? Epidemiol. Infect. 2012, 140, 207–208. [Google Scholar]
- Stokes, J.E.; Forrester, S.D. New and unusual causes of acute renal failure in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2004, 34, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Ghneim, G.S.; Viers, J.H.; Chomel, B.B.; Kass, P.H.; Descollonges, D.A.; Johnson, M.L. Use of a case-control study and geographic information systems to determine environmental and demographic risk factors for canine leptospirosis. Vet. Res. 2007, 38, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Gartner, B.; Jacquier, L.; Petignat, P.A.; Rochat, T. An uncommon cause of diffuse alveolar haemorrhage. Respiration 2008, 75, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM small animal consensus statement on leptospirosis: Diagnosis, epidemiology, treatment, and prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fraune, C.K.; Schweighauser, A.; Francey, T. Evaluation of the diagnostic value of serologic microagglutination testing and a polymerase chain reaction assay for diagnosis of acute leptospirosis in dogs in a referral center. J. Am. Vet. Med. Assoc. 2013, 242, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, L.D.; Langston, C.A. Acute Kidney Insufficiency. In Nephrology and Urology of Small Animals; Bartges, J., Polzin, D.J., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2011; pp. 472–523. [Google Scholar]
- IRIS, International Renal Interest Society. Available online: http://www.iris-kidney.com (accessed on 24 April 2014).
- Greene, C.E.; Sykes, J.E.; Moore, G.E.; Goldstein, R.E.; Schultz, R.D. Leptospirosis. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 431–447. [Google Scholar]
- Schweighauser, A.; Francey, T. Pulmonary haemorrhage as an emerging complication of acute kidney injury due to canine leptospirosis. J. Vet. Intern. Med. 2008, 22, 1473–1474. [Google Scholar]
- Baumann, D.; Flückiger, M. Radiographic findings in the thorax of dogs with leptospiral infection. Vet. Radiol. Ultrasound. 2001, 42, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.; Steinicke, K.; Arndt, G.; Gruber, A.D.; Guerra, B.; Jansen, A.; Kaser-Hotz, B.; Klopfleisch, R.; Lotz, F.; Luge, E.; et al. Pulmonary abnormalities in dogs with leptospirosis. J. Vet. Intern. Med. 2010, 24, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Kohn, B.; Plog, S.; Weingart, C.; Nockler, K.; Mayer-Scholl, A.; Gruber, A.D. An emerging pulmonary haemorrhagic syndrome in dogs: Similar to the human leptospiral pulmonary haemorrhagic syndrome? Vet. Med. Int. 2010, 2010. [Google Scholar] [CrossRef]
- World Health Organization; I.L.S. Human Leptospirosis: Guidance for diagnosis, surveillance and control. Available online: http://www.med.monash.edu.au/microbiology/staff/adler/leptoguidelines2003.pdf (accessed on 4 April 2014).
- Smythe, L.D.; Wuthiekanun, V.; Chierakul, W.; Suputtamongkol, Y.; Tiengrim, S.; Dohnt, M.F.; Symonds, M.L.; Slack, A.T.; Apiwattanaporn, A.; Chueasuwanchai, S.; et al. The microscopic agglutination test (MAT) is an unreliable predictor of infecting Leptospira serovar in Thailand. Am. J. Trop. Med. Hyg. 2009, 81, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Abdoel, T.H.; Houwers, D.J.; van Dongen, A.M.; Adesiyun, A.A.; Jimenez-Coelloe, M.; Cardoso, L.; Suepaul, S.M.; Ortega-Pacheco, A.; Smits, H.L. Rapid test for the serodiagnosis of acute canine leptospirosis. Vet. Microbiol. 2011, 150, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Steger-Lieb, A.; Gerber, B.; Nicolet, J.; Gaschen, F. An old disease with a new face: Canine leptospirosis does not lose its relevance. Schweiz. Arch. Tierheilkd. 1999, 141, 499–507. [Google Scholar] [PubMed]
- Goldstein, R.E.; Lin, R.C.; Langston, C.E.; Scrivani, P.V.; Erb, H.N.; Barr, S.C. Influence of infecting serogroup on clinical features of leptospirosis in dogs. J. Vet. Intern. Med. 2006, 20, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F.; McEwen, B.; Taylor, J.; Woods, J.P.; Abrams-Ogg, A.; Wilcock, B. Resurgence of leptospirosis in dogs in Ontario: Recent findings. Can. Vet. J. 2002, 43, 955–961. [Google Scholar] [PubMed]
- Birnbaum, N.; Barr, S.C.; Center, S.A.; Schermerhorn, T.; Randolph, J.F.; Simpson, K.W. Naturally acquired leptospirosis in 36 dogs: Serological and clinicopathological features. J. Small Anim. Pract. 1998, 39, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; van Treeck, U.; Lierz, M.; Espelage, W.; Zota, L.; Sarbu, A.; Czerwinski, M.; Sadkowska-Todys, M.; Avdicova, M.; Reetz, J.; et al. Resurgence of field fever in a temperate country: An epidemic of leptospirosis among seasonal strawberry harvesters in Germany in 2007. Clin. Infect. Dis. 2009, 48, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Andre-Fontaine, G. Canine leptospirosis–do we have a problem? Vet Microbiol 2006, 117, 19–24. [Google Scholar]
- Mastrorilli, C.; Dondi, F.; Agnoli, C.; Turba, M.E.; Vezzali, E.; Gentilini, F. Clinicopathologic features and outcome predictors of Leptospira interrogans australis serogroup infection in dogs: A retrospective study of 20 cases (2001–2004). J. Vet. Intern. Med. 2007, 21, 3–10. [Google Scholar] [PubMed]
- Trevejo, R.T.; Rigau-Perez, J.G.; Ashford, D.A.; McClure, E.M.; Jarquin-Gonzalez, C.; Amador, J.J.; de los Reyes, J.O.; Gonzalez, A.; Zaki, S.R.; Shieh, W.J.; et al. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J. Infect. Dis. 1998, 178, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.J.; Netto, B.A.; Lilembaum, W.; Alvim, M.E.; de Oliveira, A.V. The hemorrhagic syndrome of leptospirosis: An experimental study in Guinea pigs. Rev. Soc. Bras. Med. Trop. 1995, 28, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dolhnikoff, M.; Mauad, T.; Bethlem, E.P.; Carvalho, C.R. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz. J. Infect. Dis. 2007, 11, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Pappas, G.; Papadimitriou, P.; Siozopoulou, V.; Christou, L.; Akritidis, N. The globalization of leptospirosis: Worldwide incidence trends. Int. J. Infect. Dis. 2008, 12, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Skufca, J.; Arima, Y. Sex, gender and emerging infectious disease surveillance: A leptospirosis case study. Western Pac. Surveill. Response J. 2012, 3, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.; Connell, J.; O'Flanagan, D.; McKeown, P. Leptospirosis in Ireland: Annual incidence and exposures associated with infection. Epidemiol. Infect. 2014, 142, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Rojas, P.; Monahan, A.M.; Schuller, S.; Miller, I.S.; Markey, B.K.; Nally, J.E. Detection and quantification of leptospires in urine of dogs: A maintenance host for the zoonotic disease leptospirosis. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Scholl, A.; Luge, E.; Draeger, A.; Nockler, K.; Kohn, B. Distribution of Leptospira serogroups in dogs from Berlin, Germany. Vector Borne Zoonotic Dis. 2013, 13, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Whitney, E.A.; Ailes, E.; Myers, L.M.; Saliki, J.T.; Berkelman, R.L. Prevalence of and risk factors for serum antibodies against Leptospira serovars in US veterinarians. J. Am. Vet. Med. Assoc. 2009, 234, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Gendron, K.; Christe, A.; Walter, S.; Schweighauser, A.; Francey, T.; Doherr, M.G.; Lang, J. Serial CT features of pulmonary leptospirosis in 10 dogs. Vet. Rec. 2014, 174. [Google Scholar] [CrossRef] [PubMed]
- Bessire, N. La leptospirose-une maladie professionnelle. Schweiz. Med. Forum 2004, 4, 513–514. [Google Scholar]
- Giovannini, S.; Ryser, M.-P.; Tagliabue, S.; Pewsner, M.; Origgi, F. Leptospirosis in European Beavers (castor fiber) from Switzerland. In Proceedings of the WDA/EWDA Conference, Lyon, France, 22–27 July 2012.
- Adler, H.; Vonstein, S.; Deplazes, P.; Stieger, C.; Frei, R. Prevalence of Leptospira spp. In various species of small mammals caught in an inner-city area in Switzerland. Epidemiol. Infect. 2002, 128, 107–109. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Major, A.; Schweighauser, A.; Francey, T. Increasing Incidence of Canine Leptospirosis in Switzerland. Int. J. Environ. Res. Public Health 2014, 11, 7242-7260. https://doi.org/10.3390/ijerph110707242
Major A, Schweighauser A, Francey T. Increasing Incidence of Canine Leptospirosis in Switzerland. International Journal of Environmental Research and Public Health. 2014; 11(7):7242-7260. https://doi.org/10.3390/ijerph110707242
Chicago/Turabian StyleMajor, Andrea, Ariane Schweighauser, and Thierry Francey. 2014. "Increasing Incidence of Canine Leptospirosis in Switzerland" International Journal of Environmental Research and Public Health 11, no. 7: 7242-7260. https://doi.org/10.3390/ijerph110707242
APA StyleMajor, A., Schweighauser, A., & Francey, T. (2014). Increasing Incidence of Canine Leptospirosis in Switzerland. International Journal of Environmental Research and Public Health, 11(7), 7242-7260. https://doi.org/10.3390/ijerph110707242




