Abstract
Sulfated polysaccharides and their lower molecular weight oligosaccharide derivatives from marine macroalgae have been shown to possess a variety of biological activities. The present paper will review the recent progress in research on the structural chemistry and the bioactivities of these marine algal biomaterials. In particular, it will provide an update on the structural chemistry of the major sulfated polysaccharides synthesized by seaweeds including the galactans (e.g., agarans and carrageenans), ulvans, and fucans. It will then review the recent findings on the anticoagulant/antithrombotic, antiviral, immuno-inflammatory, antilipidemic and antioxidant activities of sulfated polysaccharides and their potential for therapeutic application.
1. Introduction
Many species of seaweed (marine macroalgae) are used as food and they have also found use in traditional medicine because of their perceived health benefits. Seaweeds are rich sources of sulfated polysaccharides, including some that have become valuable additives in the food industry because of their rheological properties as gelling and thickening agents (e.g., carrageenan). In addition, sulfated polysaccharides are recognized to possess a number of biological activities including anticoagulant, antiviral, and immuno-inflammatory activities that might find relevance in nutraceutical/functional food, cosmetic/cosmeceutical and pharmaceutical applications.
In this review, we will examine current progress in research on sulfated polysaccharides, relating to their structural diversity, bioactivities and mechanisms of action. The literature contains vast information on the structure and bioactivities of marine sulfated polysaccharides that cannot be completely considered in this short review. There are several excellent reviews where the reader may find additional information on various aspects of this subject [1–9].
2. Structural Diversity of Algal Sulfated Polysaccharides
2.1. Carrageenans and Agarans from Red Algae
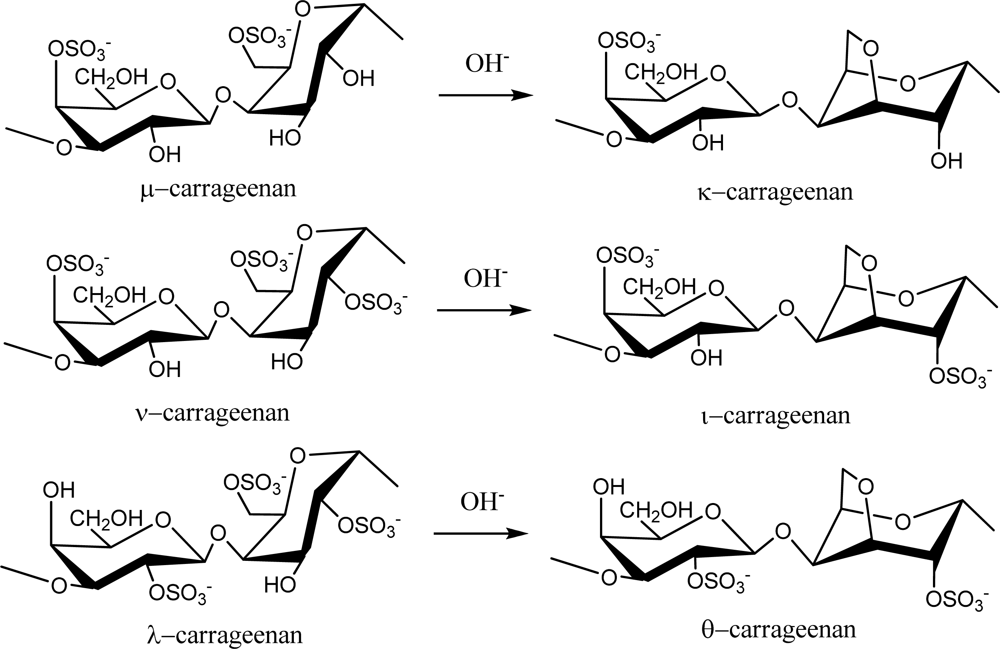
Red seaweed galactans are of great commercial importance as they are used widely in the food industry because of their rheological properties as gelling and thickening agents. These sulfated polysaccharides are primarily classified as agarans and carrageenans based on their stereochemistry, specifically galactans with 4-linked α-galactose residues of the L-series are termed agarans and those of the D-series are termed carrageneens [10]. Thus, carrageenans are high molecular weight sulfated d-galactans composed of repeating disaccharide units with alternating 3-linked β-d-galactopyranose (G-units) and 4-linked α-galactopyranose (D-units) or 3,6-anhydro-α-galactopyranose (AnGal-units).
Carrageenans are normally classified according to their structural characteristics, including their sulfation patterns and the presence or absence of AnGal on D-units. There are at least 15 different carrageenan structures [2]. The most industrially relevant carrageenans are κ, ι and λ forms, the structures of which are illustrated in Figure 1. The major source of κ-carrageenan is the red seaweed Kappaphycus alvarezii [11]. Its structure was reported as alternating 3-linked β-d-galactose 4-sulfate and 4-linked AnGal units [12]. The ι-carrageenans have an additional sulfate group on C2(O) of the AnGal residue, resulting in two sulfates per disaccharide repeating unit. Funami et al. examined ι-carrageenan extracted from Eucheuma spinosum using atomic force microscopy and suggested that ι-carrageenans were more homogeneous and flexible than κ-carrageenans [13]. The λ-carrageenans have three sulfate groups per disaccharide unit with the third sulfate group of this form at the C6 position of the 4-linked residue, but there is no 3,6-anhydride bridge on the 4-linked residues. Lambda-carrageenan is obtained from species of the Gigartina and Chondrus genera [14].
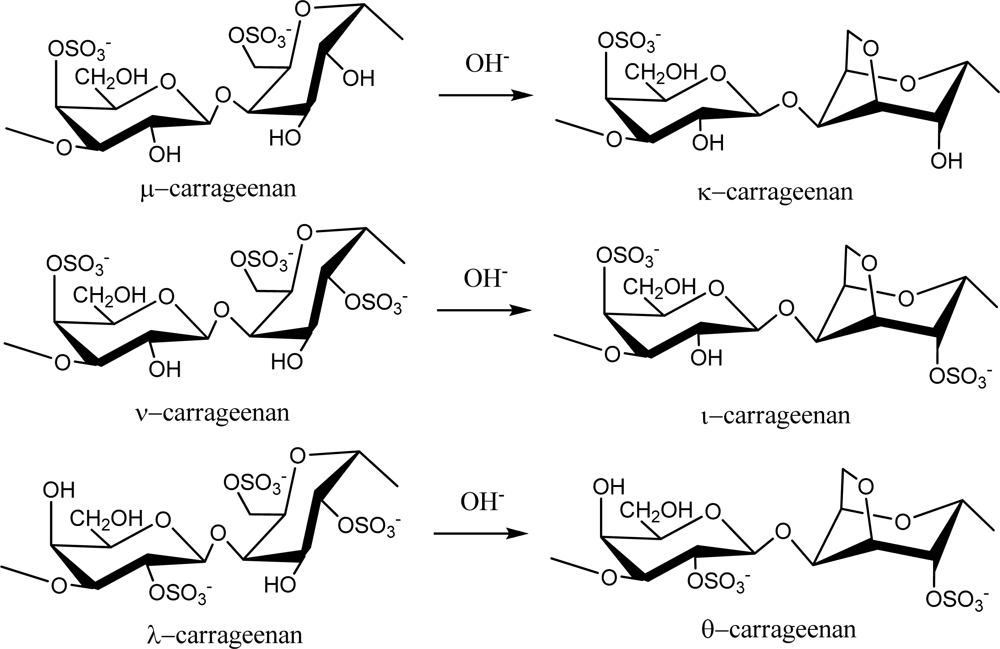
Figure 1.
Repeating disaccharide units of different types of carrageenan and their transformation by treatment with alkali.
Alternative forms of carrageenan can be obtained by chemical modification. For example, formation of an anhydride bridge in λ-carrageenan can be induced by alkali modification to produce θ-carrageenan (Figure 1). Extraction of λ-carrageenan from hand-sorted tetrasporophytes of Gigartina skottsbergii and subsequent treatment of the extract with alkaline borohydride resulted in conversion of the 4-linked residues to the 3,6-anhydride ring form, yielding θ-carrageenan with no detectable contamination of κ- or ι-carrageenans [15].
Natural carrageenans typically occur as mixtures of different hybrid types, such as κ/β-hybrids [16], κ/ι-hybrids [17–20], κ/μ-hybrids [21], or ν/ι-hybrids [22]. Additionally, methyl or pyruvic acid acetal constituents and the presence of small amount of other sugars can add to the structural complexity [23].
One of the best studied agarans is porphyran [24], obtained from Porphyra species of red algae including Porphyra capensis [25] and P. haitanensis [26,27]. Porphyran typical exhibits a linear backbone of alternating 3-linked β-d-galactose and 4-linked α-l-galactose-6-sulfate or 3,6-anhydro-α-l-galactose units. Sulfated agarans of a similar linear form are synthesized by Polysiphonia species, such as P. strictissima, P. abscissoides [28], P. nigrescens [29], and P. atterima [30]. The regular agaran backbone may be interrupted by different O-linked substitutions in addition to sulfate including methyl and xylosyl groups adding to the structural diversity. For example, Prado et al. recently reported that the sulfated agaran from P. nigrescens is highly substituted on the C-6 of β-d-galactose with sulfate, but methyl ether and β-d-xylose residues were also present [29]. Agaran from Acanthophora spicifera, is highly sulfated at the C-2 position of β-d-galactose units, with some of the residues being 4,6-pyruvylated [31]. This agaran also contains small amounts of xylose and sulfated xylose residues [31,32].
In addition to carrageenans and agarans, there are also red seaweed sulfated polysaccharides that have 4-linked d- and l-galactose sugars distributed within the same polysaccharide molecules, so-called dl-hybrids, and others with various substitutions involving sulfate groups, pyruvic acid ketals, and methoxyl groups [33]. Indeed, native polysaccharides are rarely in their uniform or “ideal” form. For example, we recently reported on the existence of a carrageenan-like sulfated galactan from Furcellaria lumbricalis composed of κ/β-carrageenan units, non-sulfated galactan units, and also smaller units containing 3-O-methyl-galactose [16]. Another example of non-ideal sulfated galactans are xylogalactans, first described in the red seaweed Corallina officinalis and termed corallinan [34], which are agarans that have β-D-xylosyl groups attached at the O-6 position of d-galactose units [35–37].
It should be noted that red seaweeds also produce other types of sulfated polysaccharides including those with mannose in their backbones [38,39]. For example, Mandal et al. described xylomannnan from Scinaia hatei consisting primarily of a backbone of α-(1→3)-linked d-mannose residues substituted at C-6, C-4, and C-2 with β-d-xylosyl residues [39].
2.2. Sulfated Polysaccharides from Green Algae
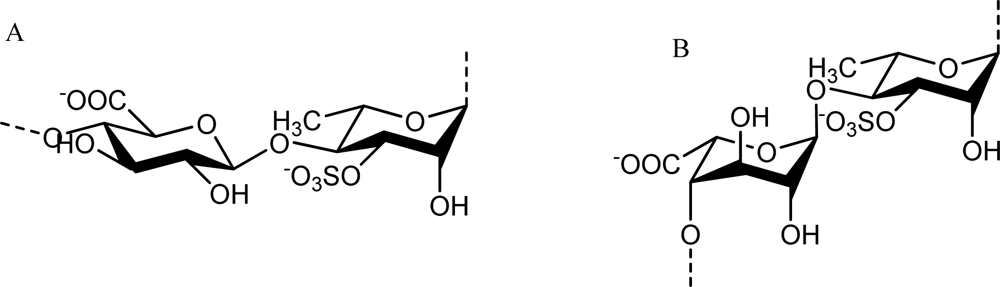
Ulvan is the major water-soluble polysaccharide found in green seaweed of the order Ulvales (Ulva and Enteromorpha sp.) that has sulfate, rhamnose, xylose, iduronic and glucuronic acids as main constituents [40,41]. As reviewed by Lahaye and Robic, ulvan structure shows great complexity and variability as evidenced by the numerous oligosaccharide repeating structural units identified in native and chemically modified ulvan preparations [3]. The main repeating disaccharide units reported are ulvanobiouronic acid 3-sulfate types containing either glucuronic or iduronic acid (Figure 2). Additionally, minor repeat units have been reported that contain sulfated xylose replacing the uronic acid or glucuronic acid as a branch on O-2 of the rhamnose-3-sulfate [40,42].
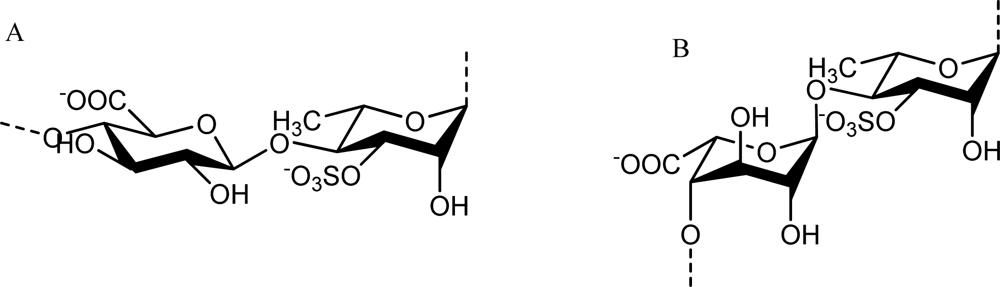
Figure 2.
The main repeating disaccharide units of ulvan. A. [→4)-β-d-Glcp-(1→4)-α-l-Rhap3S-(1→]n; B. [→4)-α-l-Idop-(1→4)-α-l-Rhap3S-(1→]n.
Although the most common source of sulfated galactans is red macroalgae, some green algae, particularly Codium species, are a significant source of sulfated galactans [43–45]. Sulfated galactans from green algae tend to be more complex and heterogeneous in structure than their counterparts from red algae. For example, C. fragile and C. cylindricum contain sulfated arabinogalactan and sulfated glucogalactan, respectively [43,46]. Bilan et al. reported a highly ramified sulfated galactan from C. yezoense that contained a linear backbone of 3-linked β-d-galactopyranose residues containing short oligosaccharides branches through (1→6) linkages [47]. Sulfate groups were found mainly at C-4 and in minor amounts at C-6. Polysaccharides containing sulfated galactans from other green seaweeds including Caulerpa and Ulva have been reported [48,49], but the galactans are minor components.
A variety of other forms of sulfated polysaccharides are synthesized by green seaweeds [41,50–53]. This includes, for example, a water-soluble heteroglycuronan from Enteromorpha compressa, composed of (1→2,4)-linked rhamnose, (1→4)-linked xylose, and (1→4)-linked glucuronic acid units [52]. Sulfate groups, when present, were situated at the C-3 of rhamnose and the C-2 of xylose. Recently, a rhamnan sulfate from Monostroma nitidum was shown to consist primarily of α-1,3-linked and α-1,2-linked rhamnose residues [51].
2.3. Fucose-containing Sulfated Polysaccharides from Brown Algae
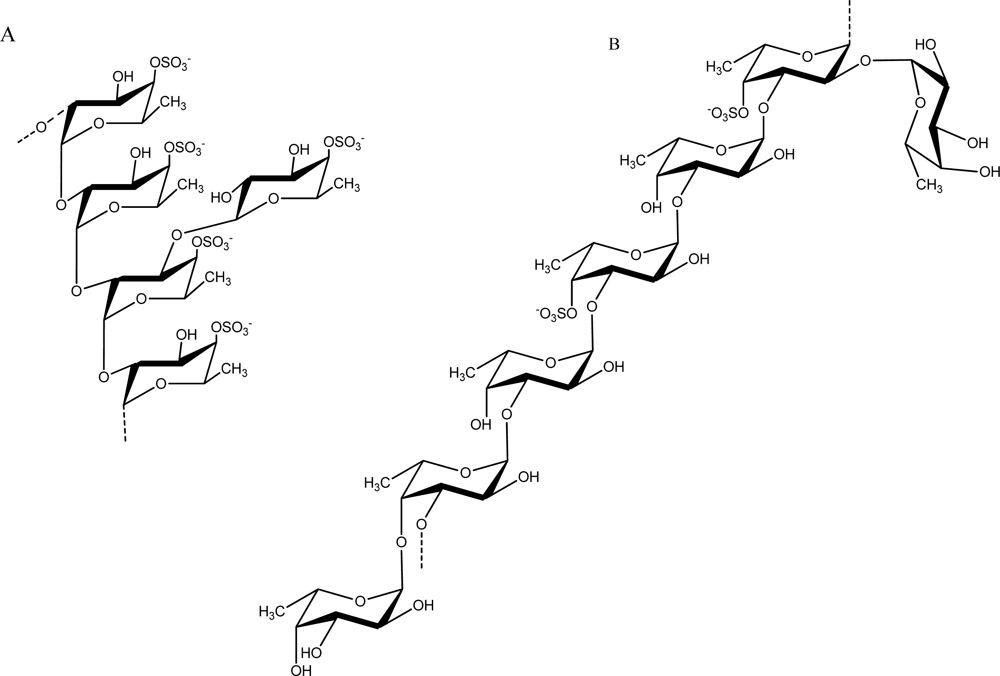
Fucans are sulfated polysaccharides that are composed of a fucose backbone. One of the best studied fucans from brown algae is fucoidan, which was first isolated by Kylin in 1913 [54]. The fucoidan from Fucus vesiculosus has been available commercially for decades (Sigma-Aldrich Chemical Company, St. Louis, MO, U.S.). Early work on its structure showed that it contained primarily (1→2) linked 4-O-sulfated fucopyranose residues [55]. However, 3-linked fucose with 4-sulfated groups were subsequently reported to be present on some of the fucose residues [56]. Additionally, it was determined to contain branches every 2–3 fucose residues. These early structures of fucoidan from F. vesiculosus are illustrated in Figure 3. Subsequently, Chevolot and colleagues reported that the fucoidan from F. vesiculosus and Ascophyllum nodosum contains a predominant disaccharide motif containing sulfate at the 2-position of the 3-linked fucose and sulfate groups on the 2- and 3-positions of the 4-linked fucose [57].
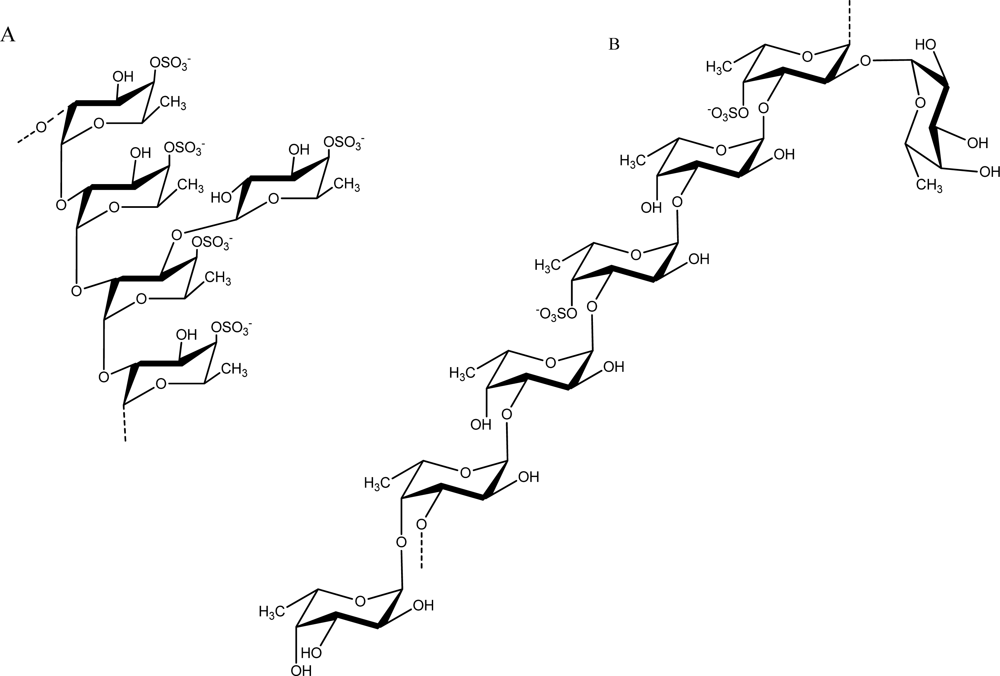
Figure 3.
Structure of fucoidan prepared from F. vesiculosus by Percival (A) [55] and Patankar (B) [56].
Fucans can differ in structure among algal species and can vary even within the same species. Because of the heterogeneity in structures within seaweed, differing extraction conditions used by researchers can give rise to the isolation of distinct fucan forms [4]. Fucans have been classified into two groups [58]. One group includes the fucans from Laminaria saccharina, L. digitata, Analipus japonicus, Cladosiphon okamuranus, and Chorda filum that have their central chains composed of (1→3)-linked α-l-fucopyranose residues. A second group included fucans isolated from Ascophyllum nodosum and Fucus species that have their central chains composed of repeating (1→3)- and (1→4)-linked α-l-fucopyranose residues. However, many studies have revealed more complex fucans some with branching structures. A fucoidan isolated from Turbinaria conoides was shown to be highly complex, with 33–34% terminals, 27–28% linked and 21–22% branched in the (1→3)-linked main chain [59]. Representative fucan structures are illustrated in Table 1.

Table 1.
Representative structures reported for fucans from brown algae.
Many fucans from brown algae contain small amounts of other monosaccharides, including glucose [68], galactose [69], mannose [70–72], xylose [69,73], uronic acids [68,74] and also acetyl groups [75].
3. Approaches in Structural Analysis of Algal Sulfated Polysaccharides
3.1. Desulfation and Methylation for Structure Analysis
Structure analysis of sulfated polysaccharides requires determination of the attached sulfate esters along their backbones and the glycosidic linkage types. Methylation is used to determine linkages between monsaccharides. By comparing the methylation of native polysaccharides to that of their desulfated counterparts, the positions of the sulfate groups can be determined. Thus, hydrolysis of the permethylated, desulfated polysaccharides, yields partially methylated monosaccharides, which following acetylation are separated and identified on gas chromatography/mass spectra [76].
Accurate structural determination requires desulfation of the polysaccharide without cleavage of the polysaccharides chain linkages. Often a solvolytic desulfation procedure is used wherein the polysaccharide as a pyridinium salt is heated in dimethyl sulfoxide [47,77,78]. Desulfation of fucoidan by methyl sulfoxide-pyridine is rapid and complete, resulting in higher yields with little degradation. However, a method using chlorotrimethylsilane (CTMS) for treatment of pyridinium salts is more appropriate for desulfation of sulfated galactans of both the agaran and carrageenan families [79]. Other approaches for desulfation that have been used involve methanolic hydrogen chloride [80], silylating reagents [81], and pyromellitic acid [82]. Chemical desulfation is relatively non-specific and usually results in a significant loss of sample material. The use of sulfatases represents a more specific approach to desulfation and would be advantageous in structural studies, yet such enzymatic approaches do not appear to be a frequent method of choice [7,83]. The reason for this is unclear but could be due to the lack of commercially available enzymes of the required specificity.
The typical methylation procedure is straightforward, involving treating the desulfated polysaccharide sample with methyl iodide in the presence of solid base, usually sodium hydroxide, in methyl sulfoxide and the procedure can be repeated to obtain a complete methylation [51,62,77,84,85].
3.2. Structural Analysis by Using NMR and MS
A powerful tool for the structural elucidation of sulfated polysaccharides is NMR spectroscopy, which can provide structural details such as the monosaccharide components, linkages, anomeric configurations, and positions of branching or sulfations. This can be done by combining various 1D and 2D-NMR techniques. The use of NMR in the structural analysis of red algal galactans and green algal ulvans has been considerable [3,86]. In part, this has been due to the relative high proportion of repeating sequences (identical sulfation pattern) in these polysaccharides that make them amenable to analysis by 13C-NMR. For example, Gonçalves and colleagues described the structural elucidation by NMR of positional isomers of sulfated oligosaccharides obtained from agarans and carrageenans [87]. This entailed partial reductive hydrolysis to produce oligosaccharides from repetitive galactans followed by separation by anion exchange and gel-filtration chromatography prior to 1D and 2D NMR analysis.
Effort has also been made to elucidate the structures of fucans by NMR. Usov et al., by 1D-NMR, found the sulfated fucans from Saccharina latissima (formerly Laminaria saccharina) to be 1→3 linked α-l-fucopyranose with a sulfate group at C4 and branched at C2 [66]. More recently, they confirmed the complex structure of this fucoidan with a more detailed structural investigation by 2D-NMR [88]. These studies also revealed the presence of three additional sulfated polysaccharide types, a fucogalactan, a fucoglucuronomannan and a fucoglucuronan, that appear to occur in minor amounts in their preparation.
Mass spectrometry (MS) is valuable in the structural analysis of polysaccharides, as it generates accurate molecular mass data for oligosaccharides and it can also provide sequence information. Compared with other analytical techniques, mass spectrometric methods have several advantages, including low sample consumption (e.g., picomole quantities) and short analysis time. While analysis of sulfated polysaccharides by MS can be problematic due to the labile nature of the sulfate groups, approaches based on electrospray ionization and matrix-assisted laser desorption/ionization (MALDI) are increasingly being developed to characterize sulfated oligosaccharides [89–92]. Negative-ion ESI-CID-MS/MS was used to characterize oligosaccharide fragments derived from mild hydrolysis of κ-carrageenan that revealed highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides [92]. Similarly, fucan oligosaccharides from A. nodosum, including a highly sulfated pentasaccharide, were analyzed successfully by negative ion ESI-MS [89].
3.3. Oversulfation of Algal Polysaccharides
Structure modification of sulfated polysaccharides, such as desulfation, oversulfation, acetylation and benzoylation, would allow the development of new and possibly more effective derivatives of naturally occurring polysaccharides [93–95]. For example, benzoylated derivatives of native ulvan from Ulva pertusa exhibited enhanced antioxidant properties [94]. Certainly, the most frequent structural modification to sulfate polysaccharides is oversulfation due to the typically strong positive correlation between their sulfate content and biological activity (discussed further in Section 4). A number of methods have been developed for polysaccharide oversulfation, such as treatment with sulfuric acid, sulfur trioxide-pyridine, chlorosulfonic acid-pyridine, dimethylformamide and sulfur trioxide-dimethylamine [96–102].
3.4. Molecular Size Modification of Algal Sulfated Polysaccharides
Lower molecular weight algal sulfated polysaccharides can be prepared by chemical, physical or enzymatic means to obtain oligosaccharides with more diverse bioactivities. For example, linear or branched sulfated galactans and fucans can be cleaved by mild acid hydrolysis [99,102] or by a radical process involving a hydrogen peroxide-cupric redox system [98,101]. The chemical methods are easy to perform but lack specificity. Minor changes in temperature or acidity can lead to variations in oligosaccharide sizes and sulfation patterns, while strong acid may alter the sulfation pattern or destroy the polysaccharide chain [97]. Enzymatic degradation of sulfated polysaccharides can be achieved by selecting enzymes such as hydrolases, fucoidanases (EC 3.2.1.44), α-l-fucosidases (EC 3.2.1.51) and galactosidases [96,103] to target glycosidic bonds while preserving the sulfate groups [100]. Fucoidanases and galactosidases have been identified in marine invertebrates and microorganisms [83,104–108]. Some bioactivities of lower molecular weight oligosaccharides prepared from algal sulfated polysaccharides are highlighted in Table 2.

Table 2.
Biological activities of selected low molecular weight oligosaccharides derived from algal sulfated polysaccharides.
4. Bioactivity and Structure-Activity Relationship
Due to the difficulties in identifying the chemical structure of algal sulfated polysaccharides, the relation between their structures and biological activities is not completely understood. One of the research approaches in establishing structure-function relationships has been to make inferences based on information obtained from studies of invertebrate sulfated polysaccharides that have a regular structure and so are more easily studied. For example, Pereira and colleagues compared branched fucans from brown seaweeds with the more simple linear versions obtained from sea cucumber and sea urchins [116]. The anticoagulant activity of seaweed fucans was shown to depend on their molecular weight, the extent of sulfation, and the distribution of sulfate groups in the repeating units. Interestingly, the algal fucans were found to inhibit coagulation through a direct inhibition of thrombin whereas the invertebrate fucans inhibited the enzyme indirectly with a requirement for antithrombin and heparin cofactor II.
In this section, we will highlight the key biological activities that have been reported for algal sulfated polysaccharides, the current knowledge regarding their mode of action and the structural requirements necessary to elicit these effects.
4.1. Anticoagulant and Antithrombotic Activities
Probably the most widely recognized and studied bioactivity of marine sulfated polysaccharides is the heparin-like anticoagulant activity exhibited by fucoidans and other fucans of brown seaweeds. This was first reported for fucoidan isolated from F. vesiculosus by Springer and colleagues who found inhibition of fibrin clot formation and antithrombin activity [117,118]. Since then, studies on fucans from various seaweeds have revealed anticoagulant and antithrombotic activities and are discussed in several recent reviews [1,4,6].
The basis for these activities is not completely understood, but a number of investigations suggest more than one mechanism of action including direct and indirect inhibition of thrombin through the activation of thrombin inhibitors (e.g antithrombin and heparin cofactor II) [116,119–121]. Recently, Cumashi and colleagues reported that fucans from 10 brown seaweeds each prolonged the clotting time of human plasma; however, only five of these fucans had significant activity against thrombin-induced platelet aggregation [122]. While the results of the latter assay are suggestive of a direct action of certain fucans on thrombin, the authors pointed out that an interfering action of thrombin binding to its receptors on platelets could not be ruled out.
The general structural features of fucans that are important in their anticoagulation activity include sugar composition, molecular weight, sulfation level and the position of sulfate groups on the sugar backbone [70,123–125]. For example, Nishino and colleagues found that a higher content of fucose and sulfate groups coincided with higher anticoagulant activities in sulfated polysaccharide fractions from E. kurome [70]. They also showed that anticoagulation activity of fucans was positively correlated with sulfate content and that only fucans with a sulfate-total sugar residue ratio greater than one possessed significant activity [93,123]. Higher molecular weight fucans (e.g., 27 and 58 kDa) showed greater anticoagulant activity than lower molecular weight ones (∼10 kDa) [126]. The relationship between molecular weight of sulfated polysaccharides and their anticoagulant activity was also considered by Pomin and colleagues who reported that linear, sulfated fucan required significantly longer chains than mammalian glycosaminoglycans to achieve anticoagulant activity [125]. Selective cleavage to reduce molecular size of the fucan by only a modest amount dramatically reduced its effect on thrombin inactivation mediated by heparin cofactor II. Lower molecular weight fucans appear to bind to heparin cofactor II but, unlike the native (full length) fucan, were unable to effectively facilitate the heparin cofactor II interaction with thrombin [125]. Chevolot et al. reported on the importance of sulfate group location on the sugar residues for anticoagulant activity [57,62]. Studying the fucoidan from A. nodosum, they found that anticoagulant activity required 2-O-sulfated and 2,3-O disulfated fucose residues, whereas sulfation at the O-4 position did not appear necessary.
Marine sulfated polysaccharides other than fucans have also been shown to possess anticoagulant activities. Reports include sulfated galactan and ulvan-like sulfated polysaccharides obtained from green algae, in particular from species of Codium and Ulva [44,45,49,127,128]. For example, Mao et al. described a sulfated polysaccharide from U. conglobata with high rhamnose content and 35% sulfate ester that prolonged clotting time through what appeared to be direct inhibition of thrombin and modulation of heparin cofactor II [49]. Hayakawa et al. tested sulfated polysaccharides from 23 green algae species for anticoagulant activity and discovered a high rhamnose-containing sulfated polysaccharide from Monostroma nitidum, the purified version of which was more potent than standard heparin [127].
Red seaweeds have also yielded a number of sulfated polysaccharides with potent anticoagulant activities [129–131]. Studies on a sulfated galactan from the red seaweed Botryocladia occidentalis are particularly illustrative. Farias et al. reported that a 2,3-di-O-sulfated d-galactan from B. occidentalis exhibited anticoagulant activity, comparable to heparin, which appeared to be due to inhibition of thrombin and factor X. Its activity was more potent than similar sulfated galactans, from invertebrate sources, that had only one sulfate per galactose residue [129]. A similar polysaccharide chain from G. crinale, but with lower amounts of 2, 3-di-O-sulfated d-galactose, was less potent in a clotting time assay when compared with that from B. occidentalis [131]. The two sulfated polysaccharides did not differ in thrombin inhibition mediated by antithrombin; however, in assays where heparin cofactor II was used in place of antithrombin, the sulfated galactan from G. crinale was less inhibitory than that from B. occidentalis. Yet the sulfated galactan from G. crinale was a more potent anticoagulant than that from B. occidentalis when Factor X was the target protease. These observations suggested that the proportion and/or the distribution of 2,3-di-sulfated galactose along the polysaccharide chain modulate the interaction of the polysaccharides with specific proteases in the coagulation system. Recently, Glauser et al. showed that the 2,3-disulfated galactan from B. occidentalis inhibits intrinsic tenase and prothrombinase complexes that are critical for factor Xa and thrombin generation, respectively [130]. The sulfated galactan interacts with the heparin-binding site on the heavy chain of factor Xa. Interestingly, the anticoagulant activities associated with the sulfated galactan and that of heparin are modulated differently by heparin cofactor II; heparin anticoagulant activity was enhanced in plasma devoid of heparin cofactor II, whereas the activity of the sulfated galactan was independent of this cofactor.
Heparin is used extensively for the prevention of venous thrombosis and the treatment of other thromboembolic disorders due to its inhibition of thrombin and other enzymes in the coagulation system. To overcome the obvious potential side-effect of bleeding, researchers have investigated means of reducing the anticoagulant activities of heparin while enhancing its anti-thrombotic activities including chemical modification and fractionation of native heparin to lower molecular forms [132–134]. Nevertheless, the development of antithrombotic algal polysaccharides would be advantageous since their use would avoid the potential for contamination with prions or viruses in commercial heparins, which are obtained from pig and bovine intestine. Moreover, with more specific activities and/or targets, the algal sulfated polysaccharides could find applications complementary to heparin [132]. To this end, one of the approaches has been to develop low molecular weight (LMW) fucoidans [121,135]. For example, a LMW fraction of approximately 8,000 derived from the fucoidan of A. nodosum reduced mean thrombus weight by 80% vs. control saline injection in a rabbit model of venous thrombosis [135]. This LMW fucoidan and related derivatives [136] are promising since they show lower effects in coagulation tests when compared to the commercial LMW heparin, dalterparin (Fragmen®, Pfizer Inc.). More recently, Rocha and colleagues reported that a sulfated galactofucan from the brown seaweed Spatoglossum schoederi has potent antithrombotic activity in a rat model of venous thrombosis [69]. Unlike heparin, which produces a rapid but transient antithrombotic effect, the in vivo action of this sulfated galactofucan progressed slowly, showing maximal effectiveness about eight hours post injection. When tested in vitro using endothelial cells, it was discovered that the galactofucan stimulates the production of heparan sulfate leading to the hypothesis that its delayed action in vivo is tied to the need for an accumulation of the heparan sulfate on blood vessel surfaces. Despite its high sulfation level, the galactofucan lacks significant anticoagulation activity, making it an ideal candidate as an antithrombotic agent [69].
4.3. Immuno-Inflammatory Activity
Sulfated polysaccharides, including those from algae, have been shown to possess immunomodulatory activities that may be of potential application in stimulating the immune response or in controlling immune cell activity to mitigate associated negative effects such as inflammation [152]. Sulfated polysaccharides may affect multiple targets in the immune and inflammatory systems that can have impact on disease progression and outcome including tumor progression and metastasis [153].
One of the interests in algal sulfated polysaccharides as anti-inflammatory agents is the growing body of evidence illustrating their ability to interfere with the migration of leukocytes to sites of inflammation. For example, in a rabbit model of bacterial meningitis, leukocyte rolling was markedly reduced by intravenous infusion of fucoidan [154]. Similarly, intravenous addition of fucoidan reduced, in a dose-dependent manner, leukocyte recruitment to peritoneum in a rat model of peritoneal inflammation [155]. These effects were ascribed to the binding of fucoidan to L- and P selectins, cell adhesion molecules essential in the recruitment process. Both of these studies used the fucoidan from Sigma-Aldrich Chemical Co (St. Louis, MO, U.S.) that is sourced from F. vesiculosus. Fucans from other seaweeds including Laminaria spp., Fucus spp., A. nodosum, and C. okamuranus also inhibit leukocyte recruitment to the abdominal cavity during acute peritonitis in rats [122]. In addition to impairing the action of selectins, algal sulfated polysaccharides inhibit tissue degradative enzymes such as heparanase and elastases that are involved in the breakdown of basement membrane integrity during inflammation [156,157].
One of the major and potentially promising activities is the potent inhibitory effect of sulfated fucans on human complement activation. The original observations showed that fucoidan fractions from A. nodosum potently inhibit both the classical and alternative pathways in human serum [158]. Tissot and colleagues have extensively studied this activity [159–162]. It was determined that low molecular weight fucoidan fractions bind to the C1q subunit of the C1 complex that triggers complement through recognition and binding of immune complexes [162]. The binding of fucoidan appears to interfere with the ability of C1q to fully trigger C1 activation [160]. Fucoidan also binds C4, thereby preventing its breakdown and generation of its cleavage product C4b, the latter being required for the formation of C3 convertase and the propagation of complement [162]. Furthermore, it was found that fucoidan binds C1q globular heads and may interfere with C1q recognition of IgG [161]. Recently, using NMR, it was found that branched fucoidan oligosaccharides are better at inhibiting complement compared to linear structures [159].
The interaction of algal sulfated polysaccharides with the complement system suggests that they may have utility in influencing innate immunity to reduce the pro-inflammatory state or other detrimental conditions such as allergic reactions arising during the innate immune response. In addition, there is a growing body of evidence that algal polysaccharides can regulate the innate immune response directly by binding to pattern recognition receptors (PRRs) such as the mannose receptor and toll-like receptors on phagocytic cells including macrophages [152]. For example, λ-carrageenan stimulated mouse T cell cultures in a toll-like receptor-4 (TLR4) dependent manner [163] generating a T helper 1 (Th1) patterned cytokine response. However, splenocytes prepared from TLR4-deficient mice still retained some ability to produce interferon-γ in response to λ-carrageenan suggesting that PRRs other than TLR4 were also elicited. In mice immunized with ovalbumin to produce an allergic reaction, oral dosing with λ-carrageenan lead to a reduction in ovalbumin-specific IgE and serum histamine release, suggesting that λ-carrageenan might be used to ameliorate allergic reactions. Similar results were reported for mekabu fucoidan from U. pinnatifida [164].
Direct stimulatory effects of algal polysaccharides on immune cells results in production of nitric oxide through induction of inducible nitric oxide synthase (iNOS) and a pro-inflammatory cytokine/chemokine profile [165]. Depending on the situation, the interaction of sulfated polysaccharides with other effectors may result in reduced inflammation. For example, fucoidan from F. vesiculosus induced iNOS in RAW264.7 macrophage cells leading to enhanced production of nitric oxide [166,167]. Yet, in the presence of lipopolysaccharide (LPS), the fucoidan impaired LPS-induced expression of iNOS and nitric oxide production [167]. Similarly, fucoidan suppresses interferon gamma-induced iNOS expression in macrophage and glial cell types [168].
These and other reports of algal sulfated polysaccharides directly stimulating the innate immune system [165,168–170] suggests that they may find therapeutic use in opposing T helper 2 (Th2)-based pathologies such as autoimmune disorders and allergy. Additionally, there is evidence that algal sulfated polysaccharides including fucoidans and carrageenans increase the cytotoxicity of natural killer cells, lymphocytes and macrophages against tumors [169,171].
The structural requirements for this immunostimulatory activity of algal sulfated polysaccharides have not been greatly studied. One report, by Leiro and colleagues, has shown greatly diminished immunostimulatory activity of ulvan-like polysaccharides from U. rigida when they were desulfated [165].
4.4. Antioxidant Activities
Algal sulfated polysaccharides, until recently, were largely ignored as sources of antioxidant activity. Studies over the last several years reveal that sulfated polysaccharides from a number of seaweeds have appreciable antioxidant capability [95,172–177]. For example, fucans from F. vesiculosus exhibited considerable ferric reducing/antioxidant power [172] and superoxide radical scavenging ability [173]. Fucan fractions from L. japonica also showed significant antioxidant capabilities in superoxide radical and hydroxyl radical scavenging assays [174,176,177]. Superoxide radical scavenging activity correlated positively with the sulfate content of the polysaccharide fractions [173,176]. Antioxidant properties of carrageenans [173] and ulvans [94] also appeared related to sulfate content. In the latter study, high sulfate content derivatives of ulvan showed improved antioxidant activities [94]. Interestingly, metal chelating, free radical and hydroxyl radical scavenging activities of fucan fractions appear to relate to their ratio of sulfate content/fucose [176].
4.5. Antilipidemic Effects
Algal sulfated polysaccharides exert lipid-lowering and other beneficial properties in hyperlipidemic animal models [178–180]. An extract from F. vesiculosus, in a dose-dependent manner, effectively reduced the elevation in serum triglyceride and total cholesterol levels in triton-induced-hyperlipemic rats. In rats fed a high cholesterol diet for 21 days, supplementation of the diet with ulvan from U. pertusa led to reductions in serum total cholesterol and LDL-cholesterol with no significant alteration in serum triglycerides [180]. The effects of ulvan were modified when it was degraded into lower molecular weight fractions. Ulvan derivatives of lower molecular weight and intrinsic viscosity did not reduce serum cholesterol but did normalize the hypertriglyceridemia of these animals and raised HDL-cholesterol. The underlying mechanisms of these actions are unclear but it does not appear to involve bile acid sequestration since ulvan and its lower molecular weight derivatives increased bile excretion to a similar extent.
Recently, it was reported that fucoidan from L. japonica reduced serum total and LDL-cholesterol and triglycerides and raised HDL-cholesterol in a hyperlipidemic rat model [179]. The treatment also increased the activities of lipoprotein lipase (LPL), hepatic lipase (HL) and lecithin cholesterol acyltranferase (LCAT) in serum. These changes in enzyme activities could be the direct result of fucoidan treatment or an indirect effect associated with improvement in lipid profile. Certainly fucoidan and other algal sulfated polysaccharides may influence LPL and HL through interaction with well-characterized heparin-binding sites on these enzymes. Consistent with this is the observation that fucoidan from F. vesiculosus releases LPL from cell surface binding sites and stabilizes LPL activity in culture medium [181].
Algal sulfated polysaccharides are showing promising effects in addressing the hyperlipidemia associated with certain drug toxicities. Fucans from S. polycystum were shown to have significant preventive effects on the elevation of cholesterol and triglycerides in serum and liver tissue resulting from acetaminophen-induced toxic hepatitis [182]. Treatment also partially reversed the reduction in hepatic LCAT and HL and improved overall histological appearance of the liver. Similarly, a sulfated polysaccharide from S. wightii reduced hyperlipidemia and normalized LPL and LCAT in plasma in cyclosporine A-induced nephrotoxicity [183]. The excretion of urea, uric acid, and creatinine were normalized by the sulfated polysaccharide treatment. In addition, the susceptibility of LDL to oxidation was reduced, suggesting that the antioxidant activity of the sulfated polysaccharide was also playing a role and may contribute to its renoprotective activity.
5. Future Perspectives
Algal sulfated polysaccharides are a source of numerous biological activities that may find therapeutic benefit. They are structurally diverse and heterogeneous, which makes studies of their structures challenging, and may also have hindered their development as therapeutic agents to date. The production of a standardized commercial product based on algal sulfated polysaccharide constituents will be a challenge since their structural and pharmacological features may vary depending on species and on location and time of harvest. For example, Bourgougnon and colleagues reported that there was a significant annual variation in the composition and the in vitro anti-HIV-1 activity of a water-soluble sulfated glucuronogalactan from Schizymenia dubyi [184]. Another issue to the therapeutic use of algal polysaccharides is their potentially low bioavailability given their often high molecular weights. It is likely, based on observations with heparin [185], that algal sulfated polysaccharides will display some, albeit low, degree of oral bioavailability. A recent pilot study in humans reported that fucoidan was ineffective as an oral anticoagulant agent [186], which underscores the issue. It also emphasizes the importance of understanding the structural requirements for biological activity and whether low molecular weight derivatives, which are potentially more bioavailable, remain active. For some applications, low bioavailability may not be a concern. First, some of the hypolipidemic effects of seaweed sulfated polysaccharides arise through effects on bile acid sequestration in the intestinal lumen. Second, for some immunomodulatory activities, the site of activation of the immune system may also be within the intestinal lumen (e.g., at Peyer’s patches) as has been hypothesized for immunomodulatory effects of polysaccharide constituents from Chlorella pyrenoidosa [187]. Finally, algal sulfated polysaccharides are already used topically in cosmetics and there is significant interest in further development for cosmetics and cosmeceuticals products [188].
Acknowledgments
This work was supported in part by Program for Changjiang Scholars and Innovative Research Team in University (IRT0944), Special Fund for Marine Scientific Research in the Public Interest (201005024), Natural Science Foundation of China (31070724), and a scholarship to G.J. jointly funded by the China Scholarship Council, Ministry of Education and the National Research Council of Canada. The authors thank K. Vanya Ewart (NRC-IMB) for her careful reading of the manuscript and helpful suggestions.
- Samples Availability: Available from the authors.
References
- Kusaykin, M; Bakunina, I; Sova, V; Ermakova, S; Kuznetsova, T; Besednova, N; Zaporozhets, T; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol J 2008, 3, 904–915. [Google Scholar]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J Appl Phycol 2001, 13, 173–184. [Google Scholar]
- Lahaye, M; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar]
- Li, B; Lu, F; Wei, X; Zhao, R. Fucoidan: structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar]
- Pomin, VH. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj J 2010, 27, 1–12. [Google Scholar]
- Pomin, VH; Mourao, PAS. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar]
- Usov, AI; Bilan, I. Fucoidans-Sulfated polysaccharides of brown algae. Russ Chem Rev 2009, 78, 785–799. [Google Scholar]
- Wijesekara, I; Pangestuti, R; Kim, S-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr Polym 2010, in press.. [Google Scholar]
- Berteau, O; Mulloy, B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar]
- Knutsen, SH; Myslabodski, DE; Larsen, B; Usov, AI. A modified system of nomenclature for red algal galactans. Bot Mar 1994, 37, 163–170. [Google Scholar]
- Anderson, NS; Dolan, TCS; Rees, DA. Carrageenans. Part VII. Polysaccharides from Eucheuma spinosum and Eucheuma cottonii. The covalent structure of l-carrageenan. J Chem Soc Perkin Trans I 1973, 2173–2176. [Google Scholar]
- Estevez, JM; Ciancia, M; Cerezo, AS. The system of low-molecular-weight carrageenans and agaroids from the room-temperature-extracted fraction of Kappaphycus alvarezii. Carbohydr Res 2000, 325, 287–299. [Google Scholar]
- Funami, T; Hiroe, M; Noda, S; Asai, I; Ikeda, S; Nishinari, K. Influence of molecular structure imaged with atomic force microscopy on the rheological behavior of carrageenan aqueous systems in the presence or absence of cations. Food Hydrocolloids 2007, 21, 617–629. [Google Scholar]
- Zhou, G; Sheng, W; Yao, W; Wang, C. Effect of low molecular [lambda]-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol Res 2006, 53, 129–134. [Google Scholar]
- Doyle, JP; Giannouli, P; Rudolph, B; Morris, ER. Preparation, authentication, rheology and conformation of theta carrageenan. Carbohydr Polym 2010, 80, 648–654. [Google Scholar]
- Yang, B; Yu, G; Zhao, X; Ren, W; Jiao, G; Fang, L; Wang, Y; Du, G; Tiller, C; Girouard, G; Barrow, CJ; Ewart, HS; Zhang, J. Structural characterisation and bioactivities of hybrid carrageenan-like sulphated galactan from red alga Furcellaria lumbricalis. Food Chem 2011, 124, 50–57. [Google Scholar]
- van de Velde, F; Antipova, AS; Rollema, HS; Burova, TV; Grinberg, NV; Pereira, L; Gilsenan, PM; Tromp, RH; Rudolph, B; Grinberg, VY. The structure of kappa/iota-hybrid carrageenans II. Coil-helix transition as a function of chain composition. Carbohydr Res 2005, 340, 1113–1129. [Google Scholar]
- Chopin, T; Kerin, BF; Mazerolle, R. Phycocolloid chemistry as a taxonomic indicator of phylogeny in the Gigartinales, Rhodophyceae: A review and current developments using Fourier transform infrared diffuse reflectance spectroscopy. Pharmacol Res 1999, 47, 167–188. [Google Scholar]
- Hilliou, L; Larotonda, FDS; Abreu, P; Ramos, AM; Sereno, AM; Gonealves, MP. Effect of extraction parameters on the chemical structure and gel properties of kappa/iota-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol Eng 2006, 23, 201–208. [Google Scholar]
- Hilliou, L; Wilhelm, M; Yamanoi, M; Gonclves, MP. Structural and mechanical characterization of [kappa]/[iota]-hybrid carrageenan gels in potassium salt using Fourier Transform rheology. Food Hydrocolloids 2009, 23, 2322–2330. [Google Scholar]
- Jouanneau, D; Guibet, M; Boulenguer, P; Mazoyer, J; Smietana, M; Helbert, W. New insights into the structure of hybrid [kappa]-/[mu]-carrageenan and its alkaline conversion. Food Hydrocolloids 2010, 24, 452–461. [Google Scholar]
- van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocolloids 2008, 22, 727–734. [Google Scholar]
- Yu, G; Hu, Y; Yang, B; Zhao, X; Wang, P; Ji, G; Wu, J; Guan, H. Extraction, isolation and structural characterization of polysaccharides from a red alga Gloiopeltis furcata. J Ocean Univ China Nat Sci 2010, 9, 193–197. [Google Scholar]
- Morrice, LM; McLean, MW; Long, WF; Williamson, FB. Porphyran primary structure. Hydrobiologia 1984, 116–117, 572–575. [Google Scholar]
- Zhang, Q; Qi, H; Zhao, T; Deslandes, E; Ismaeli, NM; Molloy, F; Critchley, AT. Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta). Carbohydr Res 2005, 340, 2447–2450. [Google Scholar]
- Zhang, Z; Zhang, Q; Wang, J; Zhang, H; Niu, X; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int J Biol Macromol 2009, 45, 22–26. [Google Scholar]
- Zhang, Q; Li, N; Liu, X; Zhao, Z; Li, Z; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr Res 2004, 339, 105–111. [Google Scholar]
- Miller, IJ; Furneaux, RH. The structural determination of the agaroid polysaccharides from four New Zealand algae in the order Ceramiales by means of 13C-NMR Spectroscopy. Bot Mar 1997, 40, 333–340. [Google Scholar]
- Prado, HJ; Ciancia, M; Matulewicz, MC. Agarans from the red seaweed Polysiphonia nigrescens (Rhodomelaceae, Ceramiales). Carbohydr Res 2008, 343, 711–718. [Google Scholar]
- Miller, IJ. Evaluation of the structures of polysaccharides from two New Zealand members of the Ceramiaceae. Bot Mar 2003, 46, 378–385. [Google Scholar]
- Gonçalves, AG; Ducatti, DRB; Duarte, MER; Noseda, MD. Sulfated and pyruvylated disaccharide alditols obtained from a red seaweed galactan: ESIMS and NMR approaches. Carbohydr Res 2002, 337, 2443–2453. [Google Scholar]
- Duarte, MER; Cauduro, JP; Noseda, DG; Noseda, MD; Gonçalves, AG; Pujol, CA; Damonte, EB; Cerezo, AS. The structure of the agaran sulfate from Acanthophora spicifera (Rhodomelaceae, Ceramiales) and its antiviral activity. Relation between structure and antiviral activity in agarans. Carbohydr Res 2004, 339, 335–347. [Google Scholar]
- Stortz, CA; Cerezo, AS. Novel findings in carrageenans, agaroids and “hybrids” red seaweed galactans. Curr Top Phytochem 2000, 4, 121–134. [Google Scholar]
- Cases, MR; Stortz, CA; Cerezo, AS. Structure of the ‘corallinans’-sulfated xylogalactans from Corallina officinalis. Int J Biol Macromol 1994, 16, 93–97. [Google Scholar]
- Navarro, DA; Ricci, AM; Rodríguez, MC; Stortz, CA. Xylogalactans from Lithothamnion heterocladum; a crustose member of the Corallinales (Rhodophyta). Carbohydr Polym. In Press..
- Navarro, DA; Stortz, CA. The system of xylogalactans from the red seaweed Jania rubens (Corallinales, Rhodophyta). Carbohydr Res 2008, 343, 2613–2622. [Google Scholar]
- Martone, PT; Navarro, DA; Stortz, CA; Estevez, JM. Differences in polysaccharide structure between calcified and uncalcified segments in the coralline Calliarthron cheilosporioides (Corallinales, Rhodaphyta). J Phycol 2010, 46, 507–515. [Google Scholar]
- Lim, BL; Ryu, IH. Purification, structural characterization, and antioxidant activity of antioxidant substance from the red seaweed Gloiopeltis tenax. J Med Food 2009, 12, 442–451. [Google Scholar]
- Mandal, P; Pujol, CA; Carlucci, MJ; Chattopadhyay, K; Damonte, EB; Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry 2008, 69, 2193–2199. [Google Scholar]
- Lahaye, M; Ray, B. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta)-NMR analysis of ulvan oligosaccharides. Carbohydr Res 1996, 283, 161–173. [Google Scholar]
- Percival, E; McDowell, RH. Chemistry and Enzymology of Marine Algal Polysaccharides; Academic Press: New York, NY, USA, 1967; p. 219. [Google Scholar]
- Lahaye, M; Brunel, M; Bonnin, E. Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr Res 1997, 304, 325–333. [Google Scholar]
- Love, J; Percival, E. The polysaccharides of the green seaweed Codium fragile. Part II. The water-soluble sulphated polysaccharides. J Chem Soc 1964, 3338–3345. [Google Scholar]
- Matsubara, K; Matsuura, Y; Bacic, A; Liao, ML; Hori, K; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int J Biol Macromol 2001, 28, 395–399. [Google Scholar]
- Farias, EHC; Pomin, VH; Valente, AP; Nader, HB; Rocha, HAO; Mourao, PAS. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar]
- Matsubara, K; Matsuura, Y; Bacic, A; Liao, ML; Hori, K; Miyazawa, K. Anticoagulant properties of a sulfated galactan preparation from a marine green alga, Codium cylindricum. Int J Biol Macromol 2001, 28, 395–399. [Google Scholar]
- Bilan, MI; Vinogradova, EV; Shashkov, AS; Usov, AI. Structure of a highly pyruvylated galactan sulfate from the Pacific green alga Codium yezoense (Bryopsidales, Chlorophyta). Carbohydr Res 2007, 342, 586–596. [Google Scholar]
- Shevchenko, N; Burtseva, Y; Zvyagintseva, T; Makar'eva, T; Sergeeva, O; Zakharenko, A; Isakov, V; Thi Linh, N; Xuan Hoa, N; Minh Ly, B; Van Huyen, P. Polysaccharides and sterols from green algae Caulerpa lentillifera and C sertularioides. Chem Nat Compd 2009, 45, 1–5. [Google Scholar]
- Mao, W; Zang, X; Li, Y; Zhang, H. Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J Appl Phycol 2006, 18, 9–14. [Google Scholar]
- Ghosh, P; Adhikari, U; Ghosal, PK; Pujol, CA; Carlucci, MJ; Damonte, EB; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 2004, 65, 3151–3157. [Google Scholar]
- Lee, J-B; Koizumi, S; Hayashi, K; Hayashi, T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr Polym 2010, in press.. [Google Scholar]
- Ray, B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr Polym 2006, 66, 408–416. [Google Scholar]
- Harada, N; Maeda, M. Chemical structure of antithrombin-active Rhamnan sulfate from Monostrom nitidum. Biosci Biotechnol Biochem 1998, 62, 1647–1652. [Google Scholar]
- Kylin, H. biochemistry of sea algae. Phys Chem 1913, 83, 171–197. [Google Scholar]
- Conchie, J; Percival, EGV. Fucoidin. Part II. The hydrolysis of a methylated fucoidin prepared from Fucus vesiculosus. J Chem Soc 1950, 827–832. [Google Scholar]
- Patankar, MS; Oehninger, S; Barnett, T; Williams, RL; Clark, GF. A revised structure for fucoidan may explain some of its biological activities. J Biol Chem 1993, 268, 21770–21776. [Google Scholar]
- Chevolot, L; Mulloy, B; Ratiskol, J; Foucault, A; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr Res 2001, 330, 529–535. [Google Scholar]
- Ushakova, NA; Morozevich, GE; Ustyuzhanina, NE; Bilan, MI; Usov, AI; Nifantiev, NE; Preobrazhenskaya, ME. Anticoagulant activity of fucoidans from brown algae. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry 2009, 3, 77–83. [Google Scholar]
- Chattopadhyay, N; Ghosh, T; Sinha, S; Chattopadhyay, K; Karmakar, P; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem 2010, 118, 823–829. [Google Scholar]
- Bilan, MI; Zakharova, AN; Grachev, AA; Shashkov, AS; Nifant’ev, NE; Usov, AI. Polysaccharides of algae: 60. Fucoidan from the Pacific brown alga Analipus japonicus (Harv.) Winne (Ectocarpales, Scytosiphonaceae). Bioorg Khim 2007, 33, 44–53. [Google Scholar]
- Marais, MF; Joseleau, JP. A fucoidan fraction from Ascophyllum nodosum. Carbohydr Res 2001, 336, 155–159. [Google Scholar]
- Chizhov, AO; Dell, A; Morris, HR; Haslam, SM; McDowell, RA; Shashkov, AS; Nifant’ev, NE; Khatuntseva, EA; Usov, AI. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr Res 1999, 320, 108–119. [Google Scholar]
- Bilan, MI; Grachev, AA; Ustuzhanina, NE; Shashkov, AS; Nifantiev, NE; Usov, AI. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr Res 2004, 339, 511–517. [Google Scholar]
- Bilan, MI; Grachev, AA; Ustuzhanina, NE; Shashkov, AS; Nifantiev, NE; Usov, AI. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr Res 2002, 337, 719–730. [Google Scholar]
- Bilan, MI; Grachev, AA; Shashkov, AS; Nifantiev, NE; Usov, AI. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr Res 2006, 341, 238–245. [Google Scholar]
- Usov, AI; Smirnova, GP; Bilan, MI; Shashkov, AS. Polysaccharides of algae: 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Bioorg Khim 1998, 24, 382–389. [Google Scholar]
- Adhikari, U; Mateu, CG; Chattopadhyay, K; Pujol, CA; Damonte, EB; Ray, B. Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar]
- Nagaoka, M; Shibata, H; Kimura-Takagi, I; Hashimoto, S; Kimura, K; Makino, T; Aiyama, R; Ueyama, S; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus TOKIDA. Glycoconj J 1999, 16, 19–26. [Google Scholar]
- Rocha, HAO; Moraes, FA; Trindade, ES; Franco, CRC; Torquato, RJS; Veiga, SS; Valente, AP; Mourao, PAS; Leite, EL; Nader, HB; Dietrich, CP. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spagtoglossum schroederi: An ideal antithrombotic agent? J Biol Chem 2005, 280, 41278–41288. [Google Scholar]
- Nishino, T; Yokoyama, G; Dobashi, K; Fujihara, M; Nagumo, T. Isolation, purification, and characterization of fucose-containing sulfated polysaccharides from the brown seaweed Ecklonia kurome and their blood-anticoagulant activities. Carbohydr Res 1989, 186, 119–129. [Google Scholar]
- Duarte, MER; Cardoso, MA; Noseda, MD; Cerezo, AS. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr Res 2001, 333, 281–293. [Google Scholar]
- Li, B; Wei, XJ; Sun, JL; Xu, SY. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed Hizikia fusiforme. Carbohydr Res 2006, 341, 1135–1146. [Google Scholar]
- Leite, EL; Medeiros, MGL; Rocha, HAO; Farias, GGM; da Silva, LF; Chavante, SF; de Abreu, LD; Dietrich, CP; Nader, HB. Structure and pharmacological activities of a sulfated xylofucoglucuronan from the alga Spatoglossum schroderi. Plant Sci 1998, 132, 215–228. [Google Scholar]
- Ponce, NMA; Pujol, CA; Damonte, EB; Flores, ML; Stortz, CA. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr Res 2003, 338, 153–165. [Google Scholar]
- Teruya, T; Tatemoto, H; Konishi, T; Tako, M. Structural characteristics and in vitro macrophage activation of acetyl fucoidan from Cladosiphon okamuranus. Glycoconj J 2009, 26, 1019–1028. [Google Scholar]
- Bjorndal, H; Hellerqvist, CG; Lindberg, B; Svensson, S. Gas-liquid chromatography and mass spectrometry in methylation analysis of polysaccharides. Angew Chem Int Ed Engl 1970, 9, 610–619. [Google Scholar]
- Bilan, MI; Vinogradova, EV; Tsvetkova, EA; Grachev, AA; Shashkov, AS; Nifantiev, NE; Usov, AI. A sulfated glucuronofucan containing both fucofuranose and fucopyranose residues from the brown alga Chordaria flagelliformis. Carbohydr Res 2008, 343, 2605–2612. [Google Scholar]
- Usov, AI; Adamyants, KS; Miroshnikova, LI; Shaposhnikova, AA; Kochetkov, NK. Solvolytic desulphation of sulphated carbohydrates. Carbohydr Res 1971, 18, 336–338. [Google Scholar]
- Kolender, AA; Matulewicz, MC. Desulfation of sulfated galactans with chlorotrimethylsilane. Characterization of beta-carrageenan by 1H NMR spectroscopy. Carbohydr Res 2004, 339, 1619–1629. [Google Scholar]
- Kantor, TG; Schubert, M. A method for the desulfation of Chondroitin Sulfate1. J Am Chem Soc 1957, 79, 152–153. [Google Scholar]
- Takano, R; Matsuo, M; Kamei-Hayashi, K; Hara, S; Hirase, S. A novel regioselective desulfation method specific to carbohydrate 6-sulfate using silylating reagents. Biosci Biotechnol Biochem 1992, 56, 1577–1580. [Google Scholar]
- Miller, IJ; Blunt, JW. Desulfation of algal galactans. Carbohydr Res 1998, 309, 39–43. [Google Scholar]
- Kusaykin, MI; Chizhov, AO; Grachev, AA; Alekseeva, SA; Bakunina, IY; Nedashkovskaya, OI; Sova, VV; Zvyagintseva, TN. A comparative study of specificity of fucoidanases from marine microorganisms and invertebrates. J Appl Phycol 2006, 18, 369–373. [Google Scholar]
- Ciucanu, I; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res 1984, 131, 209–217. [Google Scholar]
- Zibetti, RGM; Duarte, MER; Noseda, MD; Colodi, FG; Ducatti, DRB; Ferreira, LG; Cardoso, MA; Cerezo, AS. Galactans from Cryptonemia species. Part II: Studies on the system of galactans of Cryptonemia seminervis (Halymeniales) and on the structure of major fractions. Carbohydr Res 2009, 344, 2364–2374. [Google Scholar]
- Usov, AI; Yarotsky, SV; Shashkov, AS. 13C-NMR spectroscopy of red algal galactans. Biopolymers 1980, 19, 977–990. [Google Scholar]
- Gonçalves, AG; Ducatti, DRB; Paranha, RG; Eugênia, M; Duarte, R; Noseda, MD. Positional isomers of sulfated oligosaccharides obtained from agarans and carrageenans: preparation and capillary electrophoresis separation. Carbohydr Res 2005, 340, 2123–2134. [Google Scholar]
- Bilan, MI; Grachev, AA; Shashkov, AS; Kelly, M; Sanderson, CJ; Nifantiev, NE; Usov, AI. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr Res 2010, 345, 2038–2047. [Google Scholar]
- Daniel, R; Chevolot, L; Carrascal, M; Tissot, B; Mourao, PAS; Abian, J. Electrospray ionization mass spectrometry of oligosaccharides derived from fucoidan of Ascophyllum nodosum. Carbohydr Res 2007, 342, 826–834. [Google Scholar]
- Fatema, MK; Nonami, H; Ducatti, DRB; Gonçalves, AG; Duarte, MER; Noseda, MD; Cerezo, AS; Erra-Balsells, R; Matulewicz, MC. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis of oligosaccharides and oligosaccharide alditols obtained by hydrolysis of agaroses and carrageenans, two important types of red seaweed polysaccharides. Carbohydr Res 2010, 345, 275–283. [Google Scholar]
- Goncalves, AG; Ducatti, DR; Grindley, TB; Duarte, ME; Noseda, MD. ESI-MS differential fragmentation of positional isomers of sulfated oligosaccharides derived from carrageenans and agarans. J Am Soc Mass Spectrom 2010, 21, 1404–1416. [Google Scholar]
- Yang, B; Yu, G; Zhao, X; Jiao, G; Ren, S; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J 2009, 276, 2125–2137. [Google Scholar]
- Nishino, T; Nagumo, T. Anticoagulant and antithrombin activities of oversulfated fucans. Carbohydr Res 1992, 229, 355–362. [Google Scholar]
- Qi, H; Zhang, Q; Zhao, T; Chen, R; Zhang, H; Niu, X; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol 2005, 37, 195–199. [Google Scholar]
- Qi, H; Zhang, Q; Zhao, T; Hu, R; Zhang, K; Li, Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg Med Chem Lett 2006, 16, 2441–2445. [Google Scholar]
- Bhattacharyya, S. Carrageenan-induced innate immune response is modified by enzymes that hydrolyze distinct galactosidic bonds. J Nutr Biochem 2010, 21, 906–913. [Google Scholar]
- Holtkamp, AD; Kelly, S; Ulber, R; Lang, S. Fucoidans and fucoidanases-focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl Microbiol Biotechnol 2009, 82, 1–11. [Google Scholar]
- Nardella, A; Chaubet, F; Boisson-Vidal, C; Blondin, C; Durand, P; Jozefonvicz, J. Anticoagulant low molecular weight fucans produced by radical process and ion exchange chromatography of high molecular weight fucans extracted from the brown seaweed Ascophyllum nodosum. Carbohydr Res 1996, 289, 201–208. [Google Scholar]
- Pomin, VH; Valente, AP; Pereira, MS; Mourao, PAS. Mild acid hydrolysis of sulfated fucans: A selective 2-desulfation reaction and an alternative approach for preparing tailored sulfated oligosaccharides. Glycobiology 2005, 15, 1376–1385. [Google Scholar]
- Qiu, X; Amarasekara, A; Doctor, V. Effect of oversulfation on the chemical and biological properties of fucoidan. Carbohydr Polym 2006, 63, 224–228. [Google Scholar]
- Tao, S; Huina, T; Jing, X; Shuo, Z; Xin, X. Degradation and antioxidant activity of kappa-carrageenans. J Appl Phycol 2010, 117, 194–199. [Google Scholar]
- Teruya, T; Takeda, S; Tamaki, Y; Tako, M. Fucoidan isolated from Laminaria angustata var. longissima induced macrophage activation. Biosci Biotechnol Biochem 2010, 74, 1960–1962. [Google Scholar]
- Michel, G; Nyval-Collen, P; Barbeyron, T; Czjzek, M; Helbert, W. Bioconversion of red seaweed galactans: A focus on bacterial agarases and carrageenases. Appl Microbiol Biotechnol 2006, 71, 23–33. [Google Scholar]
- Barbeyron, T; L’Haridon, S; Michel, G; Czjzek, M. Mariniflexile fucanivorans sp. nov., a marine member of the Flavobacteriaceae that degrades sulphated fucans from brown algae. Int J Syst Evol Microbiol 2008, 58, 2107–2113. [Google Scholar]
- Barbeyron, T; Michel, G; Potin, P; Henrissat, B; Kloareg, B. Iota-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of kappa-carrageenases. J Biol Chem 2000, 275, 35499–35505. [Google Scholar]
- Burtseva, YV; Kusaikin, MI; Sova, VV; Shevchenko, NM; Skobun, AS; Zvyagintseva, TN. Distribution of fucoidan hydrolases and some glycosidases among marine invertebrates. Russ J Mar Biol 2000, 26, 453–456. [Google Scholar]
- Guibet, M; Colin, S; Barbeyron, T; Genicot, S; Kloareg, B; Michel, G; Helbert, W. Degradation of lambda-carrageenan by Pseudoalteromonas carrageenovora lambda-carrageenase: A new family of glycoside hydrolases unrelated to kappa-and iota-carrageenases. Biochem J 2007, 404, 105–114. [Google Scholar]
- Lemoine, M; Collen, PN; Helbert, W. Physical state of iota-carrageenan modulates the mode of action of iota-carrageenase from Pseudoalteromonas carrageenovora. Biochem J 2009, 419, 545–553. [Google Scholar]
- Colliec-Jouault, S; Millet, J; Helley, D; Sinquin, C; Fischer, AM. Effect of low-molecular-weight fucoidan on experimental arterial thrombosis in the rabbit and rat. J Thromb Haemost 2003, 1, 1114–1115. [Google Scholar]
- Toyama, MH; Toyama, DO; Torres, VM; Pontes, GC; Farias, WRL; Melo, FR; Oliveira, SCB; Fagundes, FHR; Diz Filho, EBS; Cavada, BS. Effects of Low Molecular Weight Sulfated Galactan Fragments From Botryocladia occidentalis on the Pharmacological and Enzymatic Activity of Spla2 From Crotalus Durissus Cascavella. Protein J 2010, 1–5. [Google Scholar]
- Bondu, S; Deslandes, E; Fabre, MS; Berthou, C; Yu, G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr Polym 2010, 81, 448–460. [Google Scholar]
- Mou, H; Xiaolu, J; Huashi, G. A kappa-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J Appl Phycol 2003, 15, 297–303. [Google Scholar]
- Kitamura, K; Matsuo, M; Yasui, T. Enzymic degradation of fucoidan by fucoidanase from the hepatopancreas of Patinopecten yessoensis. Biosci Biotechnol Biochem 1992, 56, 490–494. [Google Scholar]
- Klarzynski, O; Descamps, V; Plesse, B; Yvin, JC; Kloareg, B; Fritig, B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol Plant Microbe Interact 2003, 16, 115–122. [Google Scholar]
- Kim, KJ; Lee, OH; Lee, HH; Lee, BY. A 4-week repeated oral dose toxicity study of fucoidan from the Sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar]
- Pereira, MS; Mulloy, B; Mourao, PAS. Structure and anticoagulant activity of sulfated fucans. Comparison between the regular, repetitive, and linear fucans from echinoderms with the more heterogeneous and branched polymers from brown algae. J Biol Chem 1999, 274, 7656–7667. [Google Scholar]
- Bernardi, G; Springer, GF. Properties of highly purified fucan. J Biol Chem 1962, 237, 75–80. [Google Scholar]
- Springer, GF; Wurzel, HA; McNeal, GM, Jr; Ansell, NJ; Doughty, MF. Isolation of anticoagulant fractions from crude fucoidin. Proc Soc Exp Biol Med 1957, 94, 404–409. [Google Scholar]
- Grauffel, V; Kloareg, B; Mabeau, S; Durand, P; Jozefonvicz, J. New natural polysaccharides with potent antithrombic activity: Fucans from brown algae. Biomaterials 1989, 10, 363–368. [Google Scholar]
- Kuznetsova, TA; Besednova, NN; Mamaev, AN; Momot, AP; Shevchenko, NM; Zvyagintseva, TN. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull Exp Biol Med 2003, 136, 471–473. [Google Scholar]
- Mauray, S; Sternberg, C; Theveniaux, J; Millet, J; Sinquin, C; Tapon-Bretaudiere, J; Fischer, AM. Venous antithrombotic and anticoagulant activities of a fucoidan fraction. Thromb Haemost 1995, 74, 1280–1285. [Google Scholar]
- Cumashi, A; Ushakova, NA; Preobrazhenskaya, ME; D’Incecco, A; Piccoli, A; Totani, L; Tinari, N; Morozevich, GE; Berman, AE; Bilan, MI; Usov, AI; Ustyuzhanina, NE; Grachev, AA; Sanderson, CJ; Kelly, M; Rabinovich, GA; Iacobelli, S; Nifantiev, NE. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Nishino, T; Nagumo, T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr Res 1991, 214, 193–197. [Google Scholar]
- Nishino, T; Kiyohara, H; Yamada, H; Nagumo, T. An anticoagulant fucoidan from the brown seaweed Ecklonia kurome. Phytochemistry 1991, 30, 535–539. [Google Scholar]
- Pomin, VH; Pereira, MS; Valente, AP; Tollefsen, DM; Pavao, MSG; Mourao, PAS. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar]
- Nishino, T; Aizu, Y; Nagumo, T. The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thromb Res 1991, 64, 723–731. [Google Scholar]
- Hayakawa, Y; Hayashi, T; Lee, J-B; Srisomporn, P; Maeda, M; Ozawa, T; Sakuragawa, N. Inhibition of thrombin by sulfated polysaccharides isolated from green algae. Biochim Biophys Acta 2000, 1543, 86–94. [Google Scholar]
- Shanmugam, M; Mody, KH. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr Sci 2000, 79, 1672–1683. [Google Scholar]
- Farias, WRL; Valente, AP; Pereira, MS; Mourao, PAS. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem 2000, 275, 29299–29307. [Google Scholar]
- Glauser, BF; Rezende, RM; Melo, FR; Pereira, MS; Francischetti, IMB; Monteiro, RQ; Rezaie, AR; Mourao, PAS. Anticoagulant activity of a sulfated galactan: Serpin-independent effect and specific interaction with factor Xa. Thromb Haemost 2009, 102, 1183–1193. [Google Scholar]
- Pereira, MG; Benevides, NMB; Melo, MRS; Valente, AP; Melo, FR; Mourao, PAS. Structure and anticoagulant activity of a sulfated galactan from the red alga, Gelidium crinale. Is there a specific structural requirement for the anticoagulant action? Carbohydr Res 2005, 340, 2015–2023. [Google Scholar]
- Mourao, PAS; Pereira, MS. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc Med 1999, 9, 225–232. [Google Scholar]
- Anderson, JA; Fredenburgh, JC; Stafford, AR; Guo, YS; Hirsh, J; Ghazarossian, V; Weitz, JI. Hypersulfated low molecular weight heparin with reduced affinity for antithrombin acts as an anticoagulant by inhibiting intrinsic tenase and prothrombinase. J Biol Chem 2001, 276, 9755–9761. [Google Scholar]
- Barrow, RT; Parker, ET; Krishnaswamy, S; Lollar, P. Inhibition by heparin of the human blood coagulation intrinsic pathway factor X activator. J Biol Chem 1994, 269, 26796–26800. [Google Scholar]
- Millet, J; Jouault, SC; Mauray, S; Theveniaux, J; Sternberg, C; Vidal, CB; Fischer, AM. Antithrombotic and anticoagulant activities of a low molecular weight fucoidan by the subcutaneous route. Thromb Haemost 1999, 81, 391–395. [Google Scholar]
- Boisson-Vidal, C; Chaubet, F; Chevolot, L; Sinquin, C; Theveniaux, J; Millet, J; Sternberg, C; Mulloy, B; Fischer, AM. Relationship between antithrombotic activities of fucans and their structure. Drug Dev Res 2000, 51, 216–224. [Google Scholar]
- Witvrouw, M; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol 1997, 29, 497–511. [Google Scholar]
- Schaeffer, DJ; Krylov, VS. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf 2000, 45, 208–227. [Google Scholar]
- Damonte, EB; Matulewicz, MC; Cerezo, AS. Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem 2004, 11, 2399–2419. [Google Scholar]
- Luescher-Mattli, M. Algae, A Possible Source for New Drugs in the Treatment of HIV and Other Viral Diseases. Curr Med Chem 2003, 2, 219–225. [Google Scholar]
- Gerber, P; Dutcher, JD; Adams, EV; Sherman, JH. Protective effect of seaweed extracts for chicken embryos infected with influenza B or mumps virus. Proc Soc Exp Biol Med 1958, 99, 590–593. [Google Scholar]
- Nahmias, AJ; Kibrick, S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol 1964, 87, 1060–1066. [Google Scholar]
- Ghosh, T; Chattopadhyay, K; Marschall, M; Karmakar, P; Mandal, P; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Hidari, KIPJ; Takahashi, N; Arihara, M; Nagaoka, M; Morita, K; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun 2008, 376, 91–95. [Google Scholar]
- Talarico, LB; Duarte, MER; Zibetti, RGM; Noseda, MD; Damonte, EB. An algal-derived DL-galactan hybrid is an efficient preventing agent for in vitro dengue virus infection. Planta Med 2007, 73, 1464–1468. [Google Scholar]
- Talarico, LB; Damonte, EB. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar]
- Talarico, LB; Pujol, CA; Zibetti, RGM; Farea, PCS; Noseda, MD; Duarte, MER; Damonte, EB. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antiviral Res 2005, 66, 103–110. [Google Scholar]
- Ghosh, T; Pujol, CA; Damonte, EB; Sinha, S; Ray, B. Sulfated xylomannans from the red seaweed Sebdenia polydactyla: structural features, chemical modification and antiviral activity. Antivir Chem Chemother 2009, 19, 235–242. [Google Scholar]
- Harden, EA; Falshaw, R; Carnachan, SM; Kern, ER; Prichard, MN. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antiviral Res 2009, 83, 282–289. [Google Scholar]
- Mohsen, MSA; Mohamed, SF; Ali, FM; El-Sayed, OH. Chemical Structure and Antiviral Activity of Water-soluble Sulfated Polysaccharides from Sargassum latifolium. J Appl Sci Res 2007, 3, 1178–1185. [Google Scholar]
- Carlucci, MJ; Scolaro, LA; Noseda, MD; Cerezo, AS; Damonte, EB. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res 2004, 64, 137–141. [Google Scholar]
- Chen, D; Wu, XZ; Wen, ZY. Sulfated polysaccharides and immune response: promoter or inhibitor? Panminerva Med 2008, 50, 177–183. [Google Scholar]
- Groth, I; Grunewald, N; Alban, S. Pharmacological profiles of animal- and nonanimal-derived sulfated polysaccharides--comparison of unfractionated heparin, the semisynthetic glucan sulfate PS3, and the sulfated polysaccharide fraction isolated from Delesseria sanguinea. Glycobiology 2009, 19, 408–417. [Google Scholar]
- Granert, C; Raud, J; Xie, X; Lindquist, L; Lindbom, L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Invest 1994, 93, 929–936. [Google Scholar]
- Preobrazhenskaya, ME; Berman, AE; Mikhailov, VI; Ushakova, NA; Mazurov, AV; Semenov, AV; Usov, AI; Nifant’ev, NE; Bovin, NV. Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem Mol Biol Int 1997, 43, 443–451. [Google Scholar]
- Senni, K; Gueniche, F; Foucault-Bertaud, A; Igondjo-Tchen, S; Fioretti, F; Colliec-Jouault, S; Durand, P; Guezennec, J; Godeau, G; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch Biochem Biophys 2006, 445, 56–64. [Google Scholar]
- Parish, CR; Freeman, C; Hulett, MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta 2001, 1471, M99–M108. [Google Scholar]
- Blondin, C; Fischer, E; Boisson-Vidal, C; Kazatchkine, MD; Jozefonvicz, J. Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol Immunol 1994, 31, 247–253. [Google Scholar]
- Clement, MJ; Tissot, B; Chevolot, L; Adjadj, E; Du, Y; Curmi, PA; Daniel, R. NMR characterization and molecular modeling of fucoidan showing the importance of oligosaccharide branching in its anticomplementary activity. Glycobiology 2010, 20, 883–894. [Google Scholar]
- Tissot, B; Daniel, R. Biological properties of sulfated fucans: The potent inhibiting activity of algal fucoidan against the human complement system. Glycobiology 2003, 13, 29G–31G. [Google Scholar]
- Tissot, B; Gonnet, F; Iborra, A; Berthou, C; Thielens, N; Arlaud, GJ; Daniel, R. Mass spectrometry analysis of the oligomeric C1q protein reveals the B chain as the target of trypsin cleavage and interaction with fucoidan. Biochemistry 2005, 44, 2602–2609. [Google Scholar]
- Tissot, B; Montdargent, B; Chevolot, L; Varenne, A; Descroix, S; Gareil, P; Daniel, R. Interaction of fucoidan with the proteins of the complement classical pathway. Biochim Biophys Acta 2003, 1651, 5–16. [Google Scholar]
- Tsuji, RF; Hoshino, K; Noro, Y; Tsuji, NM; Kurokawa, T; Masuda, T; Akira, S; Nowak, B. Suppression of allergic reaction by lambda-carrageenan: toll-like receptor 4/MyD88-dependent and -independent modulation of immunity. Clin Exp Allergy 2003, 33, 249–258. [Google Scholar]
- Maruyama, H; Tamauchi, H; Hashimoto, M; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int Arch Allergy Immunol 2005, 137, 289–294. [Google Scholar]
- Leiro, JM; Castro, R; Arranz, JA; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int Immunopharmacol 2007, 7, 879–888. [Google Scholar]
- Nakamura, T; Suzuki, H; Wada, Y; Kodama, T; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-kB-dependent signaling pathways through macrophage scavenger receptors. Biochem Biophys Res Commun 2006, 343, 286–294. [Google Scholar]
- Yang, JW; Yoon, SY; Oh, SJ; Kim, SK; Kang, KW. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun 2006, 346, 345–350. [Google Scholar]
- Do, H; Pyo, S; Sohn, EH. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J Nutr Biochem 2010, 21, 671. [Google Scholar]
- Choi, EM; Kim, AJ; Kim, YO; Hwang, JK. Immunomodulating activity of arabinogalactan and focoidan in vitro. J Med Food 2005, 8, 446–453. [Google Scholar]
- Kim, M-H; Joo, H-G. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett 2008, 115, 138–143. [Google Scholar]
- Zhou, G; Sun, Y; Xin, H; Zhang, Y; Li, Z; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol Res 2004, 50, 47–53. [Google Scholar]
- Ruparez, P; Ahrazem, O; Leal, JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem 2002, 50, 840–845. [Google Scholar]
- Rocha de Souza, M; Marques, C; Guerra Dore, C; Ferreira da Silva, F; Oliveira Rocha, H; Leite, E. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J Appl Phycol 2007, 19, 153–160. [Google Scholar]
- Zhao, X; Xue, C; Cai, Y; Wang, D; Fang, Y. Study of antioxidant activities of fucoidan from Laminaria japonica. High Tech Lett 2005, 11, 91–94. [Google Scholar]
- Costa, LS; Fidelis, GP; Cordeiro, SL; Oliveira, RM; Sabry, DA; Ciara, RBG; Nobre, LTDB; Costa, MSSP; Almeida-Lima, J; Farias, EHC; Leite, EL; Rocha, HAO. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed Pharmacother 2010, 64, 21–28. [Google Scholar]
- Wang, J; Zhang, Q; Zhang, Z; Song, H; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int J Biol Macromol 2010, 46, 6–12. [Google Scholar]
- Wang, J; Liu, L; Zhang, Q; Zhang, Z; Qi, H; Li, P. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem 2009, 114, 1285–1290. [Google Scholar]
- Vaquez-Freire, MJ; Lamela, M; Calleja, JM. Hypolipidaemic activity of a polysaccharide extract from Fucus vesiculosus. Phytother Res 1996, 10, 647–650. [Google Scholar]
- Huang, L; Wen, K; Gao, X; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm Biol 2010, 48, 422–426. [Google Scholar]
- Pengzhan, Y; Ning, L; Xiguang, L; Gefei, Z; Quanbin, Z; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol Res 2003, 48, 543–549. [Google Scholar]
- Yokota, T; Nagashima, M; Ghazizadeh, M; Kawanami, O. Increased effect of fucoidan on lipoprotein lipase secretion in adipocytes. Life Sci 2009, 84, 523–529. [Google Scholar]
- Raghavendran, HR; Sathivel, A; Devaki, T. Effect of Sargassum polycystum (Phaeophyceae)-sulphated polysaccharide extract against acetaminophen-induced hyperlipidemia during toxic hepatitis in experimental rats. Mol Cell Biochem 2005, 276, 89–96. [Google Scholar]
- Josephine, A; Veena, CK; Amudha, G; Preetha, SP; Varalakshmi, P. Protective role of sulphated polysaccharides in abating the hyperlipidemic nephropathy provoked by cyclosporine A. Arch Toxicol 2007, 81, 371–379. [Google Scholar]
- Bourgougnon, N; Lahaye, M; Quemener, B; Chermann, JC; Rimbert, M; Cormaci, M; Furnari, G; Kornprobst, JM. Annual variation in composition and in vitro anti-HIV-1 activity of the sulfated glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartinales). J Appl Phycol 1996, 8, 155–161. [Google Scholar]
- Hiebert, LM. Oral heparins. Clin Lab 2002, 48, 111–116. [Google Scholar]
- Irhimeh, MR; Fitton, JH; Lowenthal, RM. Pilot clinical study to evaluate the anticoagulant activity of fucoidan. Blood Coagul Fibrin 2009, 20, 607–610. [Google Scholar]
- Ewart, HS; Bloch, O; Girouard, GS; Kralovec, J; Barrow, CJ; Ben-Yehudah, G; Suárez, ER; Rapoport, MJ. Stimulation of cytokine production in human peripheral blood mononuclear cells by an aqueous Chlorella extract. Planta Med 2007, 73, 762–768. [Google Scholar]
- Fitton, JH; Irimeh, M; Falk, N. Macroalgal fucoidan extracts: a new opportunity for marine cosmetics. Cosmet Toiletries 2007, 122, 55–64. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).