Oral Administration of Skin Gelatin Isolated from Chum Salmon (Oncorhynchus keta) Enhances Wound Healing in Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Animal Characteristics
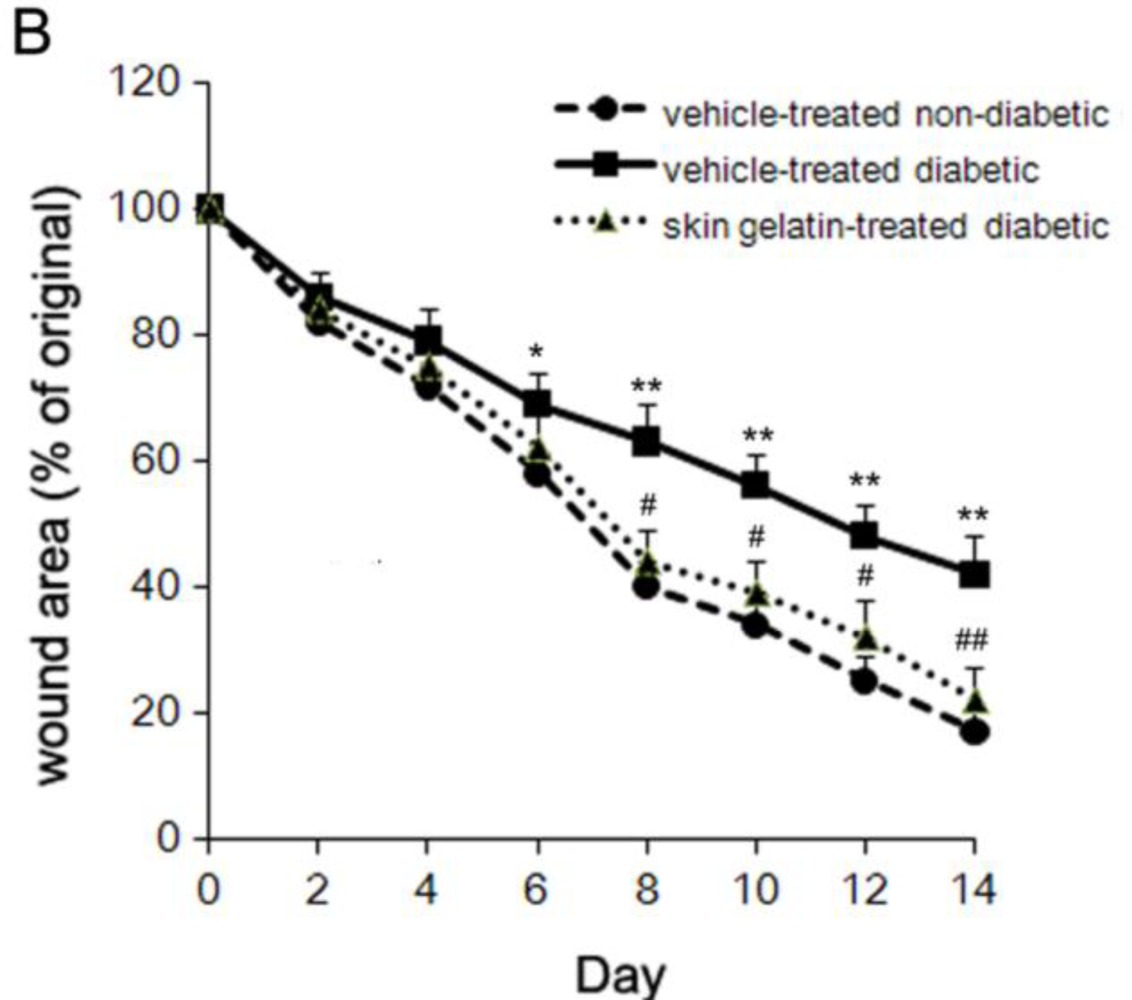
2.2. Effect of Chum Salmon Skin Gelatin Treatment on Wound Closure
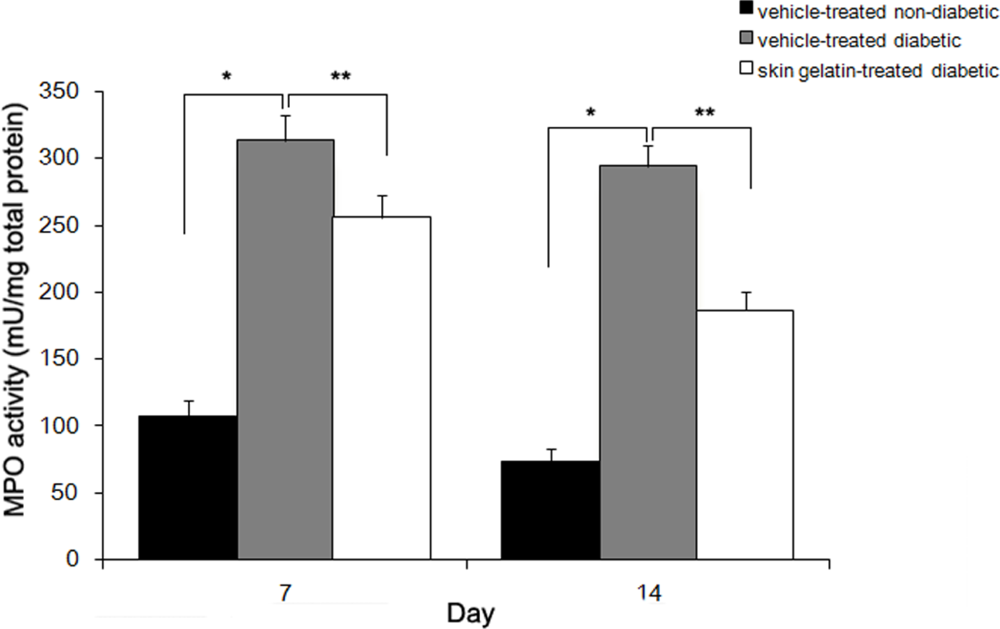
2.3. Effect of Skin Gelatin on Wound MPO Activity
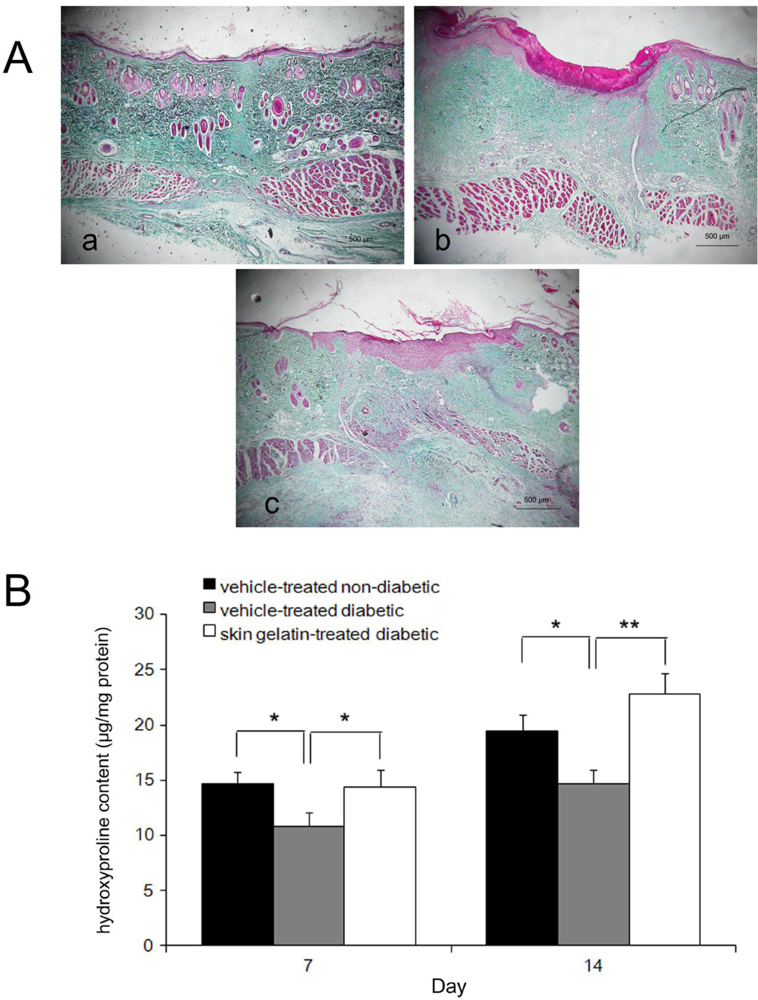
2.4. Histological Examinations
2.5. Collagen Accumulation
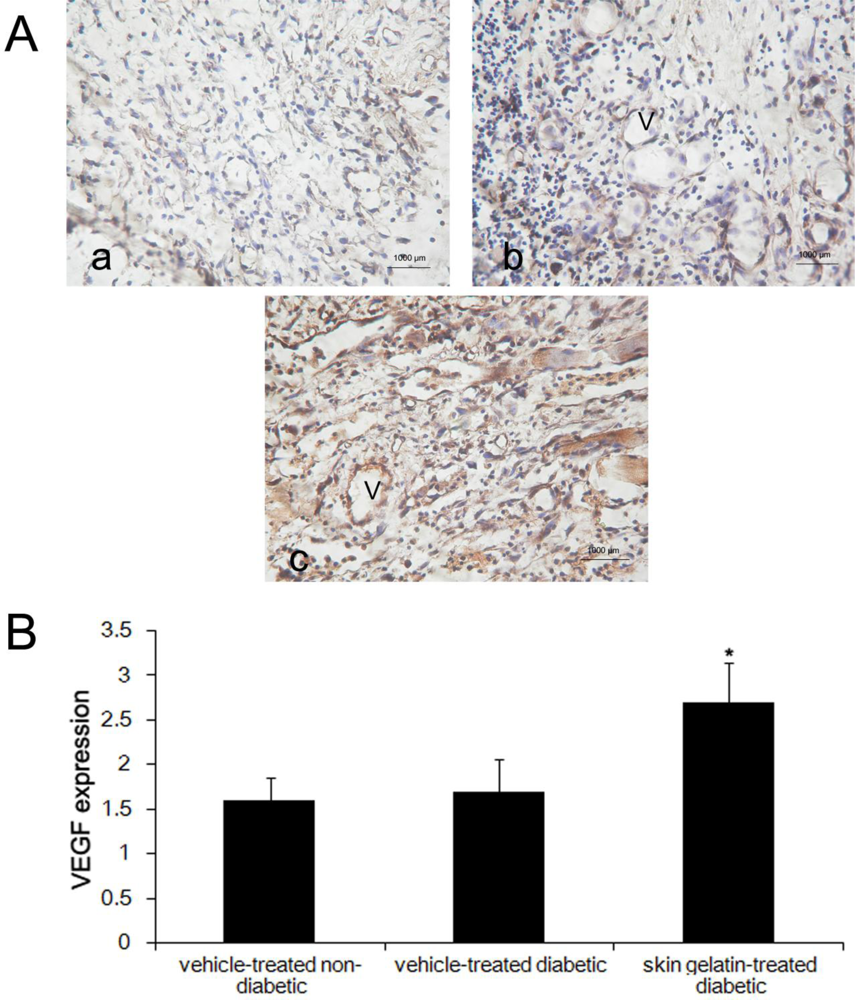
2.6. Evaluation of Immunostaining for VEGF
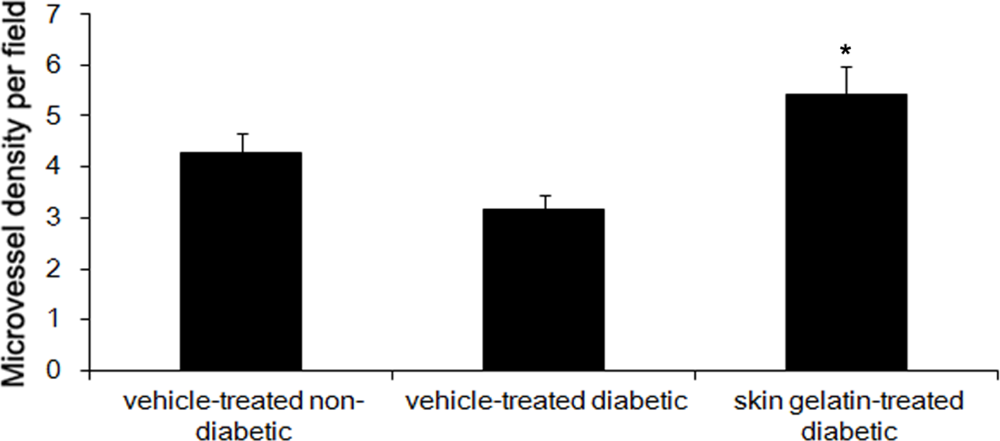
2.7. Effect of Skin Gelatin on MVD
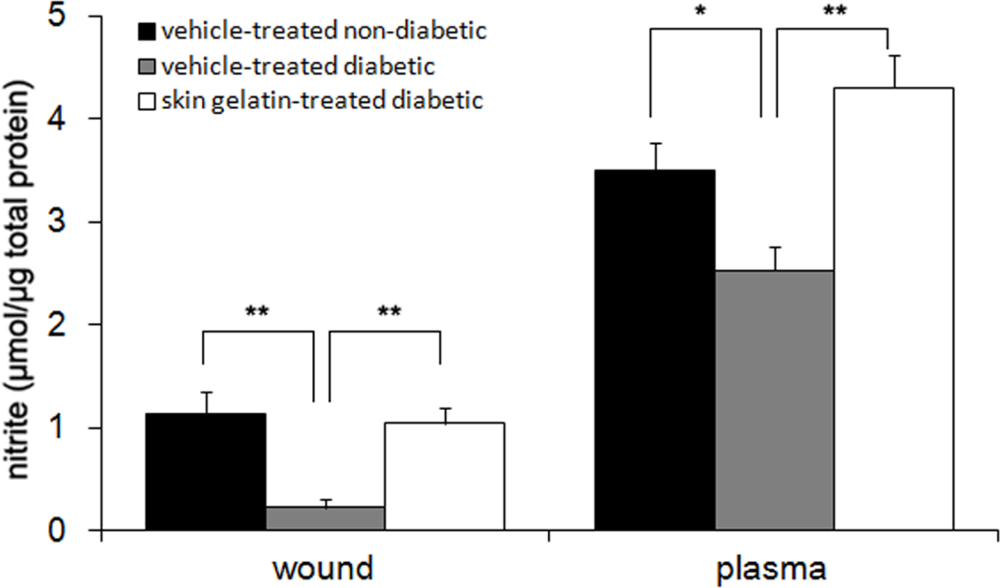
2.8. Measurement of Nitrite Levels
3. Discussion
4. Methods
4.1. Preparation of Skin Gelatin
4.2. Formation of Diabetic Rats
4.3. Full-Thickness Skin Wound Preparation
4.4. Wound Closure Measurement
4.5. Histological Analysis
4.6. Immunohistochemistry
4.7. Assess of MVD
4.8. MPO Activity Assay
4.9. Determination of Nitrite Concentration
4.10. Determination of the Hydroxyproline Content in the Wounds
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Greenhalgh, DG. Wound healing and diabetes mellitus. Clin Plast Surg 2003, 30, 37–45. [Google Scholar]
- Stadelmann, WK; Digenis, AG; Tobin, GR. Impediments to wound healing. Am J Surg 1998, 176, 39S–47S. [Google Scholar]
- Galeano, M; Bitto, A; Altavilla, D; Minutoli, L; Polito, F; Calo, M; Lo Cascio, P; Stagno d’Alcontres, F; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen 2008, 16, 208–217. [Google Scholar]
- Han, X; Xu, Y; Wang, J; Pei, X; Yang, R; Li, N; Li, Y. Effects of cod bone gelatin on bone metabolism and bone microarchitecture in ovariectomized rats. Bone 2009, 44, 942–947. [Google Scholar]
- Lee, SB; Jeon, HW; Lee, YW; Lee, YM; Song, KW; Park, MH; Nam, YS; Ahn, HC. Bio-artificial skin composed of gelatin and (1→3), (1→6)-beta-glucan. Biomaterials 2003, 24, 2503–2511. [Google Scholar]
- Wang, TW; Sun, JS; Wu, HC; Tsuang, YH; Wang, WH; Lin, FH. The effect of gelatin-chondroitin sulfate-hyaluronic acid skin substitute on wound healing in SCID mice. Biomaterials 2006, 27, 5689–5697. [Google Scholar]
- Kawai, K; Suzuki, S; Tabata, Y; Nishimura, Y. Accelerated wound healing through the incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis using a pressure-induced decubitus ulcer model in genetically diabetic mice. Br J Plast Surg 2005, 58, 1115–1123. [Google Scholar]
- Thilagar, S; Jothi, NA; Omar, AR; Kamaruddin, MY; Ganabadi, S. Effect of keratin-gelatin and bFGF-gelatin composite film as a sandwich layer for full-thickness skin mesh graft in experimental dogs. J Biomed Mater Res B Appl Biomater 2009, 88, 12–16. [Google Scholar]
- Hori, K; Sotozono, C; Hamuro, J; Yamasaki, K; Kimura, Y; Ozeki, M; Tabata, Y; Kinoshita, S. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release 2007, 118, 169–176. [Google Scholar]
- Li, J; Chen, J; Kirsner, R. Pathophysiology of acute wound healing. Clin Dermatol 2007, 25, 9–18. [Google Scholar]
- Brem, H; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 2007, 117, 1219–1222. [Google Scholar]
- Stadelmann, WK; Digenis, AG; Tobin, GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 1998, 176, 26S–38S. [Google Scholar]
- Schwentker, A; Vodovotz, Y; Weller, R; Billiar, TR. Nitric oxide and wound repair: Role of cytokines. Nitric Oxide 2002, 7, 1–10. [Google Scholar]
- Witte, MB; Kiyama, T; Barbul, A. Nitric oxide enhances experimental wound healing in diabetes. Brit J Surg 2002, 89, 1594–1601. [Google Scholar]
- Blakytny, R; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006, 23, 594–608. [Google Scholar]
- Moulin, V; Auger, FA; Garrel, D; Germain, L. Role of wound healing myofibroblasts on re-epithelialization of human skin. Burns 2000, 26, 3–12. [Google Scholar]
- Martin, A; Komada, MR; Sane, DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003, 23, 117–145. [Google Scholar]
- Roy, H; Bhardwaj, S; Yla-Herttuala, S. Biology of vascular endothelial growth factors. FEBS Lett 2006, 580, 2879–2887. [Google Scholar]
- Bitto, A; Minutoli, L; Altavilla, D; Polito, F; Fiumara, T; Marini, H; Galeano, M; Calo, M; Lo Cascio, P; Bonaiuto, M; Migliorato, A; Caputi, AP; Squadrito, F. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol Res 2008, 57, 159–169. [Google Scholar]
- Cha, J; Kwak, T; Butmarc, J; Kim, TA; Yufit, T; Carson, P; Kim, SJ; Falanga, V. Fibroblasts from non-healing human chronic wounds show decreased expression of beta ig-h3, a TGF-beta inducible protein. J Dermatol Sci 2008, 50, 15–23. [Google Scholar]
- Santos, SC; Miguel, C; Domingues, I; Calado, A; Zhu, Z; Wu, Y; Dias, S. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res 2007, 313, 1561–1574. [Google Scholar]
- Galiano, RD; Tepper, OM; Pelo, CR; Bhatt, KA; Callaghan, M; Bastidas, N; Bunting, S; Steinmetz, HG; Gurtner, GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 2004, 164, 1935–1947. [Google Scholar]
- Bitto, A; Minutoli, L; Galeano, MR; Altavilla, D; Polito, F; Fiumara, T; Calo, M; Lo Cascio, P; Zentilin, L; Giacca, M; Squadrito, F. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin Sci (Lond) 2008, 114, 707–718. [Google Scholar]
- Romana-Souza, B; Nascimento, AP; Monte-Alto-Costa, A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol 2009, 611, 77–84. [Google Scholar]
- Senel, O; Cetinkale, O; Ozbay, G; Ahcioglu, F; Bulan, R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg 1997, 39, 516–523. [Google Scholar]
- Pei, XR; Yang, RY; Zhang, ZF; Gao, LF; Wang, JB; Xu, YJ; Zhao, M; Han, XL; Liu, ZG; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem 2009, 118, 333–340. [Google Scholar]
- Kim, SK; Kim, YT; Byun, HG; Nam, KS; Joo, DS; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J Agric Food Chem 2001, 49, 1984–1989. [Google Scholar]
- Khantaphant, S; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp Biochem Physiol B Biochem Mol Biol 2008, 151, 410–419. [Google Scholar]
- Songchotikunpan, P; Tattiyakul, J; Supaphol, P. Extraction and electrospinning of gelatin from fish skin. Int J Biol Macromol 2008, 42, 247–255. [Google Scholar]
- Taheri, A; Abedian Kenari, AM; Gildberg, A; Behnam, S. Extraction and physicochemical characterization of greater Lizardfish (Saurida tumbil) skin and bone gelatin. J Food Sci 2009, 74, E160–E165. [Google Scholar]
- Li, ZJ; Wang, ZZ; Zheng, YZ; Xu, B; Yang, RC; Scadden, DT; Han, ZC. Kinetic expression of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) during embryonic stem cell differentiation. J Cell Biochem 2005, 95, 559–570. [Google Scholar]
- Stark, JM; van Egmond, AW; Zimmerman, JJ; Carabell, SK; Tosi, MF. Detection of enhanced neutrophil adhesion to parainfluenza-infected airway epithelial cells using a modified myeloperoxidase assay in a microtiter format. J Virol Methods 1992, 40, 225–242. [Google Scholar]

| Vehicle-treated non-diabetic (n = 8) | Vehicle-treated diabetic (n = 8) | Skin gelatin-treated diabetic (n = 8) | P-value (between groups after treatment) | ||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | ||
| Body weight (g) | 263 ± 11 | 297 ± 13 | 248 ± 10 | 232 ± 16 | 250 ± 12 | 234 ± 14 | <0.05 |
| Blood glucose levels (mg/dL) | 115 ± 6.3 | 119 ± 5.8 | 398 ± 26 | 384 ± 23 | 402 ± 27 | 389 ± 28 | <0.05 |
| Fibroblasts | Vascularization | Collagen | Epithelialization | |
|---|---|---|---|---|
| Day 7 | ||||
| Vehicle-treated non-diabetic | 1.4 ± 0.2 | 1.2 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.2 |
| Vehicle-treated diabetic | 1.3 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.2 ** | 1.2 ± 0.3 ** |
| Skin gelatin-treated diabetic | 1.8 ± 0.2 # | 1.4 ± 0.1 # | 1.3 ± 0.1 # | 1.5 ± 0.2 # |
| Day 14 | ||||
| Vehicle-treated non-diabetic | 1.8 ± 0.2 | 1.9 ± 0.1 | 1.9 ± 0.1 | 2.6 ± 0.3 |
| Vehicle-treated diabetic | 1.7 ± 0.2 | 1.6 ± 0.2 * | 1.2 ± 0.1 ** | 1.8 ± 0.2 ** |
| Skin gelatin-treated diabetic | 2.3 ± 0.2 # | 2.3 ± 0.2 # | 1.5 ± 0.2 # | 2.2 ± 0.2 # |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Z.; Zhao, M.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral Administration of Skin Gelatin Isolated from Chum Salmon (Oncorhynchus keta) Enhances Wound Healing in Diabetic Rats. Mar. Drugs 2011, 9, 696-711. https://doi.org/10.3390/md9050696
Zhang Z, Zhao M, Wang J, Ding Y, Dai X, Li Y. Oral Administration of Skin Gelatin Isolated from Chum Salmon (Oncorhynchus keta) Enhances Wound Healing in Diabetic Rats. Marine Drugs. 2011; 9(5):696-711. https://doi.org/10.3390/md9050696
Chicago/Turabian StyleZhang, Zhaofeng, Ming Zhao, Junbo Wang, Ye Ding, Xiaoqian Dai, and Yong Li. 2011. "Oral Administration of Skin Gelatin Isolated from Chum Salmon (Oncorhynchus keta) Enhances Wound Healing in Diabetic Rats" Marine Drugs 9, no. 5: 696-711. https://doi.org/10.3390/md9050696
APA StyleZhang, Z., Zhao, M., Wang, J., Ding, Y., Dai, X., & Li, Y. (2011). Oral Administration of Skin Gelatin Isolated from Chum Salmon (Oncorhynchus keta) Enhances Wound Healing in Diabetic Rats. Marine Drugs, 9(5), 696-711. https://doi.org/10.3390/md9050696





