A New Anthracene Derivative from Marine Streptomyces sp. W007 Exhibiting Highly and Selectively Cytotoxic Activities
Abstract
:1. Introduction
2. Results and Discussion
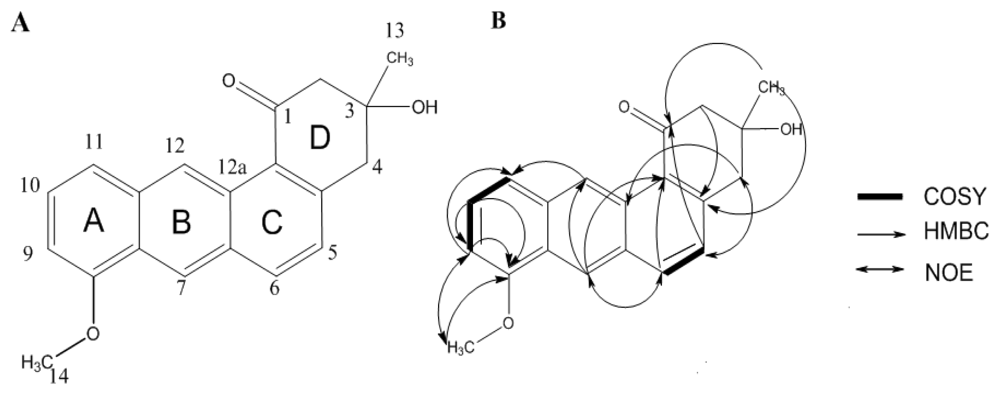
2.1. Structure Analysis of Compound 1
2.2. Cytotoxicity and Antifungal Activities
3. Experimental Section
3.1. General Experimental Procedures
3.2. Strain and Medium for Isolation and Fermentation
3.3. Fermentation
3.4. Extraction and Separation
3.5. Cytotoxicity Tests and Antifungal Activities
4. Conclusions
Supporting Information
marinedrugs-09-01502-s001.pdfAcknowledgments
- Samples Availability: Available from the authors.
References
- Newman, DJ; Cragg, GM; Snader, KM. Natural Products as sources of new drugs over the period 1981–2002. J. Nat. Prod 2003, 66, 1022–1037. [Google Scholar]
- Solanki, R; Khanna, M; Lal, R. Bioactive compounds from marine actinomycetes. Indian J. Microbiol 2008, 48, 410–431. [Google Scholar]
- Wu, SJ; Fotso, S; Li, F; Qin, S; Laatsch, H. Amorphane Sesquiterpenes from a Marine Streptomyces sp. J. Nat. Prod 2007, 70, 304–306. [Google Scholar]
- Williams, PG; Asolkar, RN; Kondratyuk, T; Pezzuto, JM; Jensen, PR; Fenical, W. Saliniketals A and B, Bicyclic Polyketides from the Marine Actinomycete Salinispora arenicola. J. Nat. Prod 2006, 70, 83–88. [Google Scholar]
- Sujatha, P; Bapi Raju, KVVSN; Ramana, T. Studies on a new marine streptomycete BT-408 producing polyketide antibiotic SBR-22 effective against methicillin resistant Staphylococcus aureus. Microbiol. Res 2005, 160, 119–126. [Google Scholar]
- Miller, ED; Kauffman, CA; Jensen, PR; Fenical, W. Piperazimycins: Cytotoxic Hexadepsipeptides from a Marine-Derived Bacterium of the Genus Streptomyces. J. Org. Chem 2006, 72, 323–330. [Google Scholar]
- Moore, BS; Trischman, JA; Seng, D; Kho, D; Jensen, PR; Fenical, W. Salinamides, antiinflammatory depsipeptides from a marine streptomycete. J. Org. Chem 1999, 64, 1145–1150. [Google Scholar]
- Dharmaraj, S. Marine Streptomyces as a novel source of bioactive substances. World J. Microbiol. Biotechnol 2010, 26, 2123–2139. [Google Scholar]
- Li, F; Maskey, RP; Qin, S; Sattler, I; Fiebig, HH; Maier, A; Zeeck, A; Laatsch, H. Chinikomycins A and B: Isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. Isolate M045. J. Nat. Prod 2005, 68, 349–353. [Google Scholar]
- Maskey, RP; Li, FC; Song, Q; Fiebig, HH; Laatsch, H. Chandrananimycins A–C: Production of novel anticancer antibiotics from a marine Actinomadura sp. Isolate M048 by variation of medium composition and growth conditions. J. Antibiot 2003, 56, 622–629. [Google Scholar]
- Wu, SJ; Fotso, S; Li, F; Qin, S; Kelter, G; Fiebig, HH; Laatsch, H. N-carboxamido-staurosporine and selina-4(14),7(11)-diene-8,9-diol, new metabolites from a marine Streptomyces sp. J. Antibiot 2006, 59, 331–337. [Google Scholar]
- Ding, L; Pfoh, R; Ruhl, S; Qin, S; Laatsch, H. T-Muurolol sesquiterpenes from the marine Streptomyces sp. M491 and revision of the configuration of previously reported amorphanes. J. Nat. Prod 2008, 72, 99–101. [Google Scholar]
- Chiriboga, X; Gilardoni, G; Magnaghi, I; Finzi, PV; Zanoni, G; Vidari, G. New anthracene derivatives from Coussarea macrophylla. J. Nat. Prod 2003, 66, 905–909. [Google Scholar]
- Beekman, AC; Barentsen, ARW; Woerdenbag, HJ; van Uden, W; Pras, N; Konings, AWT; El-Feraly, FS; Galal, AM; Wikström, HV. Stereochemistry-dependent cytotoxicity of some artemisinin derivatives. J. Nat. Prod 1997, 60, 325–330. [Google Scholar]
- Iwasa, K; Moriyasu, M; Yamori, T; Turuo, T; Lee, DU; Wiegrebe, W. In vitro cytotoxicity of the protoberberine-type alkaloids. J. Nat. Prod 2001, 64, 896–898. [Google Scholar]
- Kouam, SF; Yapna, DB; Krohn, K; Ngadjui, BT; Ngoupayo, J; Choudhary, MI; Schulz, B. Antimicrobial prenylated anthracene derivatives from the leaves of Harungana madagascariensis. J. Nat. Prod 2007, 70, 600–603. [Google Scholar]
| Position | d (J in Hz) | δC |
|---|---|---|
| 1 | 198.76 | |
| 2 | 2.97 (2H, dd, 10, 15) | 54.28 |
| 3 | 70.55 | |
| 4 | 3.35 (2H, dd, 15.0, 20.0) | 45.41 |
| 4a | 145.61 | |
| 5 | 7.37 (1H, d, 8.6) | 127.37 |
| 6 | 8.26 (1H, d, 8.6) | 135.19 |
| 6a | 130.69 | |
| 7 | 8.78 (1H, s) | 121.52 |
| 7a | 124.29 | |
| 8 | 155.38 | |
| 9 | 6.89 (1H, d, 7.55) | 102.5 |
| 10 | 7.45 (1H, t, 8.25, 7.45) | 126.35 |
| 11 | 7.65 (1H, d, 8.25) | 121.13 |
| 11a | 134.54 | |
| 12 | 10.10 (1H, s) | 125.17 |
| 12a | 128.74 | |
| 12b | 125.46 | |
| 13 | 1.46 (3H, s) | 28.47 |
| 14 | 4.09 (3H, s) | 55.10 |
| Cancer cell line | Rate of inhibition of sample (%) | Concentration (M)
| ||||
|---|---|---|---|---|---|---|
| 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | ||
| human leukemic cells line HL-60 | Rate of inhibition of compound 1 | 0 | 0 | 0 | 0 | 0 |
| Rate of inhibition of adriamycin | 100 | 88.5 | 89.5 | 88.2 | 0 | |
| human hepatoma cell line BEL-7402 | Rate of inhibition of compound 1 | 37.5 | 37.0 | 25.7 | 19.5 | 0 |
| Rate of inhibition of adriamycin | 80.9 | 85.7 | 63.4 | 32.8 | 15.5 | |
| human lung adenocarcinoma cell line A549 | Rate of inhibition of compound 1 | 65.5 | 62.8 | 61.8 | 47.8 | 48.8 |
| Rate of inhibition of adriamycin | 100 | 61.8 | 50.8 | 21.4 | 4.3 | |
| Microbial activities | The radius of the zone of inhibition (mm) |
|---|---|
| Blank | 6 |
| Antifungal (Mf) | 8 |
| Antifungal (Cl) | 9 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, H.; Wang, H.; Cui, H.; Li, Z.; Xie, Z.; Pu, Y.; Li, F.; Qin, S. A New Anthracene Derivative from Marine Streptomyces sp. W007 Exhibiting Highly and Selectively Cytotoxic Activities. Mar. Drugs 2011, 9, 1502-1509. https://doi.org/10.3390/md9091502
Zhang H, Wang H, Cui H, Li Z, Xie Z, Pu Y, Li F, Qin S. A New Anthracene Derivative from Marine Streptomyces sp. W007 Exhibiting Highly and Selectively Cytotoxic Activities. Marine Drugs. 2011; 9(9):1502-1509. https://doi.org/10.3390/md9091502
Chicago/Turabian StyleZhang, Hongyu, Hongpeng Wang, Hongli Cui, Zonggang Li, Zeping Xie, Yang Pu, Fuchao Li, and Song Qin. 2011. "A New Anthracene Derivative from Marine Streptomyces sp. W007 Exhibiting Highly and Selectively Cytotoxic Activities" Marine Drugs 9, no. 9: 1502-1509. https://doi.org/10.3390/md9091502
APA StyleZhang, H., Wang, H., Cui, H., Li, Z., Xie, Z., Pu, Y., Li, F., & Qin, S. (2011). A New Anthracene Derivative from Marine Streptomyces sp. W007 Exhibiting Highly and Selectively Cytotoxic Activities. Marine Drugs, 9(9), 1502-1509. https://doi.org/10.3390/md9091502






