In Vivo and in Vitro Anti-Inflammatory Activity of Neorogioltriol, a New Diterpene Extracted from the Red Algae Laurencia glandulifera
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Neorogioltriol on Carrageenan-Induced Paw Edema in Rats
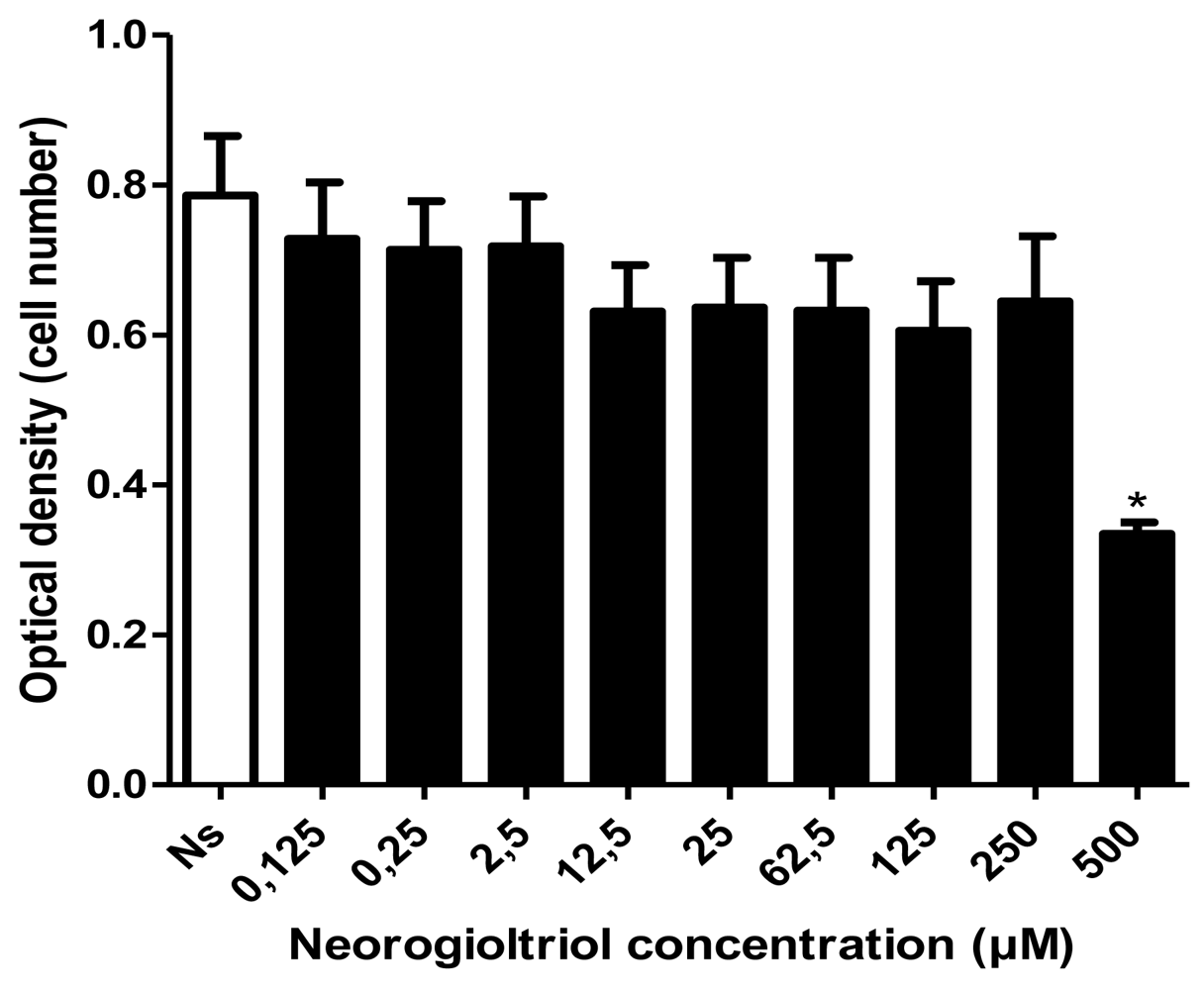
2.2. Cell Viability
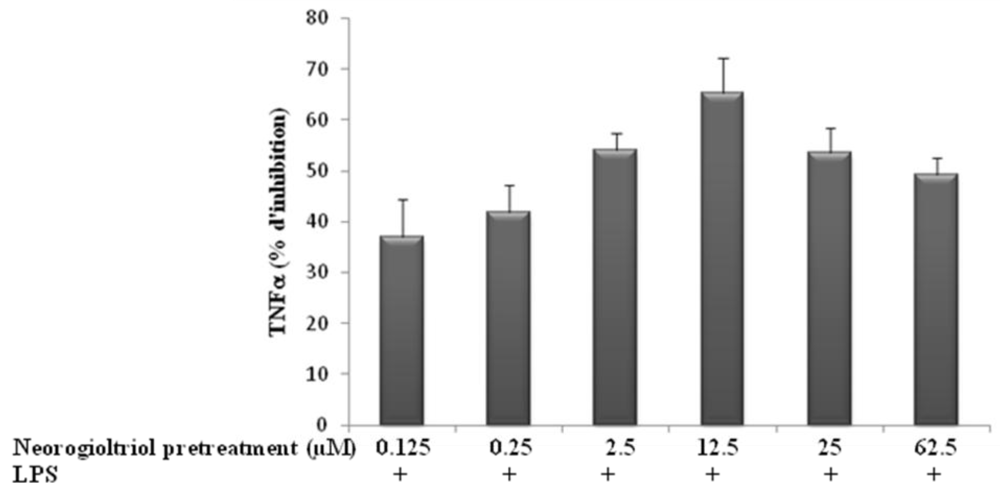
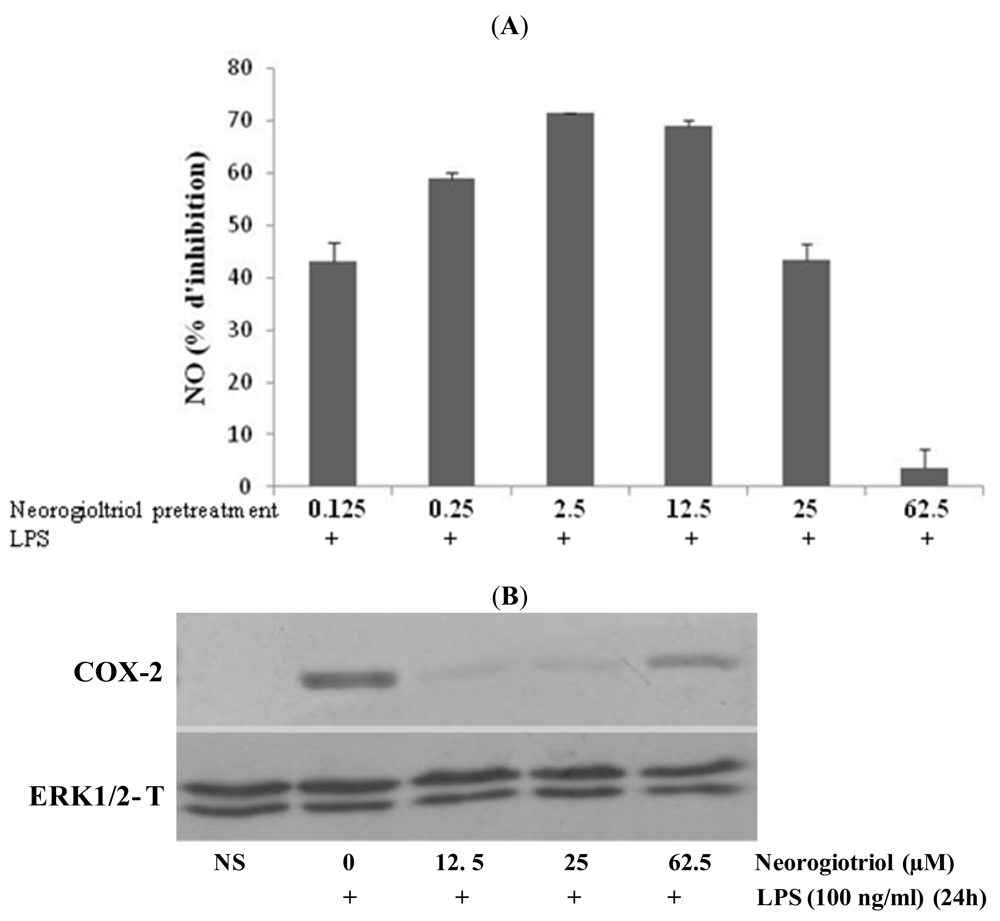
2.3. Effect of Neorogioltriol on TNFα Release, NO Production and COX-2 Expression in LPS-Stimulated Raw264.7 Cells
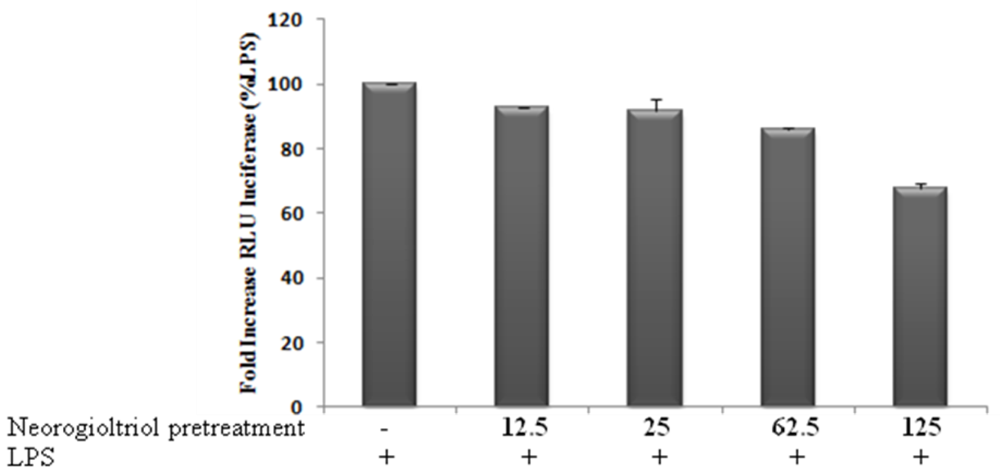
2.4. Effects of Neorogioltriol on NF-κB Transactivation in LPS-Stimulated Raw264.7 Cells
2.5. Effect of Neorogioltriol on MAPK in LPS-Stimulated Raw264.7 Cells
3. Experimental Section
3.1. Isolation of Neorogioltriol
3.2. Carrageenan-Induced Paw Edema
3.3. Reagents and Antibodies
3.4. Cells Culture
3.5. Cytotoxicity Test
3.6. Transfection and Luciferase Assay
3.7. Western Blot Analysis
3.8. Measurements of Nitrite Production and Cytokine Assays
3.9. Statistical Analysis
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Balunas, MJ; Kinghorn, AD. Drug discovery from medicinal plants. Life Sci 2005, 78, 431–441. [Google Scholar]
- Jha, RK; Zi-rong, X. Biomedical compounds from Marine organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar]
- Mayer, AM; Rodriguez, AD; Berlinck, RG; Hamann, MT. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar]
- Salminen, A; Lehtonen, M; Suuronen, T; Kaarniranta, K; Huuskonen, J. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell Mol. Life Sci 2008, 65, 2979–2999. [Google Scholar]
- Folmer, F; Jaspars, M; Dicato, M; Diederich, M. Marine natural products as targeted modulators of the transcription factor NF-κB. Biochem. Pharmacol 2008, 75, 603–617. [Google Scholar]
- Konig, GM; Wright, AD. Sesquiterpene content of the antibacterial dichloromethane extract of the marine red alga Laurencia obtusa. Planta Med 1997, 63, 186–187. [Google Scholar]
- Iliopoulou, D; Mihopoulos, N; Vagias, C; Papazafiri, P; Roussis, V. Novel cytotoxic brominated diterpenes from the red alga Laurencia obtusa. J. Org. Chem 2003, 68, 7667–7674. [Google Scholar]
- Kladi, M; Vagias, C; Stavri, M; Rahman, MM; Gibbons, S; Roussis, V. C15 acetogenins with antistaphylococcal activity from the red alga Laurencia glandulifera. Phytochem. Lett 2008, 1, 31–36. [Google Scholar]
- El Sayed, KA; Dunbar, DC; Perry, TL; Wilkins, SP; Hamann, MT; Greenplate, JT. Marine natural products as prototype insecticidal agents. J. Agric. Food Chem 1997, 45, 2735–2739. [Google Scholar]
- Vairappan, CS. Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomol. Eng 2003, 20, 255–259. [Google Scholar]
- Jung, WK; Choi, I; Oh, S; Park, SG; Seo, SK; Lee, SW; Lee, DS; Heo, SJ; Jeon, YJ; Je, JY; et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem. Toxicol 2009, 47, 293–297. [Google Scholar]
- Guzman, S; Gato, A; Calleja, JM. Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res 2001, 15, 224–230. [Google Scholar]
- Dar, A; Baig, HS; Saifullah, SM; Ahmad, VU; Yasmeen, S; Nizamuddin, M. Effect of seasonal variation on the anti-inflammatory activity of Sargassum wightii growing on the N. Arabian Sea coast of Pakistan. J. Exp. Mar. Biol. Ecol 2007, 351, 1–9. [Google Scholar]
- Jin, DQ; Lim, CS; Sung, JY; Choi, HG; Ha, I; Han, JS. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett 2006, 402, 154–158. [Google Scholar]
- Yoon, WJ; Ham, YM; Kim, KN; Park, SY; Lee, NH; Hyun, CG; Lee, WJ. Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage Raw264.7. J. Med. Plants Res 2009, 3, 1–8. [Google Scholar]
- Chatter, R; Kladi, M; Tarhouni, S; Maatoug, R; Kharrat, R; Vagias, C; Roussis, V. Neorogioltriol: A brominated diterpene with analgesic activity from Laurencia glandulifera. Phytochem. Lett 2009, 2, 25–28. [Google Scholar]
- Holsapple, MP; Schnur, M; Yim, GK. Pharmacological modulation of edema mediated by prostaglandin, serotonin and histamine. Agents Actions 1980, 10, 368–373. [Google Scholar]
- Di Rosa, M; Giroud, JP; Willoughby, DA. Studies of the acute inflammatory response induced in rat in different sites by carrageenan and turpentine. J. Pathol 1971, 104, 15–29. [Google Scholar]
- Garcia Leme, J; Hamamura, L; Leite, MP; Rocha e Silva, M. Pharmacological analysis of the acute inflammatory process induced in the rat’s paw by local injection of carrageenin and by heating. Br. J. Pharmacol 1973, 48, 88–96. [Google Scholar]
- Cunha, FQ; Poole, S; Lorenzetti, BB; Ferreira, SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol 1992, 107, 660–664. [Google Scholar]
- Salvemini, D; Wang, ZQ; Wyatt, PS; Bourdon, DM; Marino, MH; Manning, PT; Currie, MG. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol 1996, 118, 829–838. [Google Scholar]
- Omote, K; Hazama, K; Kawamata, T; Kawamata, M; Nakayaka, Y; Toriyabe, M; Namiki, A. Peripheral nitric oxide in carrageenan-induced inflammation. Brain Res 2001, 912, 171–175. [Google Scholar]
- de las Heras, B; Hortelano, S. Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy Drug Targets 2009, 8, 28–39. [Google Scholar]
- Xie, QW; Kashiwabara, Y; Nathan, C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem 1994, 269, 4705–4708. [Google Scholar]
- Chen, F; Kuhn, DC; Sun, SC; Gaydos, LJ; Demers, LM. Dependence and reversal of nitric oxide production on NF-κB in silica and lipopolysaccharide-induced macrophages. Biochem. Biophys. Res. Commun 1995, 214, 839–846. [Google Scholar]
- Schmedtje, JF, Jr; Ji, YS; Liu, WL; DuBois, RN; Runge, MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem 1997, 272, 601–608. [Google Scholar]
- Chen, CC; Wang, JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in Raw264.7 macrophages. Mol. Pharmacol 1999, 55, 481–488. [Google Scholar]
- Necela, BM; Su, W; Thompson, EA. Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor gamma and nuclear factor-κB in macrophages. Immunology 2008, 125, 344–358. [Google Scholar]
- Winter, CA; Risley, EA; Nuss, GW. Carrageenin-induced edema in hind paw of the rat as assay for anti-inflammatory drug. Proc. Soc. Exp. Biol. Med 1962, 111, 544–547. [Google Scholar]


| Times after carageenan injection (h) | 1 h | 3 h | 5 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Treatment | n | Dose (mg/kg) | EV | EI | EV | EI | EV | EI |
| (10−2 mL) | (%) | (10−2 mL) | (%) | (10−2 mL) | (%) | ||||
| Control | Control | 6 | - | 18.3 ± 2.7 | - | 56.3 ± 5.8 | - | 69 ± 7.7 | - |
| Tests | Neorogioltriol | 6 | 0.5 | 15.5 ± 3.1 | 15.3 | 42 ± 5.5 (*) | 33.3 | 49.1 ± 6.1 | 28.8 |
| 1 | 14.1 ± 3 (*) | 22.95 | 23.5 ± 3.7 (***) | 58 | 33 ± 3.5 (**) | 52.27 | |||
| References | Aspirin (ASA) | 6 | 300 | 16.5 ± 3.1 | 9.83 | 25.1 ± 6.7 (**) | 55.17 | 28.1 ± 2.2 (**) | 59.27 |
| Dexamethasone | 6 | 1 | 9.83 ± 1.8 | 47.54 | 39.3 ± 3.8 (***) | 42.5 | 32.5 ± 5.7 (***) | 52.86 | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chatter, R.; Othman, R.B.; Rabhi, S.; Kladi, M.; Tarhouni, S.; Vagias, C.; Roussis, V.; Guizani-Tabbane, L.; Kharrat, R. In Vivo and in Vitro Anti-Inflammatory Activity of Neorogioltriol, a New Diterpene Extracted from the Red Algae Laurencia glandulifera. Mar. Drugs 2011, 9, 1293-1306. https://doi.org/10.3390/md9071293
Chatter R, Othman RB, Rabhi S, Kladi M, Tarhouni S, Vagias C, Roussis V, Guizani-Tabbane L, Kharrat R. In Vivo and in Vitro Anti-Inflammatory Activity of Neorogioltriol, a New Diterpene Extracted from the Red Algae Laurencia glandulifera. Marine Drugs. 2011; 9(7):1293-1306. https://doi.org/10.3390/md9071293
Chicago/Turabian StyleChatter, Rim, Rym Ben Othman, Sameh Rabhi, Maria Kladi, Safa Tarhouni, Constantinos Vagias, Vassilios Roussis, Lamia Guizani-Tabbane, and Riadh Kharrat. 2011. "In Vivo and in Vitro Anti-Inflammatory Activity of Neorogioltriol, a New Diterpene Extracted from the Red Algae Laurencia glandulifera" Marine Drugs 9, no. 7: 1293-1306. https://doi.org/10.3390/md9071293
APA StyleChatter, R., Othman, R. B., Rabhi, S., Kladi, M., Tarhouni, S., Vagias, C., Roussis, V., Guizani-Tabbane, L., & Kharrat, R. (2011). In Vivo and in Vitro Anti-Inflammatory Activity of Neorogioltriol, a New Diterpene Extracted from the Red Algae Laurencia glandulifera. Marine Drugs, 9(7), 1293-1306. https://doi.org/10.3390/md9071293






