Protective Roles of Sodium Selenite against Aflatoxin B1-Induced Apoptosis of Jejunum in Broilers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Clinical Signs and Body Weight
| Composition | Content (%) | Nutrient | Content (%) |
|---|---|---|---|
| corn | 51.95 | crude protein (CP) | 21.50 |
| soybean | 39.50 | Methionine (Met) | 0.50 |
| rapeseed oil | 4.10 | calcium (Ca) | 1.00 |
| D,L-metionine | 0.20 | all phosphorus (P) | 0.70 |
| calcium hydrogen phosphate | 1.85 | Methionine + cysteine (Met+Cys) | 0.84 |
| calcium carbonate | 1.30 | lysine (Lys) | 1.15 |
| sodium chloride | 0.40 | Threonine (Thr) | 0.83 |
| trace element premix a | 0.50 | metabolizable energy (ME) (MJ/Kg) | 29.90 |
| choline | 0.17 | ||
| multivitamins b | 0.03 | ||
| total | 100 |
2.3. TUNEL Immunohistochemistry
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Statistical Analysis
3. Results
3.1. Clinical Signs and Body Weight
| Time | Treatments | |||
|---|---|---|---|---|
| Control | AFB1 | +Se | AFB1 + Se | |
| 1 day (n = 60) | 41.56 ± 3.36 | 41.81 ± 3.23 | 41.59 ± 3.26 | 41.86 ± 3.02 |
| 7day (n = 60) | 121.81 ± 7.45 | 120.66 ± 7.38 | 120.56 ± 8.77 | 121.93 ± 6.84 |
| 14day (n = 42) | 328.62 ± 17.36 | 323.30 ± 22.26 | 324.51 ± 20.14 | 327.49 ± 20.70 |
| 21day (n = 24) | 695.71 ± 37.77 a | 672.25 ± 37.95 b | 681.80 ± 26.84 | 694.44 ± 41.85 |
3.2. TUNEL Immunohistochemistry
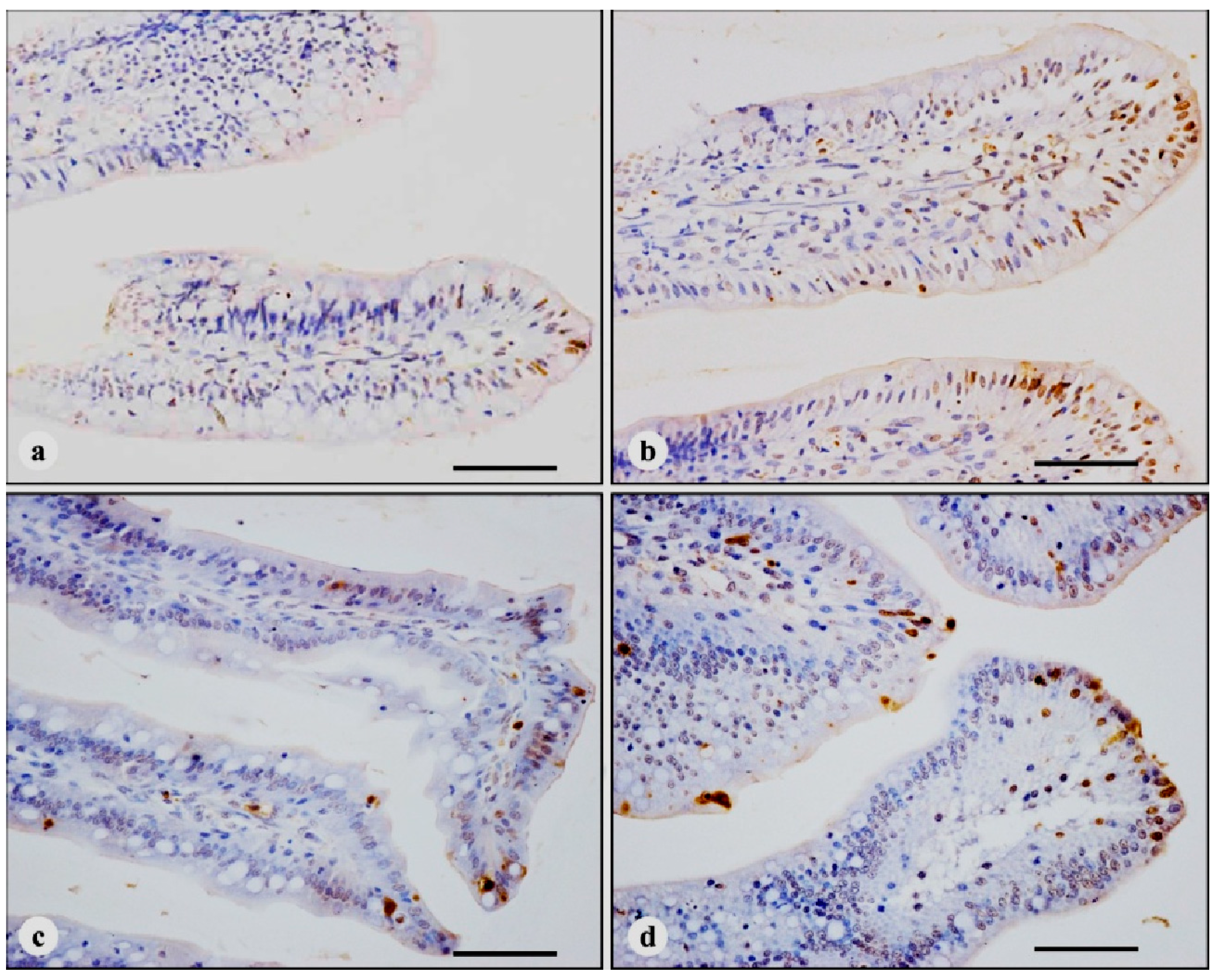
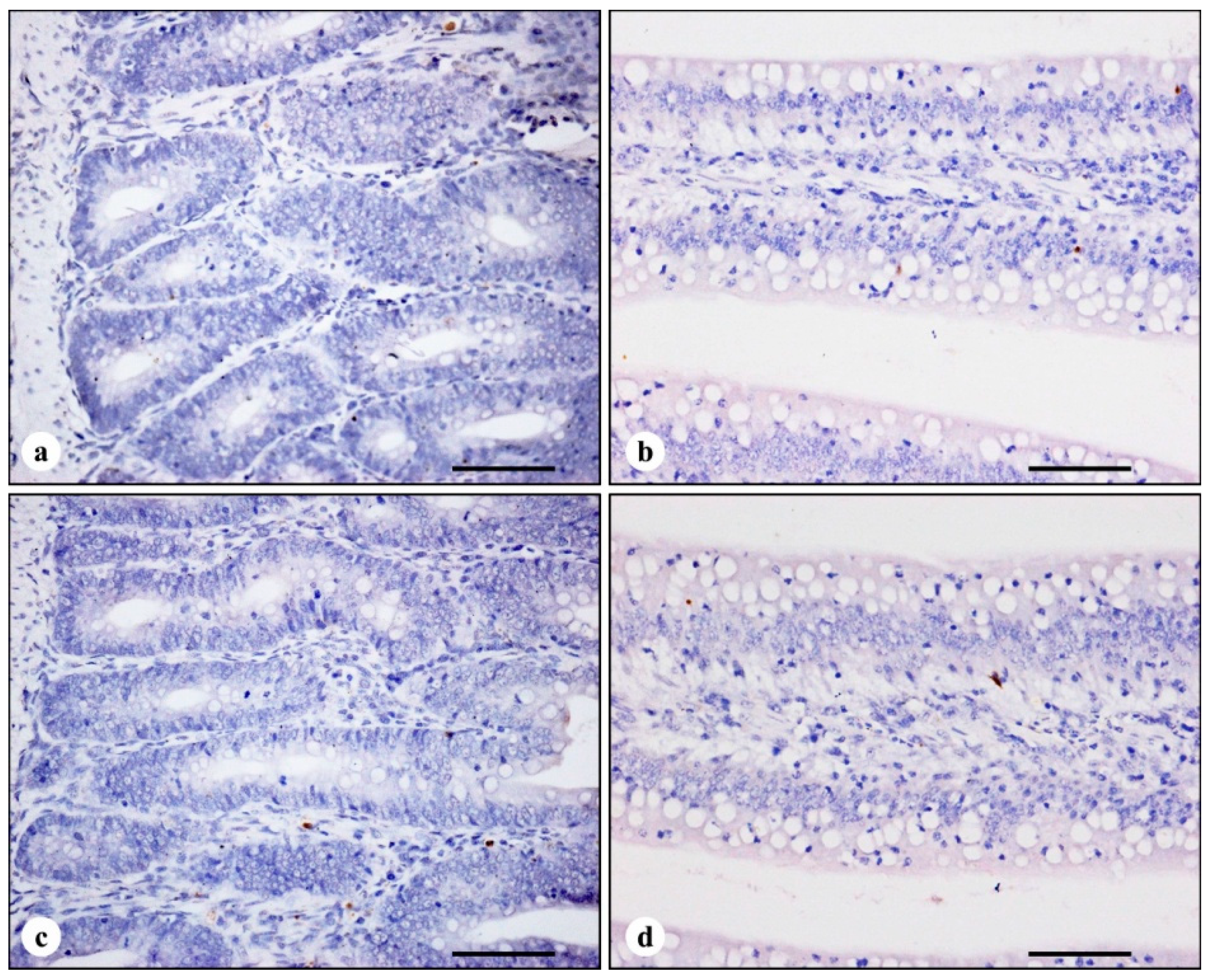
| Time | Treatments | |||
|---|---|---|---|---|
| control | AFB1 | + Se | AFB1 + Se | |
| 7 day | 5.200 ± 0.227 C | 18.900 ± 0.273 A B | 5.533 ± 0.184 C | 11.767 ± 0.243 B B |
| 14 day | 5.600 ± 0.212 C | 18.833 ± 0.322 A B | 5.267 ± 0.33 C | 13.300 ± 0.437 B A |
| 21 day | 5.100 ± 0.175 C | 19.767 ± 0.493 A A | 5.567 ± 0.233 C | 12.100 ± 0.175 B A |
3.3. Quantitative Real-Time PCR (qRT-PCR)
| Item | Time | Treatments | |||
|---|---|---|---|---|---|
| Control | AFB1 | + Se | AFB1 + Se | ||
| Bax | 7 day | 1.003 ± 0.054 B | 1.218 ± 0.028 A b | 0.936 ± 0.035 B | 1.024 ± 0.019 B |
| 14 day | 1.001 ± 0.037 B | 1.235 ± 0.020 A b | 0.948 ± 0.019 Bb | 1.040 ± 0.014 Ba b | |
| 21 day | 1.003 ± 0.054 B | 1.302 ± 0.026 A a | 0.954 ± 0.030 B | 0.993 ± 0.013 B a | |
| Bcl-2 | 7 day | 1.001 ± 0.027 Aa | 0.748 ± 0.009 B | 0.940 ± 0.013 Ab | 0.923 ± 0.014 Ab a |
| 14 day | 1.001 ± 0.027 Aa | 0.770 ± 0.020 B | 0.972 ± 0.025 A | 0.894 ± 0.024 Ab | |
| 21 day | 1.001 ± 0.039 A | 0.757 ± 0.018 Bc | 0.976 ± 0.016 ACa | 0.857 ± 0.031 BCb b | |
| Bcl-2/Bax | 7 day | 1.001 ± 0.030 Aa | 0.615 ± 0.013 B | 1.007 ± 0.050 Aa | 0.902 ± 0.006 Ab |
| 14 day | 1.003 ± 0.054 Aa | 0.623 ± 0.008 D | 1.025 ± 0.027 AC | 0.860 ± 0.029 ABb | |
| 21 day | 1.002 ± 0.042 Aa | 0.582 ± 0.026 B | 1.025 ± 0.031 Aa | 0.863 ± 0.042 Ab | |
| Caspase-3 | 7 day | 1.000 ± 0.016 B | 1.247 ± 0.023 Aa b | 1.029 ± 0.027 B | 1.166 ± 0.017 Ab b |
| 14 day | 1.003 ± 0.054 C | 1.302 ± 0.009 A a | 1.036 ± 0.050 BCb | 1.195 ± 0.014 ABa | |
| 21 day | 1.002 ± 0.040 B | 1.305 ± 0.010 Aa a | 0.995 ± 0.021 B | 1.214 ± 0.016 Ab a | |
4. Discussions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Massey, T.E.; Stewart, R.K.; Daniels, J.M.; Liu, L. Biochemical and molecular aspects of mammalian susceptibility to aflatoxin B1 carcinogenicity. Exp. Biol. Med. 1995, 208, 213–227. [Google Scholar] [CrossRef]
- McLean, M.; Dutton, M.F. Cellular interactions and metabolism of aflatoxin: An update. Pharmacol. Ther. 1995, 65, 163–192. [Google Scholar] [CrossRef] [PubMed]
- Ricordy, R.; Gensabella, G.; Cacci, E.; Augusti-Tocco, G. Impairment of cell cycle progression by aflatoxin B1 in human cell lines. Mutagenesis 2002, 17, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, K.K.; Sadana, J.R. Sequential pathology of experimental aflatoxicosis in quail and the effect of selenium supplementation in modifying the disease process. Mycopathologia 2004, 157, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Kim, J.E.; Coulombe, R., Jr. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef]
- Yunus, A.W.; Ghareeb, K.; Abd-El-Fattah, A.A.M.; Twaruzek, M.; Böhm, J. Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult. Sci. 2011, 90, 1683–1689. [Google Scholar] [CrossRef]
- Guo, S.; Shi, D.; Liao, S.; Su, R.; Lin, Y.; Pan, J.; Tang, Z. Influence of selenium on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B1. Biol. Trace Elem. Res. 2012, 146, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Guo, S.; Liao, S.; Su, R.; Pan, J.; Lin, Y.; Tang, Z. Influence of selenium on hepatic mitochondrial antioxidant capacity in ducklings intoxicated with aflatoxin B1. Biol. Trace Elem. Res. 2012, 145, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.L.; Yang, Z.B.; Yang, W.R.; Jiang, S.Z.; Zhang, G.G.; Johnston, S.L.; Chi, F. Toxicity of increasing aflatoxin B1 concentrations from contaminated corn with or without clay adsorbent supplementation in ducklings. Poult. Sci. 2013, 92, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.H.; Ferreira, F.L.; Da Silva, V.N.; Aquino, S.; Corrêa, B. Effects of aflatoxin B1 and fumonisin B1 on the viability and induction of apoptosis in rat primary hepatocytes. Int. J. Mol. Sci. 2010, 11, 1944–1955. [Google Scholar] [CrossRef]
- Brahmi, D.; Bouaziz, C.; Ayed, Y.; Ben Mansour, H.; Zourgui, L.; Bacha, H. Chemopreventive effect of cactus Opuntia ficus indica on oxidative stress and genotoxicity of aflatoxin B1. Nutr. MeTab. (Lond) 2011, 8. [Google Scholar] [CrossRef]
- Deng, S.X.; Tian, L.X.; Liu, F.J.; Jin, S.J.; Liang, G.Y.; Yang, H.J.; Du, Z.Y.; Liu, Y.J. Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus× O. aureus) during long-term dietary exposure. Aquaculture 2010, 307, 233–240. [Google Scholar] [CrossRef]
- Raj, H.G.; Kohli, E.; Rohil, V.; Dwarakanath, B.S.; Parmar, V.S.; Malik, S.; Adhikari, J.S.; Tyagi, Y.K.; Goel, S.; Gupta, K.; et al. Acetoxy-4-methylcoumarins confer differential protection from aflatoxin B1-induced micronuclei and apoptosis in lung and bone marrow cells. Mutat. Res. 2001, 494, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Lu, H.Y.; Li, Z.Y.; Bian, Q.; Qiu, L.L.; Li, Z.; Liu, Q.; Li, J.; Wang, X.; Wang, S.L. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 2012, 300, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Shu, G.; Peng, X.; Fang, J.; Cui, H.M.; Chen, J.; Wang, F.Y.; Chen, Z.L.; Zuo, Z.C.; Deng, J.L.; et al. Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1. Food Chem. Toxicol. 2013, 59, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Shu, G.; Peng, X.; Fang, J.; Chen, K.J.; Cui, H.M.; Chen, Z.L.; Zuo, Z.C.; Deng, J.L.; Geng, Y.; Lai, W.M. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int. J. Environ. Res. Public Health 2013, 10, 2834–2844. [Google Scholar] [CrossRef] [PubMed]
- De Conto, C.; Oevermann, A.; Burgener, I.A.; Doherr, M.G.; Blum, J.W. Gastrointestinal tract mucosal histomorphometry and epithelial cell proliferation and apoptosis in neonatal and adult dogs. J. Anim. Sci. 2010, 88, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Wilson, J.W.; Booth, C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem. Cells 1997, 15, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Gores, G.J. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am. J. Physiol. 1997, 273, G1174–G1188. [Google Scholar] [PubMed]
- Combs, G.F.; Clark, L.C.; Turnbull, B.W. An analysis of cancer prevention by selenium. Biofactors 2001, 14, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium 1, 2. Annu. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Combs, G.F., Jr. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- McKenzie, R.C.; Rafferty, T.S.; Beckett, G.J. Selenium: An essential element for immune function. Immunol. Today 1998, 19, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Perozo, F.; Rivera, S. Effect of Aflatoxin B1 exposure and selenium supplementation on immune response in broilers. Indian Vet. J. 2003, 80, 1218–1221. [Google Scholar]
- Zhang, S.Q.; Peng, X.; Fang, J.; Cui, H.M.; Zuo, Z.C.; Chen, Z.L. Effects of aflatoxin B1 exposure and sodium selenite supplementation on the histology, cell proliferation and cell cycle of jejunum in broilers. Biol. Trace Elem. Res. 2014, 160, 32–40. [Google Scholar] [CrossRef]
- Kaoud, H.A. Innovative methods for the amelioration of aflatoxin (afb1) effect in broiler chicks. Sci. J. Appl. Res. 2013, 1, 15–19. [Google Scholar]
- Peng, X.; Cui, Y.; Cui, W.; Deng, J.; Cui, H. The decrease of relative weight, lesions, and apoptosis of bursa of Fabricius induced by excess dietary selenium in chickens. Biol. Trace Elem. Res. 2009, 131, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hamidu, J.A.; Uddin, Z.; Li, M.; Fasenko, G.M.; Guan, L.L.; Barreda, D.R. Broiler egg storage induces cell death and influences embryo quality. Poult. Sci. 2011, 90, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Dusanic, D.; Bencina, D.; Oven, I.; Cizelj, I.; Bencina, M.; Narat, M. Mycoplasma synoviae induces upregulation of apoptotic genes, secretion of nitric oxide and appearance of an apoptotic phenotype in infected chicken chondrocytes. Vet. Res. 2012, 43. [Google Scholar] [CrossRef]
- Iqbal, M.; Philbin, V.J.; Smith, A.L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005, 104, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.J.; Wu, C.X.; Gong, L.M.; Song, T.; Wu, H.; Zhang, L.Y. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poultry Sci. 2012, 91, 2532–2539. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef] [PubMed]
- Polzar, B.; Zanotti, S.; Stephan, H.; Rauch, F.; Peitsch, M.C.; Irmler, M.; Tschopp, J.; Mannherz, H.G. Distribution of deoxyribonuclease I in rat tissues and its correlation to cellular turnover and apoptosis (programmed cell death). Eur. J. Cell. Biol. 1994, 64, 200–210. [Google Scholar] [PubMed]
- Barnard, J.A.; Warwick, G.J.; Gold, L.I. Localisation of transforming growth factor beta isoforms in the normal murine small intestine and colon. Gastroenterology 1993, 105, 67–73. [Google Scholar]
- Probstmeier, R.; Martini, R.; Tacke, R.; Schachner, M. Expression of the adhesion molecules L1, N-CAM and J1/tenascin during development of the murine small intestine. Differentiation 1990, 44, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Probstmeier, R.; Martini, R.; Schachner, M. Expression of J1/tenascin in the crypt-villus unit of adult mouse small intestine: Implications for its role in epithelial cell shedding. Development 1990, 109, 313–321. [Google Scholar] [PubMed]
- Shibahara, T.; Sato, N.; Waguri, S.; Iwanaga, T.; Nakahara, A.; Fukutomi, H.; Uchiyama, Y. The fate of effete epithelial cells at the villus tip of the human small intestine. Arch. Histol. Cytol. 1995, 58, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Negoescu, A.; Guillermet, C.; Lorimier, P.; Brambilla, E.; Labat-Moleur, F. Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed. Pharmacother. 1998, 52, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Deng, J.; Wu, B. The association between splenocyte apoptosis and alterations of Bax, Bcl-2, Caspase-3 mRNA expression, and oxidative stress induced by dietary nickel chloride in broilers. Int. J. Environ. Res. Public Health 2013, 10, 7310–7326. [Google Scholar] [CrossRef] [PubMed]
- Benard, O.; Madesh, M.; Anup, R.; Balasubramanian, K.A. Apoptotic process in the monkey small intestinal epithelium: 1. Association with glutathione level and its efflux. Free Radic. Biol. Med. 1999, 26, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Westcarr, S.; Farshori, P.; Wyche, J.; Anderson, W.A. Apoptosis and differentiation in the crypt-villus unit of the rat small intestine. J. Submicrosc. Cytol. Pathol. 1999, 31, 15–30. [Google Scholar] [PubMed]
- Bjerknes, M.; Cheng, H. Gastrointestinal stem cells. II. Intestinal stem cells. Am. J. Physiol. Gastrointest Liver Physiol. 2005, 289, G381–G387. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Coates, P.J.; Ansari, B.; Hopwood, D. Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J. Cell. Sci. 1994, 107, 3569–3577. [Google Scholar] [PubMed]
- Karan, S.M. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 1999, 4, D286–D298. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.X.; Ou, J.S.; Li, Y.; Su, J.J.; Ou, C.; Yang, C.; Yue, H.F.; Ban, K.C. Dynamic expression of apoptosis-related genes during development of laboratory hepatocellular carcinoma and its relation to apoptosis. World J. Gastroenterol. 2005, 11, 4740–4744. [Google Scholar] [PubMed]
- Van Vleet, T.R.; Watterson, T.L.; Klein, P.J.; Coulombe, R.A., Jr. Aflatoxin B1 alters the expression of p53 in cytochrome P450-expressing human lung cells. Toxicol. Sci. 2006, 89, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. Biol. Chem. 2006, 387, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.; Abdel-Aziem, S.H.; Abdel-Wahhab, M.A. Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutat. Res. 2011, 723, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, L.; Yildirim, A.; Agar, G. The antioxidant effects of vitamin A, C, and E on aflatoxin B1-induced oxidative stress in human lymphocytes. Toxicol. Ind. Health 2009, 25, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kanbur, M.; Eraslan, G.; Sarıca, Z.S.; Aslan, Ö. The effects of evening primrose oil on lipid peroxidation induced by subacute aflatoxin exposure in mice. Food Chem. Toxicol. 2011, 49, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Mechanisms involved in the generation of free radicals. Pathol. Biol. 1996, 44, 6–13. [Google Scholar] [PubMed]
- Choi, K.C.; Chung, W.T.; Kwon, J.K.; Yu, J.Y.; Jang, Y.S.; Park, S.M.; Lee, S.Y.; Lee, J.C. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food Chem. Toxicol. 2010, 48, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Yener, Z.; Celik, I.; Ilhan, F.; Bal, R. Effects of Urtica dioica L. seed on lipid peroxidation, antioxidants and liver pathology in aflatoxin-induced tissue injury in rats. Food Chem. Toxicol. 2009, 47, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Steinbrenner, H.; Sies, H. Selenium, oxidative stress, and health aspects. Mol. Aspects. Med. 2005, 26, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Madesh, M.; Balasubramanian, K.A. Apoptosis in the intestinal epithelium: Its relevance in normal and pathophysiological conditions. J. Gastroenterol. Hepatol. 2000, 15, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Dejean, L.M.; Martinez-Caballero, S.; Manon, S.; Kinnally, K.W. Regulation of the mitochondrial apoptosis-induced channel, MAC, by Bcl-2 family proteins. Biochim Biophys Acta. 2006, 1762, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell. Biochem. 2004, 256–257, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Samali, A.; Orrenius, S. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 2000, 29, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.F.; Tome, A.R.; Rao, V.S. Inhibition by the bioflavonoid ternatin of Aflatoxin B1-induced lipid peroxidation in rat liver. J. Pharm. Pharmacol. 1999, 51, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Ebert, R.; Ulmer, M.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Stopper, H.; Schupp, N.; Kassem, M.; Jakob, F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem. Cells 2006, 24, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhang, S.P.; Liu, C.W.; Cai, Y.Q. The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK1 cells. Toxicol. In Vitro 2009, 23, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Zhang, S.; Fang, J.; Cui, H.; Zuo, Z.; Deng, J. Protective Roles of Sodium Selenite against Aflatoxin B1-Induced Apoptosis of Jejunum in Broilers. Int. J. Environ. Res. Public Health 2014, 11, 13130-13143. https://doi.org/10.3390/ijerph111213130
Peng X, Zhang S, Fang J, Cui H, Zuo Z, Deng J. Protective Roles of Sodium Selenite against Aflatoxin B1-Induced Apoptosis of Jejunum in Broilers. International Journal of Environmental Research and Public Health. 2014; 11(12):13130-13143. https://doi.org/10.3390/ijerph111213130
Chicago/Turabian StylePeng, Xi, Shengqiang Zhang, Jing Fang, Hengmin Cui, Zhicai Zuo, and Junliang Deng. 2014. "Protective Roles of Sodium Selenite against Aflatoxin B1-Induced Apoptosis of Jejunum in Broilers" International Journal of Environmental Research and Public Health 11, no. 12: 13130-13143. https://doi.org/10.3390/ijerph111213130
APA StylePeng, X., Zhang, S., Fang, J., Cui, H., Zuo, Z., & Deng, J. (2014). Protective Roles of Sodium Selenite against Aflatoxin B1-Induced Apoptosis of Jejunum in Broilers. International Journal of Environmental Research and Public Health, 11(12), 13130-13143. https://doi.org/10.3390/ijerph111213130




