Assessment of Bioflocculant Production by Bacillus sp. Gilbert, a Marine Bacterium Isolated from the Bottom Sediment of Algoa Bay
Abstract
:1. Introduction
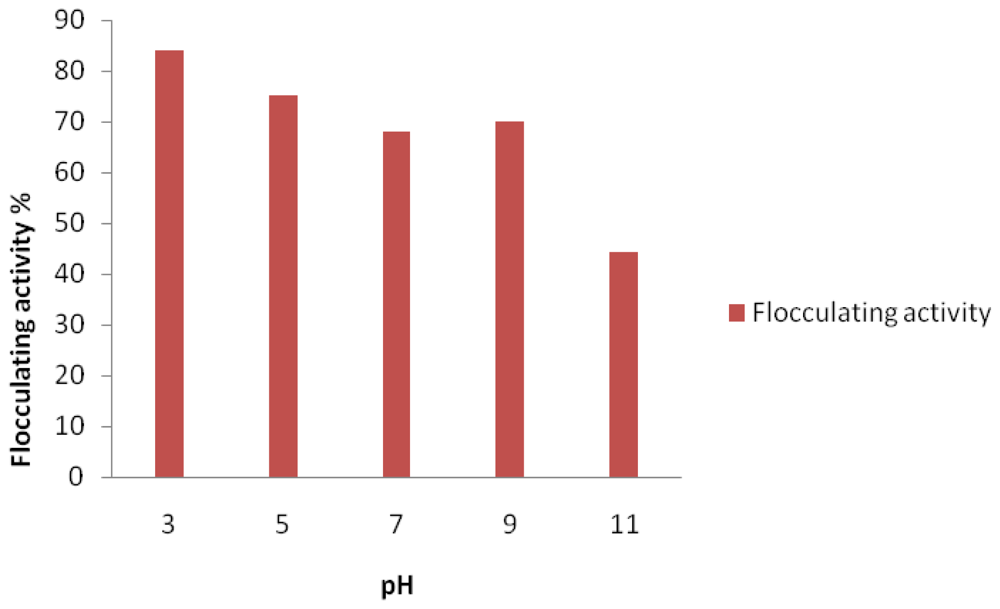
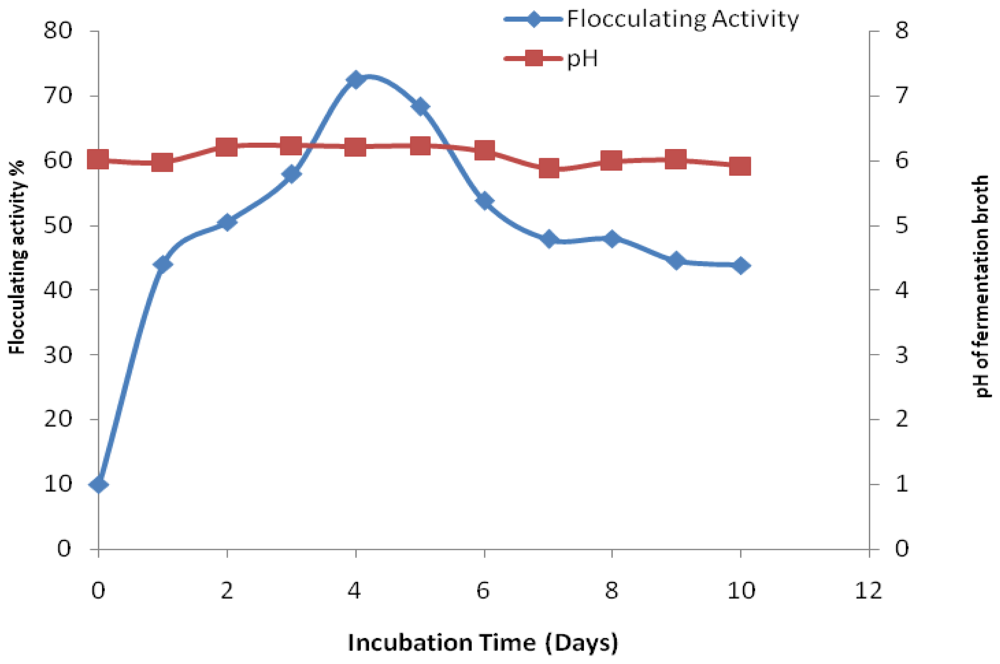
2. Results and Discussion
3. Experimental Section
3.1. Source of Bacteria and Culture Media
3.2. Screening for Flocculant Producing Microorganisms
3.3. Measurement of Flocculating Activity
3.4. Effects of Culture Conditions on Bioflocculant Production
3.5. Time Course Experiment
3.6. Bioflocculant Purification and Analyses
3.7. Identification of the Bioflocculant-Producing Bacterium
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- He, N; Li, Y; Chen, J; Lun, SY. Identification of a novel bioflocculant from a newly isolated Corynebacterium glutamicum. Biochem. Eng. J 2002, 11, 137–148. [Google Scholar]
- Salehizadeh, H; Shojaosadati, SA. Extracellular biopolymeric flocculants-recent trends and biotechnological importance. Biotechnol. Adv 2001, 19, 371–385. [Google Scholar]
- Takadi, H; Kodowaki, K. Flocculant production by Paecilomyces sp. taxanomic studies and culture conditions for production. Agric. Biol. Chem 1985, 49, 3151–3157. [Google Scholar]
- Kurane, R; Hatamochi, K; Kiyohara, T; Hirao, M; Taniguchi, Y. Production of a bioflocculant by Rhodococcus erythropolis S-1 grown on alcohols. Biosci. Biotechnol. Biochem 1994, 58, 428–429. [Google Scholar]
- Xia, S; Zhang, Z; Wang, X; Yang, A; Chen, L; Zhao, J; Leonard, D; Jaffrezic-Renault, N. Production and characterization of bioflocculant by Proteus mirabilis TJ-1. Bioresour. Technol 2008, 99, 6520–6527. [Google Scholar]
- Kwon, GS; Moon, SH; Hong, SD; Lee, HM; Kim, HS; Oh, HM; Yoon, BD. A novel flocculants biopolymer produced by Pestalotiopsis sp. KCTC 8637P. Biotechnol. Lett 1996, 18, 1459–1464. [Google Scholar]
- Deng, S; Yu, G; Ting, YP. Production of a bioflocculant by Aspergillus parasiticus and its application in dye removal. Colloids Surf. B Biointerfaces 2005, 44, 179–186. [Google Scholar]
- Turnbull, PCB. Bacillus. In Barron’s Medical Microbiology, 4th ed; Baron, S, Ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Bunk, B; Biedendieck, R; Jahn, D; Vary, PS. Bacillus megaterium and other Bacilli: Industrial applications. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Flickinger, MC, Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 1–15. [Google Scholar]
- Desouky, AM; Haleem, AE; Roda, FT; Thourya, M; Sidra, M; Fatima, H. Isolation and characterization of extracellular bioflocculants produced by bacteria isolated from Quatari Ecosystems. Pol. J. Microbiol 2008, 57, 231–239. [Google Scholar]
- Perez-Garcia, A; Romero, D; de Vicente, A. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol 2011, 22, 1–7. [Google Scholar]
- Priest, FG; Goodfellow, M; Shute, LA; Berkeley, RCW. Bacillus amyloliquefaciens sp. nov., norm. rev. Int. J. Syst. Bacteriol 1987, 37, 69–71. [Google Scholar]
- Fugita, M; Ike, M; Jang, JH; Kim, SM; Hirao, T. Bioflocculant production from lower-molecular fatty acids as a novel strategy for utilization of sludge digestion liquor. World Sci. Technol 2001, 44, 237–243. [Google Scholar]
- Thomas, CP; Duvall, ML; Robertson, EP; Barrett, KB; Bala, GA. Surfactant-based EOR me mediated by naturally occurring microorganisms. Soc. Petrol. Eng. Reservoir Eng 1993, 11, 285–291. [Google Scholar]
- Yakimov, MM; Timmis, KN; Wray, V; Fredrickson, HL. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol 1995, 61, 1706–1713. [Google Scholar]
- Margesin, R; Schiner, F. Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 2001, 5, 73–83. [Google Scholar]
- He, N; Li, Y; Chen, J. Production of a novel polygalacturonic acid bioflocculant REA-11 by Corynebacterium glutamicum. Bioresour. Technol 2004, 94, 99–105. [Google Scholar]
- Cosa, S; Mabinya, LV; Olaniran, AO; Okoh, OO; Okoh, AI. Bioflocculant Production by Virgibacillus sp. Rob Isolated from the Bottom Sediment of Algoa Bay in the Eastern Cape, South Africa. Molecules 2011, 16, 2431–2442. [Google Scholar]
- Gong, W; Wang, S; Sun, F; Liu, XW; Yue, QY; Gao, BY. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol 2008, 99, 4668–4674. [Google Scholar]
- Kurane, R; Hatatkeyama, S; Tsugeno, H. Correlation between flocculation production and morphological changes in Rhodococcus erythropolis S-1. J. Ferment. Bioeng 1991, 72, 498–500. [Google Scholar]
- Zheng, Y; Ye, ZL; Fang, XL; Li, YH; Cai, WM. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol 2008, 99, 7686–7691. [Google Scholar]
- Li, Z; Zhong, S; Lei, H; Chen, R; Yu, Q; Li, H. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol 2009, 100, 3650–3656. [Google Scholar]
- Sobeck, DC; Higgins, MJ. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res 2002, 36, 527–538. [Google Scholar]
- Zhang, Z; Lin, B; Xia, S; Wang, X; Yang, A. Production and application of a novel bioflocculant by multi-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci 2007, 19, 667–673. [Google Scholar]
- Yim, JH; Kim, SJ; Ahn, SH; Lee, HK. Characterization of novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodium impudicum KG03. Bioresour. Technol 2007, 98, 361–367. [Google Scholar]
- Lachhwani, P. Studies on polymeric bioflocculant producing microorganisms. M.Sc. Thesis, Thapar Institute of Engineering & Technology, Patiala, India, 2005. [Google Scholar]
- Shimofuruya, H; Koide, A; Shirota, K; Tsuji, T; Nakamura, M; Suzuki, J. The production of flocculating substance(s) by Streptomyces griseus. Biosci. Biotechnol. Biochem 1995, 60, 498–500. [Google Scholar]
- Fujita, M; Ike, M; Tachibana, S; Kitada, G; Kim, SM; Inoue, Z. Characterization of a bioflocculant produced by citrobacter-tkf04 from acetic and propionic acids. J. Biosci. Bioeng 2000, 89, 40–46. [Google Scholar]
- Zhang, J; Liu, Z; Wang, S; Jiang, P. Characterization of a bioflocculant produced by the marine myxobacterium Nannocystis sp. NU2. Appl. Microbiol. Biotechnol 2002, 59, 517–522. [Google Scholar]
- Li, Y; He, N; Guan, H; Du, G; Chen, J. A novel polygalacturonic acid bioflocculant REA-11 produced by Corynebacterium glutamicum: A proposed biosynthetic pathway and experimental confirmation. Appl. Microbiol. Biotechnol 2003, 6, 200–206. [Google Scholar]
- Kumar, CG; Joo, HS; Kavali, R; Choi, JW; Chang, CS. Characterization of extracellular biopolymer flocculant from a haloakalophilic Bacillus isolate. World J. Microbiol. Biotechnol 2004, 20, 837–843. [Google Scholar]
- Yokoi, H; Yoshida, T; Mori, S; Hirose, J; Hayashi, S; Takasaki, Y. Biopolymer flocculant produced by Enterobacter sp. Biotechnol. Lett 1996, 19, 572. [Google Scholar]
- Chang, WC; Soon, AY; In, HO; Sang, HP. Characterization of an extracellular flocculating substance produced by a planktonic cyanobacterium, Anabaena sp. Biotechnol. Lett 1998, 20, 643–646. [Google Scholar]
- Chen, H; Zhang, JF; Jiang, PJ; Yang, SL; Liu, ZL. Composition and characterization of microbiological flocculant SC06. Environ. Chem 2002, 21, 360–364. [Google Scholar]
- Cook, AE; Meyers, PR. Rapid identification of filamentous actinomycetes to the genus level using genus-specific 16S rRNA gene restriction fragment patterns. Int. J. Syst. Evol. Microbiol 2003, 53, 1907–1915. [Google Scholar]

| Depth (m) | Temperature (°C) | Conductivity (mS/cm) | Salinity (ppt) | pH | Turbidity (NTU) | DO (mg/L) |
|---|---|---|---|---|---|---|
| 7.25 ± 0.19 | 17.2 ± 0.03 | 46 ± 0.03 | 35.8 ± 0.04 | 8.42 ± 0.26 | 3.72 ± 1.05 | 7.12 ± 0.5 |
| Carbon source | Glucose | Sucrose | Fructose | Starch |
| Flocculating activity (%) | 65 | 72.4 | 59 | – |
| Nitrogen source | Peptone | Ammonium sulphate | Urea | Ammonium chloride |
| Flocculating activity (%) | 65 | – | 56 | 91 |
| Cations | Calcium chloride | Magnesium chloride | Iron sulphate | Potassium chloride |
| Flocculating activity (%) | 65 | 72 | 70 | 69 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nontembiso, P.; Sekelwa, C.; Leonard, M.V.; Anthony, O.I. Assessment of Bioflocculant Production by Bacillus sp. Gilbert, a Marine Bacterium Isolated from the Bottom Sediment of Algoa Bay. Mar. Drugs 2011, 9, 1232-1242. https://doi.org/10.3390/md9071232
Nontembiso P, Sekelwa C, Leonard MV, Anthony OI. Assessment of Bioflocculant Production by Bacillus sp. Gilbert, a Marine Bacterium Isolated from the Bottom Sediment of Algoa Bay. Marine Drugs. 2011; 9(7):1232-1242. https://doi.org/10.3390/md9071232
Chicago/Turabian StyleNontembiso, Piyo, Cosa Sekelwa, Mabinya V. Leonard, and Okoh I. Anthony. 2011. "Assessment of Bioflocculant Production by Bacillus sp. Gilbert, a Marine Bacterium Isolated from the Bottom Sediment of Algoa Bay" Marine Drugs 9, no. 7: 1232-1242. https://doi.org/10.3390/md9071232
APA StyleNontembiso, P., Sekelwa, C., Leonard, M. V., & Anthony, O. I. (2011). Assessment of Bioflocculant Production by Bacillus sp. Gilbert, a Marine Bacterium Isolated from the Bottom Sediment of Algoa Bay. Marine Drugs, 9(7), 1232-1242. https://doi.org/10.3390/md9071232





