Bioactive Indole Derivatives from the South Pacific Marine Sponges Rhopaloeides odorabile and Hyrtios sp.
Abstract
:1. Introduction
2. Results and Discussion
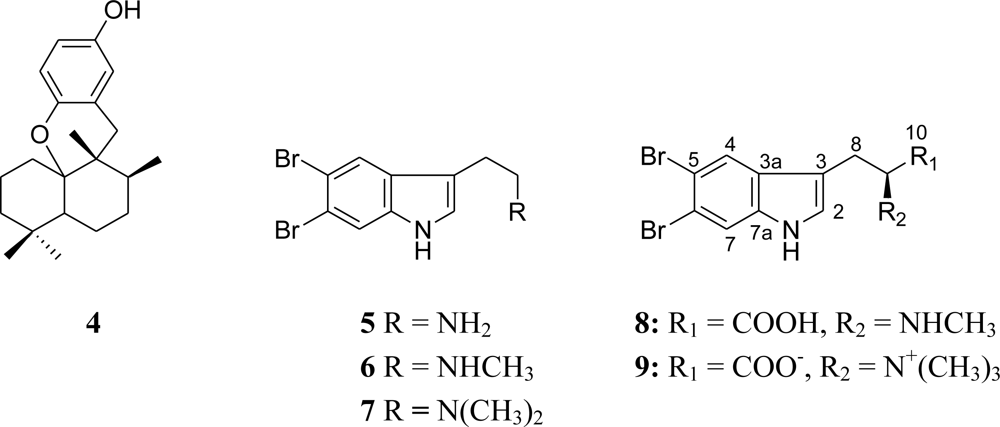
2.1. Isolation of Indole Derivatives
2.2. Structure Elucidation of 5,6-Dibromo-l-hypaphorine (9)
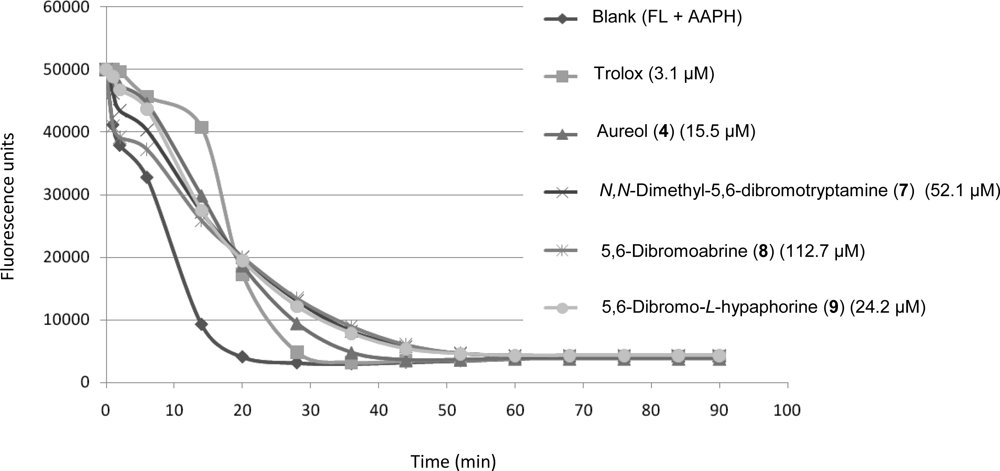
2.3. Biological Activities of Compounds 1–9
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. PLA2 Inhibition Assay
3.5. Antioxidant Assays
3.6. Cytotoxicity Assay
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Blunt, JW; Copp, BR; Munro, MH; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2011, 28, 196–268, and the previous reviews of this series.. [Google Scholar]
- Gul, W; Hamann, MT. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci 2005, 78, 442–453. [Google Scholar]
- Sugiyama, Y; Ito, Y; Suzuki, M; Hirota, A. Indole derivatives from a marine sponge-derived yeast as DPPH radical scavengers. J Nat Prod 2009, 72, 2069–2071. [Google Scholar]
- Bao, B; Zhang, P; Lee, Y; Hong, J; Lee, C-O; Jung, JH. Monoindole alkaloids from a marine sponge Spongosorites sp. Mar Drugs 2007, 5, 31–39. [Google Scholar]
- Rasmussen, T; Jensen, J; Anthoni, U; Christophersen, C; Nielsen, PH. Structure and synthesis of bromoindoles from the marine sponge Pseudosuberites hyalinus. J Nat Prod 1993, 56, 1553–1558. [Google Scholar]
- Shen, YC; Liaw, C; Ho, JR; Khalil, AT; Kuo, YH. Isolation of aureol from Smenospongia sp. and cytotoxic activity of some aureol derivatives. Nat Prod Res 2006, 20, 578–585. [Google Scholar]
- Van Lear, GE; Morton, GO; Fulmor, W. New antibacterial bromoindole metabolites from the marine sponge Polyfibrospongia maynardii. Tetrahedron Lett 1973, 4, 299–300. [Google Scholar]
- Djura, P; Stierle, DB; Sullivan, B; Faulkner, DJ. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (Polyfibrospongia) echina. J Org Chem 1980, 45, 1435–1441. [Google Scholar]
- Tasdemir, D; Bugni, TS; Mangalindan, GC; Concepcion, GP; Harper, MK; Ireland, CM. Cytotoxic bromoindole derivatives and terpenes from the Philippine marine sponge Smenospongia sp. Z Naturforsch 2002, 57c, 914–922. [Google Scholar]
- Campagnuolo, C; Fattorusso, E; Taglialatela-Scafati, O. Plakohypaphorines A–C, iodine-containing alkaloids from the Caribbean sponge Plakortis simplex. Eur J Org Chem 2003, 284–287. [Google Scholar]
- Kondo, K; Nishi, J; Ishibashi, M; Kobayashi, J. Two new tryptophan-derived alkaloids from the Okinawan marine sponge Aplysina sp. J Nat Prod 1994, 57, 1008–1011. [Google Scholar]
- Raverty, WD; Thomson, RH; King, TJ. Metabolites from the sponge Pachymatisma johnstoni; L-6-bromohypaphorine, a new amino-acid (and its crystal structure). J Chem Soc, Perkin Trans 1 1977, 1204–1211. [Google Scholar]
- Borrelli, F; Campagnuolo, C; Capassa, R; Fattorusso, E; Taglialatela-Scafati, O. Iodinated indole alkaloids from Plakortis simplex. New plakohypaphorines and an evaluation of their antihistamine activity. Eur J Org Chem 2004, 3227–3232. [Google Scholar]
- Motti, CA; Bourguet-Kondracki, M-L; Longeon, A; Doyle, JR; Llewellyn, LE; Tapiolas, DM; Yin, P. Comparison of the biological properties of several marine sponge-derived sesquiterpenoid quinone. Molecules 2007, 12, 1376–1388. [Google Scholar]
- Cao, G; Prior, RL. Measurement of Oxygen Radical Absorbance Capacity in Biological Samples. Methods Enzymol 1999, 299, 50–61. [Google Scholar]
- Ou, B; Hampsch-Woodill, M; Prior, RL. Development and validation of an improved Oxygen Radical Absorbance Capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem 2001, 49, 4619–4626. [Google Scholar]

| no. | 1H | 13C | HMBCb |
|---|---|---|---|
| 1-NH | 11.20 brs | - | 2, 3 |
| 2 | 7.27 s | 126.8 | 3, 3a, 7a |
| 3 | 109.5 | ||
| 3a | 128.2 | ||
| 4 | 8.02 s | 123.1 | 3, 5, 6, 7a |
| 5 | 112.6 c | ||
| 6 | 114.8 c | ||
| 7 | 7.72 s | 116.0 | 3a, 5, 6, 7a |
| 7a | 135.7 | ||
| 8 | 3.21 m | 24.2 | 2, 3, 3a, 9, 10 |
| 9 | 3.67 dd (10.1, 3.3) | 78.2 | 3, 8, 10 |
| 10 | 167.0 | ||
| OH | 8.45 brs | 10 | |
| N(CH3)3 | 3.17 s | 51.0 |
| Compound | PLA2a | ORAC FLb |
|---|---|---|
| (1H-Indol-3-yl)oxoacetamide (1) | 1.17 ± 0.05 | nt |
| (1H-Indol-3-yl)oxoacetic acid methyl ester (2) | 1.11 ± 0.33 | nt |
| 6-Bromoindole-3-carbaldehyde (3) | 1.27 ± 0.06 | nt |
| Aureol (4) | 0.46 ± 0.02 | 0.29 ± 0.03 |
| 5,6-Dibromotryptamine (5) | 0.62 ± 0.01 | nt |
| N-Methyl-5,6-dibromotryptamine (6) | 0.33 ± 0.03 | nt |
| N,N-Dimethyl-5,6-dibromotryptamine (7) | 0.77 ± 0.05 | 0.06 ± 0.01 |
| 5,6-Dibromoabrine (8) | 0.30 ± 0.01 | 0.07 ± 0.01 |
| 5,6-Dibromo-l-hypaphorine (9) | 0.20 ± 0.01 | 0.22 ± 0.04 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Longeon, A.; Copp, B.R.; Quévrain, E.; Roué, M.; Kientz, B.; Cresteil, T.; Petek, S.; Debitus, C.; Bourguet-Kondracki, M.-L. Bioactive Indole Derivatives from the South Pacific Marine Sponges Rhopaloeides odorabile and Hyrtios sp. Mar. Drugs 2011, 9, 879-888. https://doi.org/10.3390/md9050879
Longeon A, Copp BR, Quévrain E, Roué M, Kientz B, Cresteil T, Petek S, Debitus C, Bourguet-Kondracki M-L. Bioactive Indole Derivatives from the South Pacific Marine Sponges Rhopaloeides odorabile and Hyrtios sp. Marine Drugs. 2011; 9(5):879-888. https://doi.org/10.3390/md9050879
Chicago/Turabian StyleLongeon, Arlette, Brent R. Copp, Elodie Quévrain, Mélanie Roué, Betty Kientz, Thierry Cresteil, Sylvain Petek, Cécile Debitus, and Marie-Lise Bourguet-Kondracki. 2011. "Bioactive Indole Derivatives from the South Pacific Marine Sponges Rhopaloeides odorabile and Hyrtios sp." Marine Drugs 9, no. 5: 879-888. https://doi.org/10.3390/md9050879
APA StyleLongeon, A., Copp, B. R., Quévrain, E., Roué, M., Kientz, B., Cresteil, T., Petek, S., Debitus, C., & Bourguet-Kondracki, M.-L. (2011). Bioactive Indole Derivatives from the South Pacific Marine Sponges Rhopaloeides odorabile and Hyrtios sp. Marine Drugs, 9(5), 879-888. https://doi.org/10.3390/md9050879






